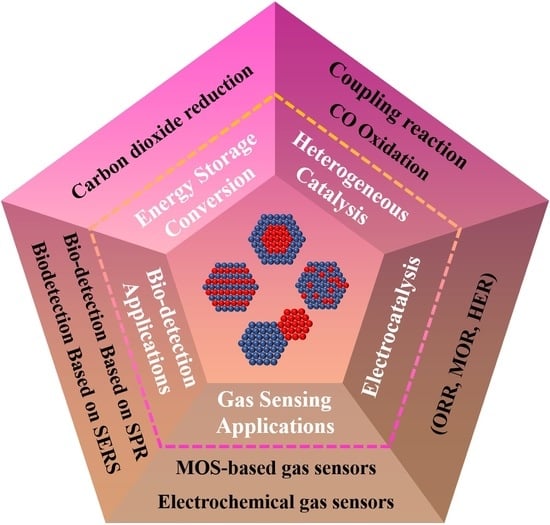
Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing
Abstract
:1. Introduction
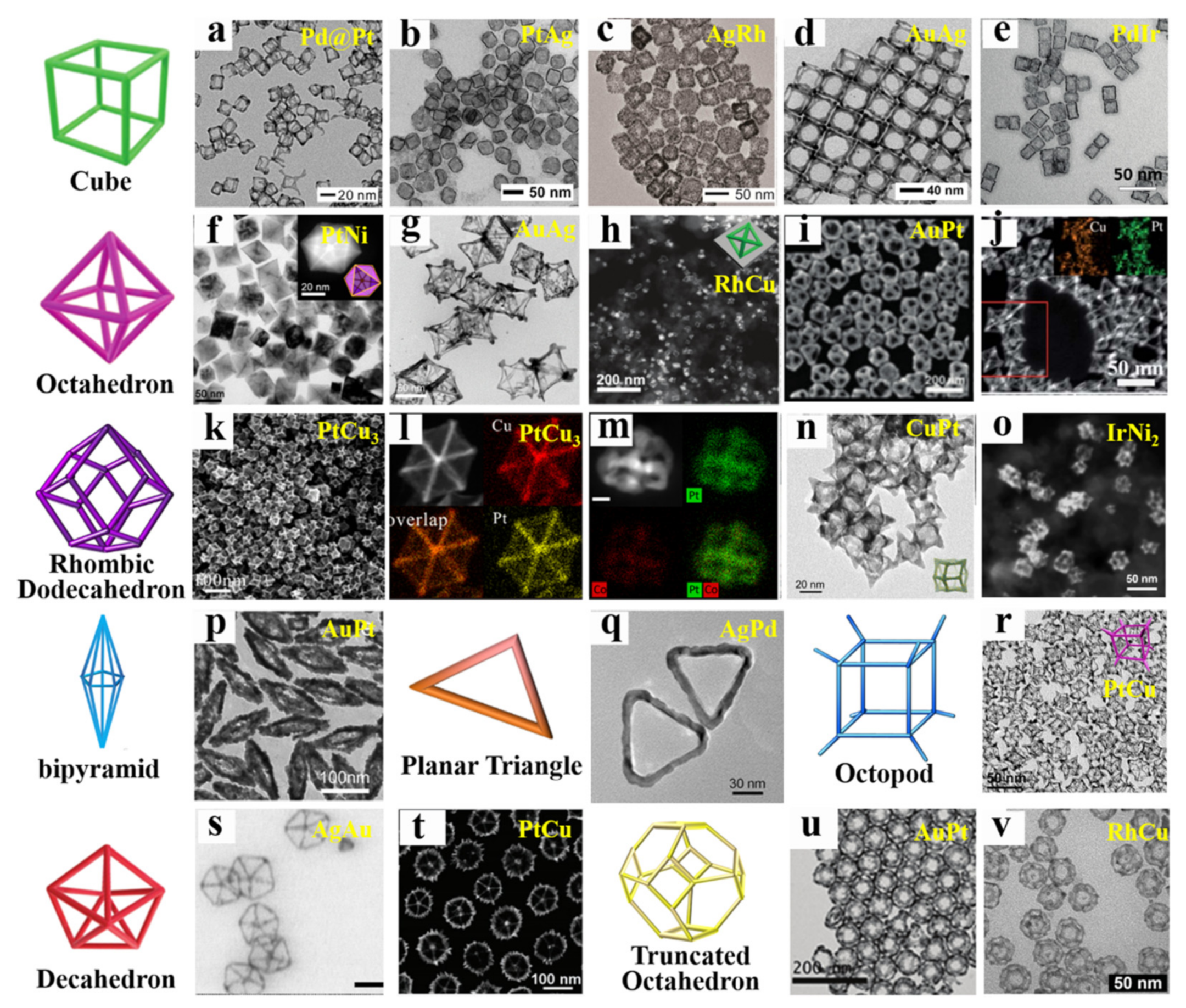
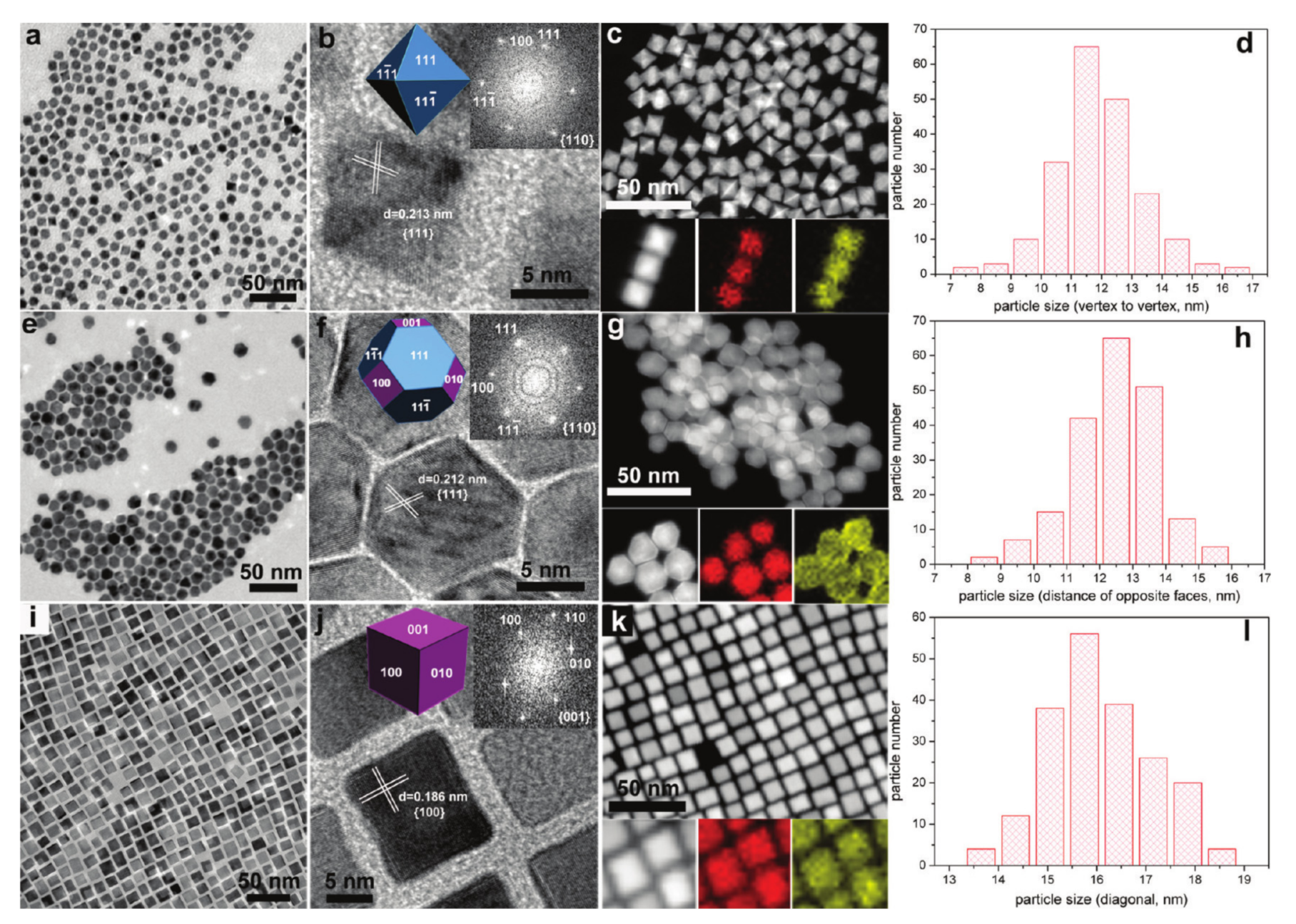
2. The Structures of Bimetallic Nanocrystals
2.1. Alloyed Structure
2.2. Core-Shell Structure
2.3. Heterostructure
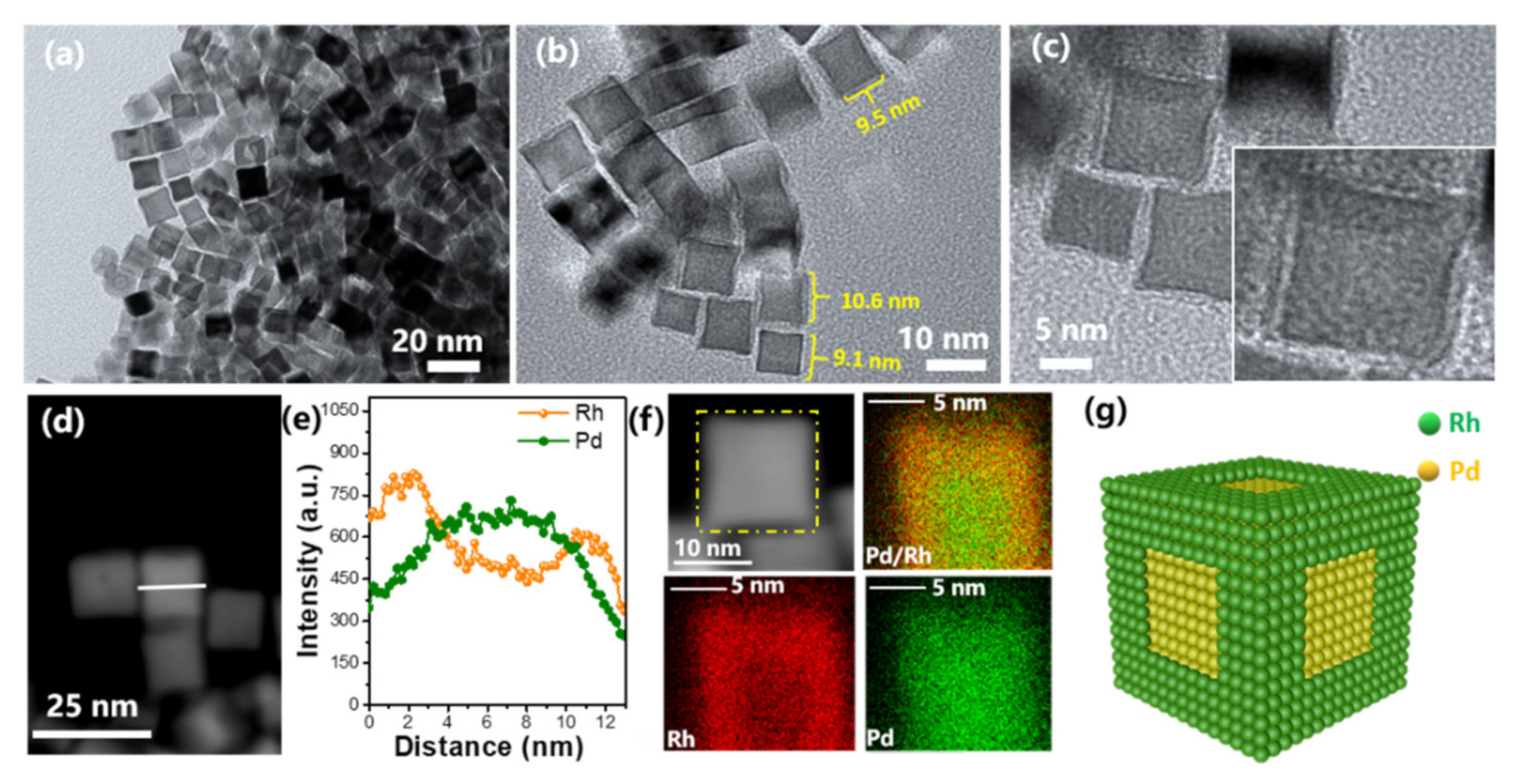
2.4. Core-Frame and Other Derivative Structure
3. Controllable Synthesis of Bimetallic Nanocrystals
3.1. Co-Reduction
3.1.1. Solvothermal Method
3.1.2. Oil Phase Method
3.2. Seed-Mediated Growth
3.3. Thermal Decomposition
3.4. Galvanic Replacement Reaction
3.5. Other Methods
4. Applications of Bimetallic Nanocrystals
4.1. Catalytic Applications
Electrocatalysis
- Oxygen Reduction Reaction (ORR)
- 2.
- Methanol Oxidation Reaction (MOR)
- 3.
- Oxygen Evolution Reaction (OER)
4.2. Heterogeneous Catalysis
4.3. Energy Conversion Applications
4.4. Sensing Applications
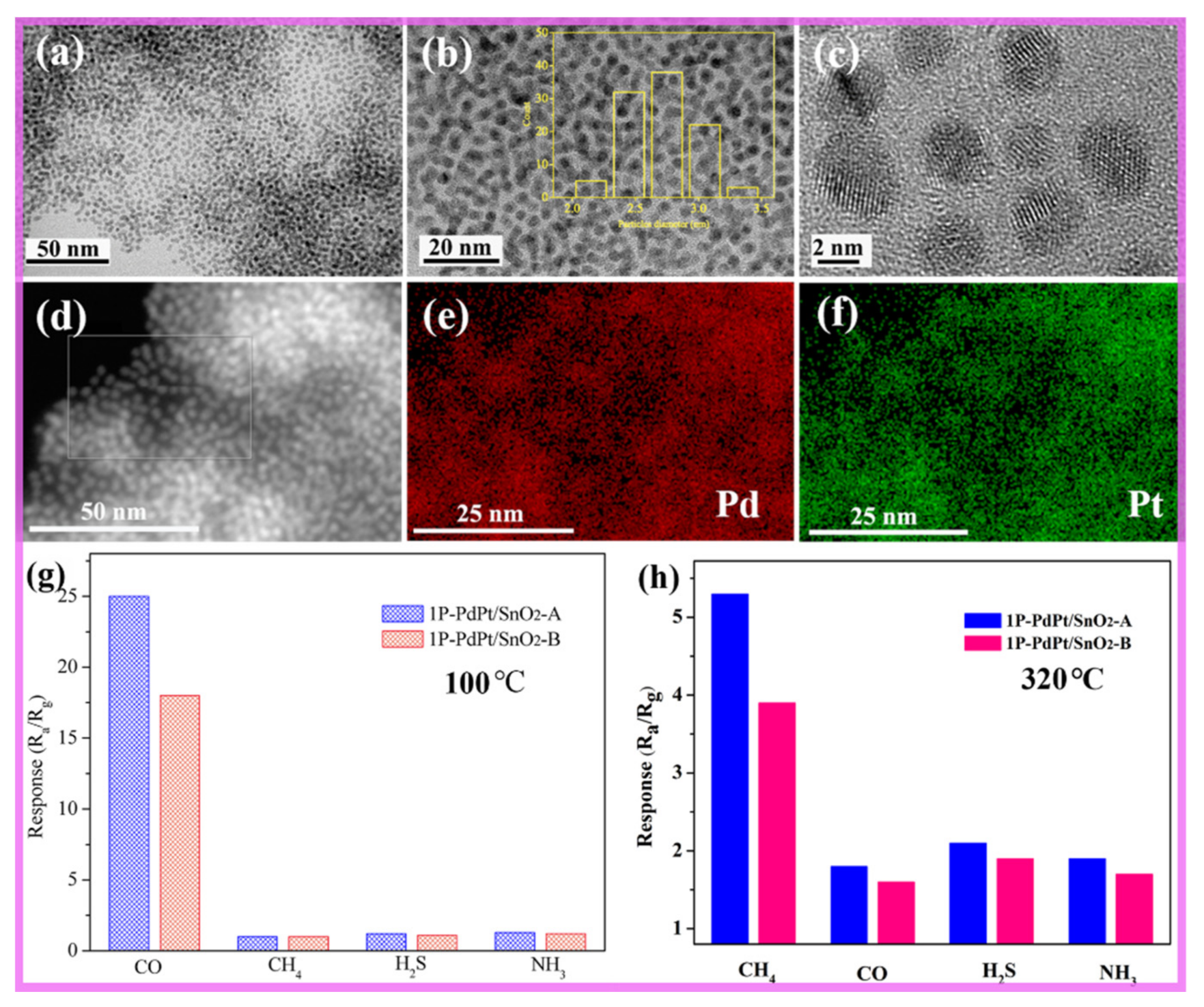
4.4.1. Metal Oxide Semiconducting (MOS) Sensors
4.4.2. Electrochemical Sensors
4.4.3. Catalytic Combustion Gas Sensor
4.5. Biodetection Applications
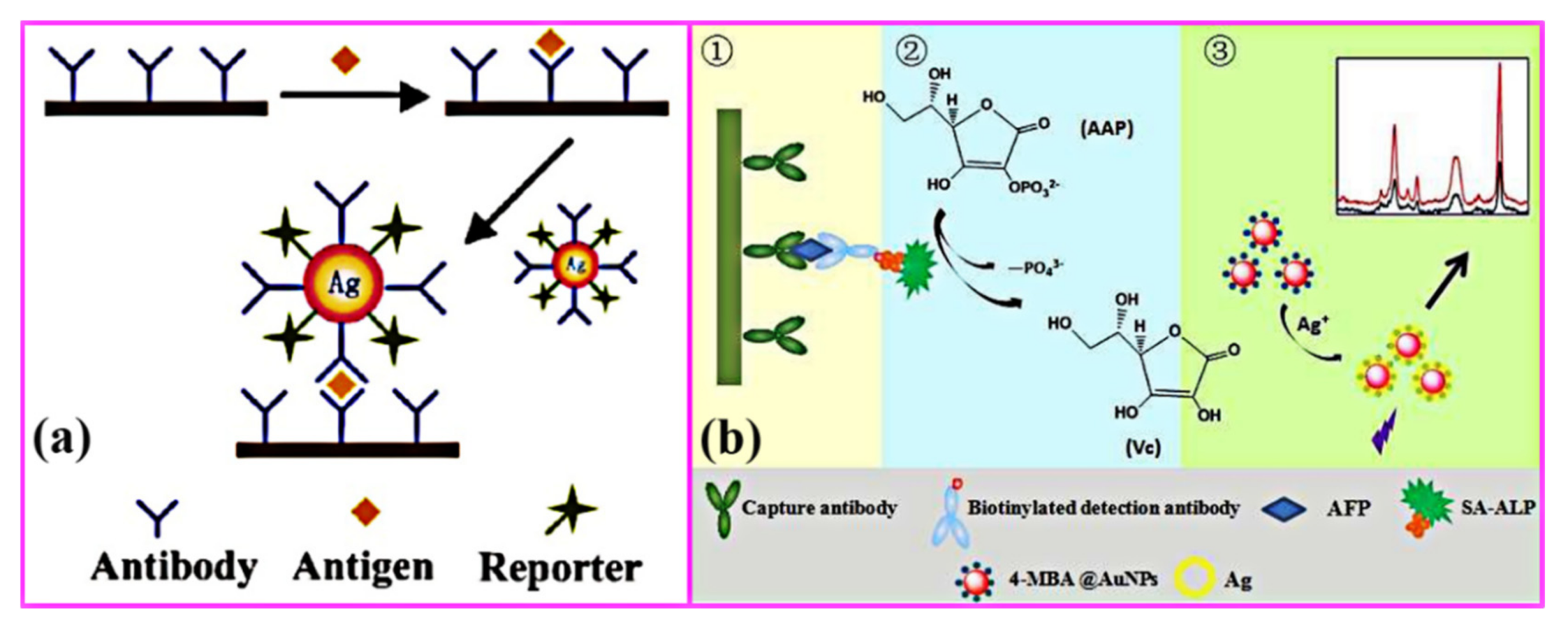
4.5.1. Biodetection Based on Surface Plasmon Resonance (SPR) and Local Surface Plasmon Resonance (LSPR)
4.5.2. Biodetection Based on Surface-Enhanced Raman Scattering (SERS)
5. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, J.F.; Fields-Zinna, C.A.; Murray, R.W. The story of a monodisperse gold nanoparticle: Au25L18. Acc. Chem. Res. 2010, 43, 1289–1296. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Small is different: Shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc. Chem. Res. 2004, 37, 326–333. [Google Scholar] [CrossRef]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Lee, H.M.; Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 1560–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, H.; Yoon, S.; An, K.; Kim, H.Y. Catalytic CO oxidation over Au nanoparticles supported on CeO2 nanocrystals: Effect of the Au–CeO2 interface. ACS Catal. 2018, 8, 11491–11501. [Google Scholar] [CrossRef]
- Liu, J.-X.; Su, Y.; Filot, I.A.; Hensen, E.J. A linear scaling relation for CO oxidation on CeO2-supported Pd. J. Am. Chem. Soc. 2018, 140, 4580–4587. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, S.; Sun, Z.; Cui, N.; Zhao, P.; Tang, Q.; Tong, Y.; Liu, Y. Enhancing the intrinsic stretchability of micropatterned gold film by covalent linkage of carbon nanotubes for wearable electronics. ACS Appl. Electron. Mater. 2019, 1, 1295–1303. [Google Scholar] [CrossRef]
- Murray, C.; Sun, S.; Doyle, H.; Betley, T. Monodisperse 3d transition-metal (Co, Ni, Fe) nanoparticles and their assembly intonanoparticle superlattices. MRS Bull. 2001, 26, 985–991. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Zhang, N.; Shao, Q.; Xiao, X.; Huang, X. Advanced catalysts derived from composition-segregated platinum–nickel nanostructures: New opportunities and challenges. Adv. Funct. Mater. 2019, 29, 1808161. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Lu, P.; Zhang, K.; Zhou, P.; Liu, Y.; Guo, Z.; Lu, X. Gold/silver bimetallic nanocrystals: Controllable synthesis and biomedical applications. J. Biomed. Nanotechnol. 2017, 13, 1178–1209. [Google Scholar] [CrossRef]
- Wang, D.; Deng, L.; Cai, H.; Yang, J.; Bao, L.; Zhu, Y.; Wang, X. Bimetallic PtCu nanocrystal sensitization WO3 hollow spheres for highly efficient 3-Hydroxy-2-butanone biomarker detection. ACS Appl. Mater. Interfaces 2020, 12, 18904–18912. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Holewinski, A.; Schweitzer, N.; Nikolla, E.; Linic, S. Electronic structure engineering in heterogeneous catalysis: Identifying novel alloy catalysts based on rapid screening for materials with desired electronic properties. Top. Catal. 2012, 55, 376–390. [Google Scholar] [CrossRef]
- Zhao, X.; Xi, C.; Zhang, R.; Song, L.; Wang, C.; Spendelow, J.S.; Frenkel, A.I.; Yang, J.; Xin, H.L.; Sasaki, K. High-Performance nitrogen-doped intermetallic PtNi catalyst for the oxygen reduction reaction. ACS Catal. 2020, 10, 10637–10645. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 16, 10414–10472. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- Kwon, T.; Jun, M.; Lee, K. Catalytic nanoframes and beyond. Adv. Mater. 2020. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random alloyed versus intermetallic nanoparticles: A comparison of electrocatalytic performance. Adv. Mater. 2018, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Li, Y.; Zhou, H.; Duan, X.; Huang, Y. Synthesis of PtPd bimetal nanocrystals with controllable shape, composition, and their tunable catalytic properties. Nano Lett. 2012, 12, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Sytwu, K.; Vadai, M.; Dionne, J.A. Bimetallic nanostructures: Combining plasmonic and catalytic metals for photocatalysis. Adv. Phys. X 2019, 4, 398–422. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.; Wang, Y. Manipulating bimetallic nanostructures with tunable localized surface plasmon resonance and their applications for sensing. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Yin, A.; Min, X.; Zhang, Y.; Yan, C. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt-Pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Liu, Q.; Zhou, M.; Mi, G.; Du, X. Hierarchically alloyed Pd–Cu microarchitecture with tunable shapes: Morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane. Int. J. Hydrogen Energy 2019, 44, 30226–30236. [Google Scholar] [CrossRef]
- Habas, S.; Lee, H.; Radmilovic, V.; Somorjai, G.; Yang, P. Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 2007, 6, 692–697. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Fang, J.; Zou, S. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef]
- Shen, M.; Xie, M.; Slack, J.; Waldrop, K.; Chen, Z.; Lyu, Z.; Cao, S.; Zhao, M.; Chi, M.; Pintauro, P.N. Pt−Co truncated octahedral nanocrystals as a highly active and durable catalyst toward the oxygen reduction reaction. Nanoscale 2020. [Google Scholar] [CrossRef]
- Fan, F.-R.; Liu, D.-Y.; Wu, Y.-F.; Duan, S.; Xie, Z.-X.; Jiang, Z.-Y.; Tian, Z.-Q. Epitaxial growth of heterogeneous metal nanocrystals: From gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951. [Google Scholar] [CrossRef]
- Yin, A.-X.; Min, X.-Q.; Zhu, W.; Wu, H.-S.; Zhang, Y.-W.; Yan, C.-H. Multiply twinned Pt–Pd nanoicosahedrons as highly active electrocatalysts for methanol oxidation. Chem. Commun. 2012, 48, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Kim, M.; Kang, S.W.; Han, S.W. Polyhedral bimetallic alloy nanocrystals exclusively bound by {110} facets: Au–Pd rhombic dodecahedra. Angew. Chem. Int. Ed. 2011, 50, 3466–3470. [Google Scholar] [CrossRef]
- Singh, G.; Sunde, S.; Seland, F. Synthesis of CO-tolerant Ni-Pt rhombic dodecahedra bimetallic electrocatalytic nanoparticles. ChemNanoMat 2020, 6, 1220–1228. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, Z.; Henderson, N.L.; Bauer, J.C.; Goodman, D.W.; Batteas, J.D.; Schaak, R.E. Synthesis of CuPt nanorod catalysts with tunable lengths. J. Am. Chem. Soc. 2009, 131, 5720–5721. [Google Scholar] [CrossRef]
- Liao, H.G.; Cui, L.; Whitelam, S.; Zheng, H. Real-time imaging of Pt3Fe nanorod growth in solution. Science 2012, 336, 1011–1014. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.X.; Hao, B.W.; Nan, L.; Yan, Y.; Xiong, W.D.L.; Xin, W. One-pot synthesis of Pt–Co alloy nanowire assemblies with tunable composition and enhanced electrocatalytic properties. Angew. Chem. Int. Ed. 2015, 54, 3797–3801. [Google Scholar] [CrossRef]
- Fang, D.; Wan, L.; Jiang, Q.; Zhang, H.; Tang, X.; Qin, X.; Shao, Z.; Wei, Z. Wavy PtCu alloy nanowire networks with abundant surface defects enhanced oxygen reduction reaction. Nano Res. 2019, 12, 2766–2773. [Google Scholar] [CrossRef]
- Tang, J.X.; Chen, Q.S.; You, L.X.; Liao, H.G.; Sun, S.G.; Zhou, S.G.; Xu, Z.N.; Chen, Y.; Guo, G.C. Screw-like PdPt nanowires as highly efficient electrocatalysts for methanol and ethylene glycol oxidation. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Lim, B.; Lu, X.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Lee, E.P.; Xia, Y. Facile synthesis of highly faceted multioctahedral Pt nanocrystals through controlled overgrowth. Nano Lett. 2008, 8, 4043–4047. [Google Scholar] [CrossRef]
- Lee, C.-L.; Tseng, C.-M.; Wu, R.-B.; Wu, C.-C.; Syu, C.-M. Porous Ag–Pd triangle nanoplates with tunable alloy ratio for catalyzing electroless copper deposition. Colloids Surf. A Physicochem. Eng. Asp. 2009, 352, 84–87. [Google Scholar] [CrossRef]
- Hu, C.; Mu, X.; Fan, J.; Ma, H.; Zhao, X.; Chen, G.; Zhou, Z.; Zheng, N. Interfacial effects in PdAg bimetallic nanosheets for selective dehydrogenation of formic acid. ChemNanoMat 2016, 2, 28–32. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Fu, G.; Xu, L.; Sun, D.; Lee, J.-M.; Tang, Y. Core–shell CuPd@Pd tetrahedra with concave structures and Pd-enriched surface boost formic acid oxidation. J. Mater. Chem. A 2018, 6, 10632–10638. [Google Scholar] [CrossRef]
- Xie, S.; Lu, N.; Xie, Z.; Wang, J.; Kim, M.J.; Xia, Y. Synthesis of Pd-Rh core–frame concave nanocubes and their conversion to Rh cubic nanoframes by selective etching of the Pd cores. Angew. Chem. Int. Ed. 2012, 124, 10412–10416. [Google Scholar] [CrossRef]
- Yin, S.; Xu, Y.; Liu, S.; Yu, H.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Binary nonmetal S and P-co-doping into mesoporous PtPd nanocages boosts oxygen reduction electrocatalysis. Nanoscale 2020, 12, 14863–14869. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.D. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Sheng, J.; Kang, J.; Ye, H.; Xie, J.; Zhao, B.; Fu, X.-Z.; Yu, Y.; Sun, R.; Wong, C.-P. Porous octahedral PdCu nanocages as highly efficient electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 3906–3912. [Google Scholar] [CrossRef]
- Hood, Z.D.; Kubelick, K.P.; Gilroy, K.D.; Vanderlaan, D.; Yang, X.; Yang, M.; Chi, M.; Emelianov, S.Y.; Xia, Y. Photothermal transformation of Au–Ag nanocages under pulsed laser irradiation. Nanoscale 2019, 11, 3013–3020. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Z.; Gong, J. Shape-controlled synthesis of Au-Pd bimetallic nanocrystals for catalytic applications. Chem. Soc. Rev. 2016, 45, 3916–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Vara, M.; Hood, Z.D.; Luo, M.; Yang, T.H.; Bao, S.; Chi, M.; Xiao, P.; Zhang, Y. Quantitative analysis of the reduction kinetics responsible for the one-pot synthesis of Pd-Pt bimetallic nanocrystals with different structures. J. Am. Chem. Soc. 2016, 138, 12263–12270. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Xia, Y. Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Bao, Y.X.; Hao, B.W.; Xin, W.; Lou, X.W.D. One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef]
- Abe, H.; Matsumoto, F.; Alden, L.R.; Warren, S.C.; Abruna, H.D.; Disalvo, F.J. Electrocatalytic performance of fuel oxidation by Pt3Ti nanoparticles. J. Am. Chem. Soc. 2008, 130, 5452–5458. [Google Scholar] [CrossRef]
- Rong, H. Kinetically controlling surface structure to construct defect-rich intermetallic nanocrystals: Effective and stable catalysts. Adv. Mater. 2016, 28, 2540–2546. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Feng, Q.; He, D.; Li, Y. Intermetallic NixMy (M = Ga and Sn) nanocrystals: A non-precious metal catalyst for semi-hydrogenation of alkynes. Adv. Mater. 2016, 28, 4747–4754. [Google Scholar] [CrossRef]
- Chung, D.; Jun, S.; Yoon, G.; Kwon, S.; Shin, D.; Seo, P.; Yoo, J.; Shin, H.; Chung, Y.; Kim, H.; et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Yan, Y.; Du, J.; Gilroy, K.; Yang, D.; Xia, Y.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29, 1605997. [Google Scholar] [CrossRef] [Green Version]
- Chi, M.; Wang, C.; Lei, Y.; Wang, G.; Li, D.; More, K.L.; Lupini, A.; Allard, L.F.; Markovic, N.M.; Stamenkovic, V.R. Surface faceting and elemental diffusion behaviour at atomic scale for alloy nanoparticles during in situ annealing. Nat. Commun. 2015, 6, 8925. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Pyo, J.B.; Ye, X.; Gordon, T.R.; Murray, C.B. Synthesis, shape control, and methanol electro-oxidation properties of Pt–Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano 2012, 6, 5642–5647. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.; DiSalvo, F.; Abruña, H.D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Zhang, Y.; Bu, L.; Jiang, K.; Guo, S.; Huang, X. Concave Pd-Pt Core–shell nanocrystals with ultrathin Pt shell feature and enhanced catalytic performance. Small 2016, 12, 706–712. [Google Scholar] [CrossRef]
- Hou, S.; Hu, X.; Wen, T.; Liu, W.; Wu, X. Core–shell noble metal nanostructures templated by gold nanorods. Adv. Mater. 2013, 25, 3857–3862. [Google Scholar] [CrossRef]
- Alayoglu, S.; Nilekar, A.U.; Mavrikakis, M.; Eichhorn, B. Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 2008, 7, 333–338. [Google Scholar] [CrossRef]
- Choi, S.I.; Young, A.; Lee, S.R.; Ma, C.; Luo, M.; Chi, M.; Tsung, C.K.; Xia, Y. Pd@Rh core–shell nanocrystals with well-defined facets and their enhanced catalytic performance towards CO oxidation. Nanoscale Horiz. 2019, 4. [Google Scholar] [CrossRef]
- Park, J.; Zhang, L.; Choi, S.I.; Roling, L.T.; Lu, N.; Herron, J.A.; Xie, S.; Wang, J.; Kim, M.J.; Mavrikakis, M. Atomic layer-by-layer deposition of platinum on palladium octahedra for enhanced catalysts toward the oxygen reduction reaction. ACS Nano 2015, 9, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Serpell, C.J.; Cookson, J.; Ozkaya, D.; Beer, P.D. Core@shell bimetallic nanoparticle synthesis via anion coordination. Nat. Chem. 2011, 3, 478–483. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.-I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.; Liu, J.; Xie, Z.; Herron, J.A.; et al. Palladium–platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sundarapandi, M.; Shanmugam, S.; Ramaraj, R. Synthesis and catalytic activities of metal shells (monolayer, bilayer, and alloy layer)-coated gold octahedra toward catalytic reduction of nitroaromatics. J. Phys. Chem. C 2019, 123. [Google Scholar] [CrossRef]
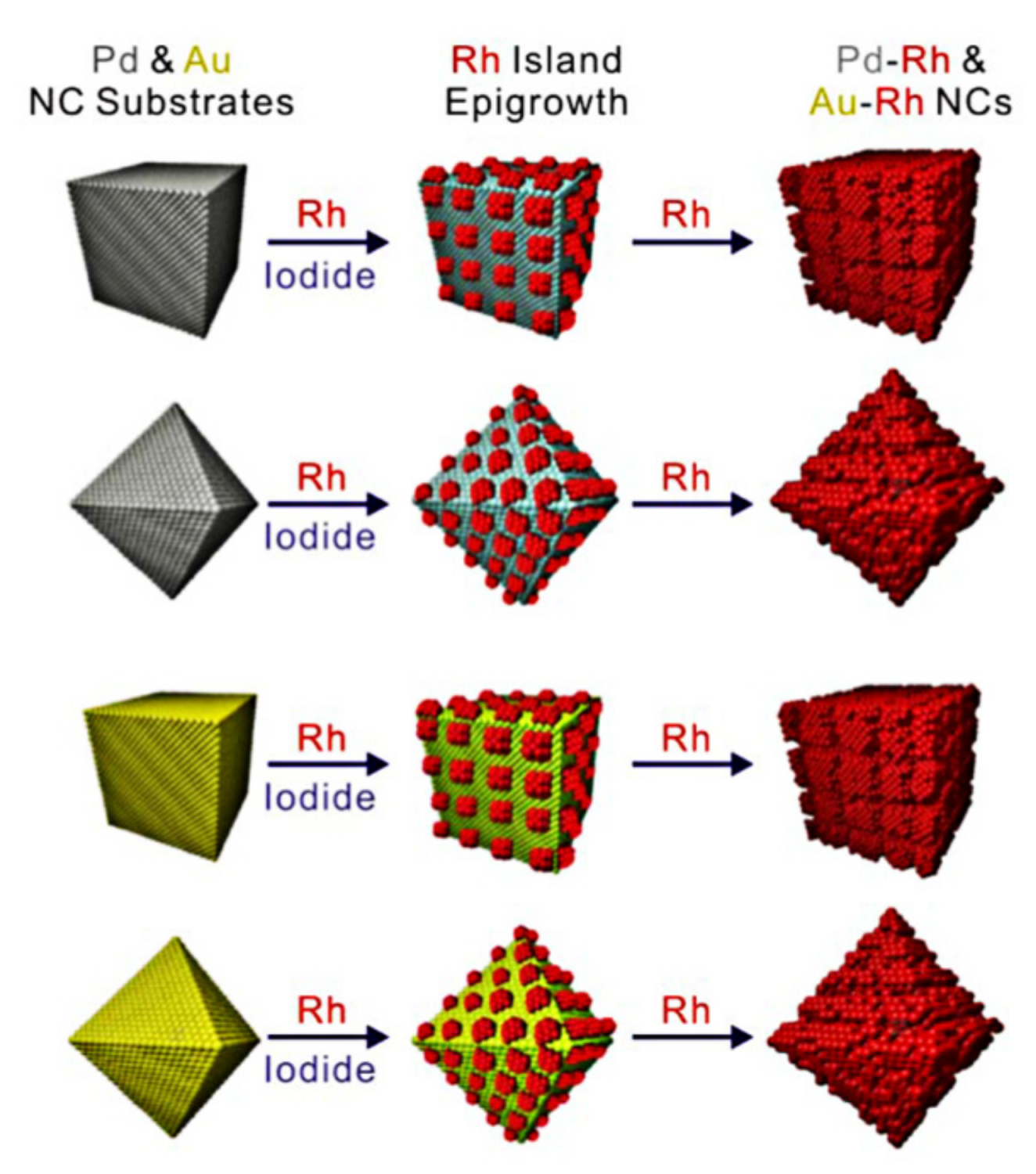
- Sneed, B.T.; Kuo, C.-H.; Brodsky, C.N.; Tsung, C.-K. Iodide-mediated control of rhodium epitaxial growth on well-defined noble metal nanocrystals: Synthesis, characterization, and structure-dependent catalytic properties. J. Am. Chem. Soc. 2012, 134, 18417–18426. [Google Scholar] [CrossRef]
- Zheng, Z.; Tachikawa, T.; Majima, T. Single-particle study of Pt-modified Au nanorods for plasmon-enhanced hydrogen generation in visible to near-infrared region. J. Am. Chem. Soc. 2014, 136, 6870–6873. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Q.; Zhao, X.; Cui, C.; Sang, Y. Electromagnetic induction effect induced high-efficiency hot charge generation and transfer in Pd-tipped Au nanorods to boost plasmon-enhanced formic acid dehydrogenation. Nano Energy 2021, 80, 105543. [Google Scholar] [CrossRef]
- Lu, N.; Wang, J.; Xie, S.; Xia, Y.; Kim, M.J. Enhanced shape stability of Pd–Rh core–frame nanocubes at elevated temperature: In situ heating transmission electron microscopy. Chem. Commun. 2013, 49, 11806–11808. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Cho, S.; Kim, D.; Ih, S.; Lee, S.; Zhang, L.; Hao, L.; Jin, Y.L.; Liu, L.; Park, S. 3D PtAu nanoframe superstructure as a high-performance carbon-free electrocatalyst. Nanoscale 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mensi, M.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag-Cu nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.P.; Doan, D.; Aitken, Z.H.; Chen, S.; Gu, X.W. Hardening in Au-Ag nanoboxes from stacking fault-dislocation interactions. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Liu, H.; Wang, J.; Kim, M.J.; Yang, D.; Xie, Z.; Liu, J.; Xia, Y. Facile Synthesis of Pd–Pt Alloy Nanocages and Their Enhanced Performance for Preferential Oxidation of CO in Excess Hydrogen. ACS Nano 2011, 5, 8212–8222. [Google Scholar] [CrossRef]
- Sheng, J.; Kang, J.; Hu, Z.; Yu, Y.; Fu, X.Z.; Sun, R.; Wong, C.P. Correction: Octahedral Pd nanocages with porous shells converted from Co(OH)2 nanocages with nanosheet surfaces as robust electrocatalysts for ethanol oxidation. J. Mater. Chem. A 2018, 6, 23905. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Wang, D.; Rong, Y.; Peng, Q.; Li, Y. Highly branched Pt–Ni nanocrystals enclosed by stepped surface for methanol oxidation. Chem. Sci. 2012, 3, 1925–1929. [Google Scholar] [CrossRef]
- Park, J.; Wang, H.; Vara, M.; Xia, Y. Platinum cubic nanoframes with enhanced catalytic activity and durability toward oxygen reduction. ChemSusChem 2016, 9, 2855–2861. [Google Scholar] [CrossRef]
- Ahn, J.; Wang, D.; Ding, Y.; Zhang, J.; Qin, D. Site-selective carving and Co-deposition: Transformation of Ag nanocubes into concave nanocrystals encased by Au–Ag alloy frames. ACS Nano 2018, 12, 298–307. [Google Scholar] [CrossRef]
- Xiong, L.; Sun, Z.; Zhang, X.; Zhao, L.; Huang, P.; Chen, X.; Jin, H.; Sun, H.; Lian, Y.; Deng, Z. Octahedral gold-silver nanoframes with rich crystalline defects for efficient methanol oxidation manifesting a CO-promoting effect. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mceachran, M.; Keogh, D.; Pietrobon, B.; Cathcart, N.; Gourevich, I.; Coombs, N.; Kitaev, V. Ultrathin gold nanoframes through surfactant-free templating of faceted pentagonal silver nanoparticles. J. Am. Chem. Soc. 2011, 133, 8066–8069. [Google Scholar] [CrossRef]
- Fang, C.; Zhao, G.; Zhang, Z.; Ding, Q.; Yu, N.; Cui, Z.; Bi, T. AuPt bipyramid nanoframes as multifunctional platforms for in situ monitoring of the reduction of nitrobenzene and enhanced electrocatalytic methanol oxidation. Chem. A Eur. J. 2019, 25, 7351–7358. [Google Scholar] [CrossRef]
- Nosheen, F.; Zhang Z-c Zhuang, J.; Wang, X. One-pot fabrication of single-crystalline octahedral Pt–Cu nanoframes and their enhanced electrocatalytic activity. Nanoscale 2013, 5, 3660–3663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Z.; Chen, B.; Wei, C.; Zhao, J.; Chen, J.; Zhang, X.; Lai, Z.; Fan, Z.; Tan, C. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shen, P.K. Concave platinum–copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis. ACS Nano 2016, 11, 11946–11953. [Google Scholar] [CrossRef]
- Ding, J.; Bu, L.; Guo, S.; Zhao, Z.; Zhu, E.; Huang, Y.; Huang, X. Morphology and phase controlled construction of Pt–Ni nanostructures for efficient electrocatalysis. Nano Lett. 2016, 16, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Shao, Q.; Zhu, X.; Huang, X. Dynamic structure evolution of composition segregated iridium-nickel rhombic dodecahedra toward efficient oxygen evolution electrocatalysis. ACS Nano 2018, 12, 7371–7379. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Cao, W.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. An ultrasensitive electrochemical immunosensor for CEA using MWCNT-NH2 supported PdPt nanocages as labels for signal amplification. J. Mater. Chem. 2015, 3, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, X.; Zhang, Y.; Ding, Y.; Su, D.; Qin, D. Pt–Ag cubic nanocages with wall thickness less than 2 nm and their enhanced catalytic activity toward oxygen reduction. Nanoscale 2017, 9, 15107–15114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ahn, J.; Liu, J.; Qin, D. Syntheses, plasmonic properties, and catalytic applications of Ag–Rh core-frame nanocubes and Rh nanoboxes with highly porous walls. Chem. Mater. 2018, 30, 2151–2159. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Xie, M.; Lyu, Z.; Chi, M.; Mavrikakis, M.; Jin, W.; Xia, Y. Iridium-based cubic nanocages with 1.1-nm-thick walls: A highly efficient and durable electrocatalyst for water oxidation in an acidic medium. Angew. Chem. Int. Ed. 2019, 58, 7244–7248. [Google Scholar] [CrossRef]
- Oh, A.; Baik, H.; Choi, D.S.; Cheon, J.Y.; Kim, B.; Kim, H.; Kwon, S.J.; Joo, S.H.; Jung, Y.; Lee, K. Skeletal octahedral nanoframe with cartesian coordinates via geometrically precise nanoscale phase segregation in a Pt@Ni core-shell nanocrystal. ACS Nano 2015, 9, 2856–2867. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Zhu, W.; Yan, C.-H.; Zhang, Y.-W. Selective synthesis of rhodium-based nanoframe catalysts by chemical etching of 3d metals. Chem. Commun. 2015, 51, 3997–4000. [Google Scholar] [CrossRef]
- Ham, S.; Jang, H.J.; Song, Y.; Shuford, K.L.; Park, S. Octahedral and cubic gold nanoframes with platinum framework. Angew. Chem. Int. Ed. 2015, 54, 9025–9028. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; Zhang, J.; Lei, Z.; Chen, Q.; Xie, Z.; Zheng, L. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Gao, M.; Jin, J.; van Spronsen, M.A.; Salmeron, M.B.; Yang, P. High-performance Pt–Co nanoframes for fuel-cell electrocatalysis. Nano Lett. 2020, 20, 1974–1979. [Google Scholar] [CrossRef]
- Lyu, L.-M.; Kao, Y.-C.; Cullen, D.A.; Sneed, B.T.; Chuang, Y.-C.; Kuo, C.-H. Spiny rhombic dodecahedral CuPt nanoframes with enhanced catalytic performance synthesized from Cu nanocube templates. Chem. Mater. 2017, 29, 5681–5692. [Google Scholar] [CrossRef]
- Xu, L.; Luo, Z.; Fan, Z.; Yu, S.; Chen, J.; Liao, Y.; Xue, C. Controllable galvanic synthesis of triangular Ag-Pd alloy nanoframes for efficient electrocatalytic methanol oxidation. Chemistry 2015, 21, 8966. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yang, Y.; Yoon, D.; Baik, H.; Haam, S.; Yang, H.; Lee, K. RhCu 3D nanoframe as a highly active electrocatalyst for oxygen evolution reaction under alkaline condition. Adv. Sci. 2016, 3, 1500252. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, R.; Liu, M.; Wang, J.; Kim, M.J.; Ye, Z.; Xia, Y. Aqueous synthesis of Pd–M (M = Pd, Pt, and Au) decahedra with concave facets for catalytic applications. Top. Catal. 2020, 1–9. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.W.; Tao, F.F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef] [Green Version]
- Lamer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, S.; Wang, D.; He, W.; Li, Y. Syntheses of water-soluble octahedral, truncated octahedral, and cubic Pt-Ni nanocrystals and their structure-activity study in model hydrogenation reactions. J. Am. Chem. Soc. 2012, 134, 8975–8981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, T.; Fang, J.; Xu, P.; Li, X.; Xu, J.; Liu, C.C. Integrated Pt2Ni alloy@Pt core–shell nanoarchitectures with high electrocatalytic activity for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 11400–11407. [Google Scholar] [CrossRef]
- Zhan, F.; Yin, J.; Zhou, J.; Jiao, T.; Zhang, L.; Xia, M.; Bai, Z.; Peng, Q. Facile preparation and highly efficient catalytic performances of Pd-Cu bimetallic catalyst synthesized via seed-mediated method. Nanomaterials 2020, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-L.; Prasad, K.S.; Wu, H.-L.; Ho, J.-A.A.; Huang, M.H. Au nanocube-directed fabrication of Au−Pd core−shell nanocrystals with tetrahexahedral, concave octahedral, and octahedral structures and their electrocatalytic activity. J. Am. Chem. Soc. 2010, 132, 14546–14553. [Google Scholar] [CrossRef]
- Gao, C.; Lu, Z.; Liu, Y.; Zhang, Q.; Chi, M.; Cheng, Q.; Yin, Y. Highly stable silver nanoplates for surface plasmon resonance biosensing. Angew. Chem. Int. Ed. 2012, 51, 5629–5633. [Google Scholar] [CrossRef]
- Wang, X.; Vara, M.; Luo, M.; Huang, H.; Ruditskiy, A.; Park, J.; Bao, S.; Liu, J.; Howe, J.; Chi, M. Pd@ Pt core–shell concave decahedra: A class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem. Mater. 2001, 13, 2313–2322. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Cho, E.; Li, Z.; Yu, T.; Zeng, J.; Xie, Z.; Xia, Y. Au@Ag core−shell nanocubes with finely tuned and well-controlled sizes, shell thicknesses, and optical properties. ACS Nano 2010, 4, 6725–6734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Tang, S.; Liu, B.; Ren, B.; Zheng, N. Enhancing the photothermal stability of plasmonic metal nanoplates by a core-shell architecture. Adv. Mater. 2011, 23, 3420–3425. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Chen, Y.; Zhang, D.; Guo, M.; Xue, Z.; Wang, X.; Cheng, Z.; Xu, J. High-sensitive MEMS hydrogen sulfide sensor made from PdRh bimetal hollow nanoframe decorated metal oxides and sensitization mechanism study. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Johnson, B.; Raja, R.; Sankar, G.; Midgley, P. High-performance nanocatalysts for single-step hydrogenations. ChemInform 2003, 34, 20–30. [Google Scholar] [CrossRef]
- Ely, T.; Amiens, C.; Chaudret, B.; Snoeck, E.; Verelst, M.; Respaud, M.; Broto, J. Synthesis of nickel nanoparticles. influence of aggregation induced by modification of poly(vinylpyrrolidone) chain length on their magnetic properties. Chem. Mater. 1999, 11, 526–529. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.; Lee, S.; Khim, Z.G.; Char, K.; Hyeon, T. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. J. Am. Chem. Soc. 2000, 122, 8581–8582. [Google Scholar] [CrossRef]
- Puntes, V.; Krishnan, K.; Alivisatos, A. Colloidal nanocrystal shape and size control: The case of cobalt. Science 2001, 291, 2115–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.U.; Jang, Y.; Park, J.; Na, H.B.; Park, H.M.; Yun, H.J.; Lee, J.; Hyeon, T. Designed synthesis of atom-economical Pd/Ni bimetallic nanoparticle-based catalysts for sonogashira coupling reactions. J. Am. Chem. Soc. 2004, 126, 5026–5027. [Google Scholar] [CrossRef]
- Lanza, R.; Bersani, M.; Conte, L.; Martucci, A.; Canu, P.; Guglielmi, M.; Mattei, G.; Bello, V.; Centazzo, M.; Rosei, R. Effect of crystalline phase and composition on the catalytic properties of PdSn bimetallic nanoparticles in the PROX reaction. J. Phys. Chem. C 2014, 118, 369–374. [Google Scholar] [CrossRef]
- Bönnemann, H.; Brand, R.A.; Brijoux, W.; Hofstadt, H.W.; Frerichs, M.; Kempter, V.; Maus-Friedrichs, W.; Matoussevitch, N.; Nagabhushana, K.S.; Voigts, F.; et al. Air stable Fe and Fe-Co magnetic fluids-synthesis and characterization. Appl. Organomet. Chem. 2005, 19, 790–796. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.T.; Xia, Y. Template-engaged replacement reaction: A one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2002, 2, 481–485. [Google Scholar] [CrossRef]
- Sun, Y.; Wiley, B.; Li, Z.Y.; Xia, Y. Synthesis and optical properties of nanorattles and multiple-walled nanoshells/nanotubes made of metal alloys. J. Am. Chem. Soc. 2004, 126, 9399–9406. [Google Scholar] [CrossRef]
- Belousov, O.V.; Belousova, N.V.; Sirotina, A.V.; Solovyov, L.A.; Zhyzhaev, A.M.; Zharkov, S.M.; Mikhlin, Y.L. Formation of bimetallic Au–Pd and Au–Pt nanoparticles under hydrothermal conditions and microwave irradiation. Langmuir 2011, 27, 11697–11703. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Farzinpour, P.; Sundar, A.; Hughes, R.A.; Neretina, S. Sacrificial templates for galvanic replacement reactions: Design criteria for the synthesis of pure Pt nanoshells with a smooth surface morphology. Chem. Mater. 2014, 26, 3340–3347. [Google Scholar] [CrossRef]
- Pasricha, R.; Bala, T.; Biradar, A.V.; Umbarkar, S.; Sastry, M. Synthesis of catalytically active porous platinum nanoparticles by transmetallation reaction and proposition of the mechanism. Small 2009, 5, 1467–1473. [Google Scholar] [CrossRef]
- Liang, H.P.; Zhang, H.M.; Hu, J.S.; Guo, Y.G.; Wan, L.J.; Bai, C.L. Pt hollow nanospheres: Facile synthesis and enhanced electrocatalysts. Angew. Chem. 2004, 116, 1566–1569. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Wang, R. Well-aligned CoPt hollow nanochains synthesized in water at room temperature. J. Phys. Chem. C 2012, 116, 5352–5357. [Google Scholar] [CrossRef]
- Mohl, M.; Dobo, D.; Kukovecz, A.; Konya, Z.; Kordas, K.; Wei, J.; Vajtai, R.; Ajayan, P.M. Formation of CuPd and CuPt bimetallic nanotubes by galvanic replacement reaction. J. Phys. Chem. C 2011, 115, 9403–9409. [Google Scholar] [CrossRef]
- Mayers, B.; Jiang, X.; Sunderland, D.; Cattle, B.; Xia, Y. Hollow nanostructures of platinum with controllable dimensions can be synthesized by templating against selenium nanowires and colloids. J. Am. Chem. Soc. 2003, 125, 13364–13365. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Chang, H.-T. Preparation of gold−tellurium hybrid nanomaterials for surface-enhanced raman spectroscopy. Langmuir 2008, 24, 365–367. [Google Scholar] [CrossRef]
- Saha, S.; Gayen, P.; Wang, Z.; Dixit, R.J.; Sharma, K.; Basu, S.; Ramani, V.K. Development of bimetallic PdNi electrocatalysts toward mitigation of catalyst poisoning in direct borohydride fuel cells. ACS Catal. 2021, 11, 8417–8430. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Wang, H.; Huang, B.; Dai, Y. Multifunctional electrocatalyst PtM with low Pt loading and high activity towards hydrogen and oxygen electrode reactions: A computational study. Appl. Catal. B Environ. 2019, 255, 117743. [Google Scholar] [CrossRef]
- Chen, F.; Wang, D.; Chen, J.; Ling, J.; Yue, H.; Gou, L.; Tang, H. PtNi nanocubes-catalyzed tyramine signal amplification electrochemiluminescence sensor for nonenzymatic and ultrasensitive detection of hepatocellular carcinoma cells. Sens. Actuators B Chem. 2020, 305, 127472. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Chi, M.; Li, D.; Strmcnik, D.; Dennis, V.D.V.; Wang, G.; Komanicky, V.; Chang, K.C.; Paulikas, A.P.; Tripkovic, D. Design and synthesis of bimetallic electrocatalyst with multilayered Pt-skin surfaces. J. Am. Chem. Soc. 2011, 133, 14396–14403. [Google Scholar] [CrossRef]
- Swami, A.; Patil, I.; Lokanathan, M.; Ingavale, S.; Kakade, B. Enhanced oxygen reduction reaction by PdPt Alloy catalyst with stabilized platinum skin. Chem. Sel. 2020, 5, 3486–3493. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X. Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mg(Pt) for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Peng, Z.; Yang, S.; Yang, H. Truncated octahedral Pt(3)Ni oxygen reduction reaction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 4984–4985. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, L.; You, H.; Gross, A.; Li, J.; Yang, H. Icosahedral platinum alloy nanocrystals with enhanced electrocatalytic activities. J. Am. Chem. Soc. 2012, 134, 11880–11883. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Kang, Y.; Chen, C.; Resasco, J.; Yang, P. Atomic structure of Pt3Ni nanoframe electrocatalysts by in situ X-ray absorption spectroscopy. J. Am. Chem. Soc. 2015, 137, 15817–15824. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Yang, H.; Pan, J.; Liu, J.; Dotse, C.; Luan, Y.; Gao, R.; Lin, C.; Zhang, J.; et al. High-indexed Pt3Ni alloy tetrahexahedral nanoframes evolved through preferential CO etching. Nano Lett. 2017, 17, 2204–2210. [Google Scholar] [CrossRef]
- Luo, S.; Tang, M.; Shen, P.K.; Ye, S. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts. Adv. Mater. 2017, 29, 1601687. [Google Scholar] [CrossRef]
- Kwon, H.; Kabiraz, M.K.; Park, J.; Oh, A.; Baik, H.; Choi, S.-I.; Lee, K. Dendrite-embedded platinum–nickel multiframes as highly active and durable electrocatalyst toward the oxygen reduction reaction. Nano Lett. 2018, 18, 2930–2936. [Google Scholar] [CrossRef]
- Chen, D.; Tong, Y. Irrelevance of carbon monoxide poisoning in the methanol oxidation reaction on a PtRu electrocatalyst. Angew. Chem. 2015, 127, 9394–9398. [Google Scholar] [CrossRef]
- Chen, D.J.; Sun, S.G.; Tong, Y.Y.J. On the chemistry of activating commercial carbon-supported PtRu electrocatalyst for methanol oxidation reaction. Chem. Commun. 2014, 50, 12963–12968. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Recent progress of ultrathin 2D Pd-based nanomaterials for fuel cell electrocatalysis. Small 2021, 17, 2005092. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ma, Y.; Li, J.; Huang, J.; Yan, Y.; Zhang, H.; Wu, J.; Yang, D. Strain-induced Stranski–Krastanov growth of Pd@Pt core–shell hexapods and octapods as electrocatalysts for methanol oxidation. Nanoscale 2017, 9, 11077–11084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Janyasupab, M.; Liu, C.W.; Li, X.; Xu, J.; Liu, C.C. Three dimensional PtRh alloy porous nanostructures: Tuning the atomic composition and controlling the morphology for the application of direct methanol fuel cells. Adv. Funct. Mater. 2012, 22, 3570–3575. [Google Scholar] [CrossRef]
- Wang, L.; Nemoto, Y.; Yamauchi, Y. Direct synthesis of spatially-controlled Pt-on-Pd bimetallic nanodendrites with superior electrocatalytic activity. J. Am. Chem. Soc. 2011, 133, 9674–9677. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamauchi, Y. Metallic nanocages: Synthesis of bimetallic Pt-Pd hollow nanoparticles with dendritic shells by selective chemical etching. J. Am. Chem. Soc. 2013, 135, 16762–16765. [Google Scholar] [CrossRef]
- Li, H.H.; Zhao, S.; Gong, M.; Cui, C.H.; He, D.; Liang, H.W.; Wu, L.; Yu, S.H. Ultrathin PtPdTe nanowires as superior catalysts for methanol electrooxidation. Angew. Chem. Int. Ed. 2013, 52, 7472–7476. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.; Sun, X.; Sun, S. Synthesis of ultrathin FePtPd nanowires and their use as catalysts for methanol oxidation reaction. J. Am. Chem. Soc. 2011, 133, 15354–15357. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Z.; Lu, X.; Zhou, Z.; Shao, J.; Zhou, H.S. Facile synthesis of three-dimensional PtPdNi fused nanoarchitecture as highly active and durable electrocatalyst for methanol oxidation. ACS Appl. Energy Mater. 2017, 1, 32–37. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Zhang, D.; Han, Y.; Wang, Z.; Qin, Y.; Lin, S.; Wu, X.; Zhao, H.; Lai, J. Surface oxygen-mediated ultrathin PtRuM (Ni, Fe, and Co) nanowires boosting methanol oxidation reaction. J. Mater. Chem. A 2020, 8, 2323–2330. [Google Scholar] [CrossRef]
- Andrews, S.P.; Stepan, A.F.; Tanaka, H.; Ley, S.V.; Smith, M.D. Heterogeneous or homogeneous? A case study involving palladium-containing perovskites in the suzuki reaction. Adv. Synth. Catal. 2005, 347, 647–654. [Google Scholar] [CrossRef]
- Yi, J.; Lee, W.H.; Choi, C.H.; Lee, Y.; Park, K.S.; Min, B.K.; Hwang, Y.J.; Oh, H.-S. Effect of Pt introduced on Ru-based electrocatalyst for oxygen evolution activity and stability. Electrochem. Commun. 2019, 104, 106469. [Google Scholar] [CrossRef]
- Zhou, M.; Bao, S.; Bard, A.J. Probing size and substrate effects on the hydrogen evolution reaction by single isolated Pt atoms, atomic clusters, and nanoparticles. J. Am. Chem. Soc. 2019, 141, 7327–7332. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.T.; Myers, D. Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew. Chem. 2014, 126, 14240–14245. [Google Scholar] [CrossRef]
- Chang, S.H.; Danilovic, N.; Chang, K.-C.; Subbaraman, R.; Paulikas, A.P.; Fong, D.D.; Highland, M.J.; Baldo, P.M.; Stamenkovic, V.R.; Freeland, J.W. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, H.; Liang, X.; Liu, Y.; Zou, X. Theoretical insights into nonprecious oxygen-evolution active sites in Ti–Ir-Based perovskite solid solution electrocatalysts. J. Mater. Chem. A 2020, 8, 218–223. [Google Scholar] [CrossRef]
- Jin, H.; Hong, Y.; Yoon, J.; Oh, A.; Chaudhari, N.K.; Baik, H.; Joo, S.H.; Lee, K. Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M= Ir and Pt) nanoframes toward efficient oxygen evolution catalysis. Nano Energy 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Pei, J.; Mao, J.; Liang, X.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Ir–Cu nanoframes: One-pot synthesis and efficient electrocatalysts for oxygen evolution reaction. Chem. Commun. 2016, 52, 3793–3796. [Google Scholar] [CrossRef]
- Pi, Y.; Guo, J.; Shao, Q.; Huang, X. Highly efficient acidic oxygen evolution electrocatalysis enabled by porous Ir–Cu nanocrystals with three-dimensional electrocatalytic surfaces. Chem. Mater. 2018, 30, 8571–8578. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H.; Zhang, J.; Xu, J. IrNi nanoparticle-decorated flower-shaped NiCo2O4 nanostructures: Controllable synthesis and enhanced electrochemical activity for oxygen evolution reaction. Sci. China Mater. 2017, 60, 119–130. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.-T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Miyamura, H.; Suzuki, A.; Yasukawa, T.; Kobayashi, S. Polysilane-immobilized Rh–Pt bimetallic nanoparticles as powerful arene hydrogenation catalysts: Synthesis, reactions under batch and flow conditions and reaction mechanism. J. Am. Chem. Soc. 2018, 140, 11325–11334. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, Y.; Park, J.; Liang, X. Atomic layer deposited Pt-Co bimetallic catalysts for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols. J. Catal. 2018, 366, 61–69. [Google Scholar] [CrossRef]
- Taylor, M.J.; Beaumont, S.K.; Islam, M.J.; Tsatsos, S.; Parlett, C.A.; Issacs, M.A.; Kyriakou, G. Atom efficient PtCu bimetallic catalysts and ultra dilute alloys for the selective hydrogenation of furfural. Appl. Catal. B Environ. 2020, 284, 119737. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, C.; Yang, G.; Sun, Y.; Wang, D.; Liu, J. High-performance microstructured Au-Ag bimetallic catalyst for oxidative coupling of methanol to methyl formate. Catal. Commun. 2019, 129, 105741. [Google Scholar] [CrossRef]
- Xiao, Y.; Varma, A. Highly selective nonoxidative coupling of methane over Pt-Bi bimetallic catalysts. ACS Catal. 2018, 8, 2735–2740. [Google Scholar] [CrossRef]
- Ba, Q.; Jia, X.; Huang, L.; Li, X.; Chen, W.; Mao, L. Alloyed PdNi hollow nanoparticles as cocatalyst of CdS for improved photocatalytic activity toward hydrogen production. Int. J. Hydrogen Energy 2019, 44, 5872–5880. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.; Yan, Y.; Luo, S.; Li, X.; Huang, J.; Zhang, H.; Yang, D. Coupling PtNi ultrathin nanowires with MXenes for boosting electrocatalytic hydrogen evolution in both acidic and alkaline solutions. Small 2019, 15, 1805474. [Google Scholar] [CrossRef]
- Wei, R.; Chen, Z.; Lv, H.; Zheng, X.; Ge, X.; Sun, L.; Song, K.; Kong, C.; Zhang, W.; Liu, B. Ultrafine RhNi nanocatalysts confined in hollow mesoporous carbons for a highly efficient hydrogen production from ammonia borane. Inorg. Chem. 2021, 60, 6820–6828. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Pi, Y.; Shao, Q.; Wang, P.; Guo, J.; Huang, X. General Formation of Monodisperse IrM (M = Ni, Co, Fe) Bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting. Adv. Funct. Mater. 2017, 27, 1700886. [Google Scholar] [CrossRef]
- Huynh, T.-T.; Huang, W.-H.; Tsai, M.-C.; Nugraha, M.; Haw, S.-C.; Lee, J.-F.; Su, W.-N.; Hwang, B.J. Synergistic hybrid support comprising TiO2–carbon and ordered PdNi alloy for direct hydrogen peroxide synthesis. ACS Catal. 2021, 11, 8407–8416. [Google Scholar] [CrossRef]
- Fan, A.; Qin, C.; Zhang, X.; Yang, J.; Ge, J.; Wang, S.; Yuan, X.; Wang, S.; Dai, X. Engineering FeNi alloy nanoparticles via synergistic ultralow Pt doping and nanocarbon capsulation for efficient hydrogen evolution. J. Mater. Chem. A 2019, 7, 24347–24355. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Tian, W.; Meng, A.; Yang, L. CoNi bimetal cocatalyst modifying a hierarchical ZnIn2S4 nanosheet-based microsphere noble-metal-free photocatalyst for efficient visible-light-driven photocatalytic hydrogen production. ACS Sustain. Chem. Eng. 2019, 7, 20190–20201. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Niu, H.-J.; Han, Z.; Feng, J.-J.; Huang, H.; Wang, A.-J. Simple fabrication of trimetallic platinum-nickel-cobalt hollow alloyed 3D multipods for highly boosted hydrogen evolution reaction. J. Colloid Interface Sci. 2020, 570, 205–211. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Su, J.; Jiang, P.; Xia, G.; Chen, Q. Enhanced activity for hydrogen evolution reaction over CoFe catalysts by alloying with small amount of Pt. ACS Appl. Mater. Interfaces 2017, 9, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, F.; Kubo, A.; Hayashi, T. Generation of tertiary phosphine-coordinated Pd(0) species from Pd(OAc)2 in the catalytic heck reaction. Chem. Lett. 1992, 21, 2177–2180. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon−carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Core-shell-like Ni-Pd nanoparticles supported on carbon black as a magnetically separable catalyst for green Suzuki-Miyaura coupling reactions. Appl. Catal. B Environ. 2017, 200, 39–46. [Google Scholar] [CrossRef]
- Hahn, C.; Abram, D.N.; Hansen, H.A.; Hatsukade, T.; Jackson, A.; Johnson, N.C.; Hellstern, T.R.; Kuhl, K.P.; Cave, E.R.; Feaster, J.T. Synthesis of thin film AuPd alloys and their investigation for electrocatalytic CO2 reduction. J. Mater. Chem. A 2015, 3, 20185–20194. [Google Scholar] [CrossRef]
- Jedidi, A.; Rasul, S.; Masih, D.; Cavallo, L.; Takanabe, K. Generation of Cu–In alloy surfaces from CuInO2 as selective catalytic sites for CO2 electroreduction. J. Mater. Chem. A 2015, 3, 19085–19092. [Google Scholar] [CrossRef] [Green Version]
- Takashima, T.; Suzuki, T.; Irie, H. Electrochemical reduction of carbon dioxide to formate on palladium-copper alloy nanoparticulate electrode. Electrochemistry 2019, 87, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yan, L.; Wang, Y.; Zhang, Z.; Xu, J. PdPt bimetal-functionalized SnO2 nanosheets: Controllable synthesis and its dual selectivity for detection of carbon monoxide and methane. ACS Appl. Mater. Interfaces 2019, 11, 26116–26126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Xu, P.; Wen, W.; Li, X.; Xu, J. PtW/MoS2 hybrid nanocomposite for electrochemical sensing of H2O2 released from living cells. Biosens. Bioelectron. 2016, 80, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, Y.; Xu, J.; Dong, J.; Liu, C.-C. Monodisperse AuM (M = Pd, Rh, Pt) bimetallic nanocrystals for enhanced electrochemical detection of H2O2. Sens. Actuators B Chem. 2015, 207, 404–412. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhang, Y.; Fang, J.; Xu, P.; Xu, J.; Li, X.; Liu, C.-C.; Wen, W. Highly effective and specific way for the trace analysis of carbaryl insecticides based on Au42Rh58 alloy nanocrystals. J. Mater. Chem. A 2017, 5, 7064–7071. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, L.; He, Y.; Wang, L.; Huang, Z.; Jiang, Y.; Gao, J. Hierarchical nanocomposites with an N-doped carbon shell and bimetal core: Novel enzyme nanocarriers for electrochemical pesticide detection. Biosens. Bioelectron. 2018, 121, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ji, D.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Liu, C.-C.; Wen, W. Detection of phenylketonuria markers using a ZIF-67 encapsulated PtPd alloy nanoparticle (PtPd@ ZIF-67)-based disposable electrochemical microsensor. ACS Appl. Mater. Interfaces 2019, 11, 20734–20742. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Yuan, Y. Mesoporous PdO/Pt/Al2O3 film produced by reverse-micro-emulsion and its application for methane micro-sensor. RSC Adv. 2014, 5, 4586–4591. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Stewart, M.; Anderton, C.; Thompson, L.; Maria, J.; Gray, S.; Rogers, J.; Nuzzo, R. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Jain, P.K.; Qian, W.; El-Sayed, M.A. Ultrafast cooling of photoexcited electrons in gold nanoparticle-thiolated DNA conjugates involves the dissociation of the gold-thiol bond. J. Am. Chem. Soc. 2006, 128, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Yonzon, C.R.; Stuart, D.A.; Zhang, X.; Mcfarland, A.D.; Haynes, C.L.; Duyne, R.P.V. Towards advanced chemical and biological nanosensors—An overview. Talanta 2005, 67, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Arsalani, S.; Ghodselahi, T.; Neishaboorynejad, T.; Baffa, O. DNA detection based on localized surface plasmon resonance spectroscopy of Ag@Au biocomposite nanoparticles. Plasmonics 2019. [Google Scholar] [CrossRef]
- Yu, C.; Irudayaraj, J. Multiplex Biosensor Using Gold Nanorods. Anal. Chem. 2007, 79, 572–579. [Google Scholar] [CrossRef]
- Dong, P.; Lin, Y.; Deng, J.; Di, J. Ultrathin gold-shell coated silver nanoparticles onto a glass platform for improvement of plasmonic sensors. Acs Appl. Mater. Interfaces 2013, 5, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.X.; Jiang, R.; Rong, J.; Yan, C.H. Time-temperature indicator for perishable products based on kinetically programmable Ag overgrowth on Au nanorods. ACS Nano 2013, 7, 4561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Cao, Y.; Chen, J.; Wang, X.; Yu, J.; Yu, B.; Yan, Z.; Chen, X. Au@Ag core/shell nanoparticles as colorimetric probes for cyanide sensing. Nanoscale 2014, 6, 9939–9943. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhou, X.; Wen, C.Y.; Yu, J.; Han, X.; Li, X.; Yan, Z.F.; Zeng, J. A convenient colorimetric method for sensitive and specific detection of cyanide using Ag@Au core–shell nanoparticles. Sens. Actuators B Chem. 2016, 228, 366–372. [Google Scholar] [CrossRef]
- Jiang, R.; Chen, H.; Lei, S.; Qian, L.; Wang, J. Unraveling the evolution and nature of the plasmons in (Au Core)-(Ag Shell) nanorods. Adv. Mater. 2012, 24, 200–207. [Google Scholar] [CrossRef]
- Samal, A.K.; Polavarapu, L.; Rodal-Cedeira, S.; Liz-Marzán, L.M.; Pérez-Juste, J.; Pastoriza-Santos, I. Size Tunable Au@Ag core–shell nanoparticles: Synthesis and surface-enhanced raman scattering properties. Langmuir 2013, 29, 15076–15082. [Google Scholar] [CrossRef] [Green Version]
- Grubisha, D.S.; Lipert, R.J.; Park, H.Y.; Driskell, J.; Porter, M.D. Femtomolar detection of prostate-specific antigen: An immunoassay based on surface-enhanced raman scattering and immunogold labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef]
- Cui, Y.; Ren, B.; Yao, J.L.; Gu, R.A.; Tian, Z.Q. Synthesis of Ag core-Au shell bimetallic nanoparticles for immunoassay based on surface-enhanced Raman spectroscopy. J. Phys. Chem. B 2006, 110, 4002–4006. [Google Scholar] [CrossRef]
- Yang, L.; Gao, M.X.; Zhan, L.; Gong, M.; Zhen, S.J.; Huang, C.Z. An enzyme-induced Au@Ag core–shell nanoStructure used for an ultrasensitive surface-enhanced Raman scattering immunoassay of cancer biomarkers. Nanoscale 2017, 9, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kanti Kabiraz, M.; Kwon, H.; Park, S.; Baik, H.; Choi, S.-I.; Lee, K. Radially phase segregated PtCu@ PtCuNi dendrite@ frame nanocatalyst for the oxygen reduction reaction. ACS Nano 2017, 11, 10844–10851. [Google Scholar] [CrossRef] [PubMed]













| Structure | Shapes | Models | Bimetals and References |
|---|---|---|---|
| Zero-dimensional | Tetrahedron |  | PdPt [26], PdCu [27] |
| Octahedron and truncated octahedron |   | Pt@Pd [28], Pt3Ni [29] PtCo [30] | |
| Cube and truncated cube |   | Au-Pd [31], Pt-Pd [32] | |
| Icosahedron |  | Pt-Pd [32] | |
| Rhombic dodecahedron |  | AuPd [33], NiPt [34] | |
| One-dimensional | Nanorods |  | CuPt [35], Pt3Fe [36] |
| Wavy wire |  | PtCo [37], PtCu [38], PdPt [39] | |
| Two-dimensional | Triangular plates |  | Pd@Pt [40], Ag-Pd [41] |
| Hexagonal plates |  | Pd@Pt [40], PdAg [42] | |
| Hollow structure | Nanoframework |   | CuPd [43], PdRh [44] |
| Nanocage |    | PdPt [45], PtNi [46] PdCu [47], AuAg [48] |
| Reduction Reaction | Eo (V vs. SHE) a |
|---|---|
Au3+ + 3e−  Au Au | 1.50 |
Pt2+ + 2e−  Pt Pt | 1.18 |
Ir3+ + 3e−  Ir Ir | 1.16 |
Pd2+ + 2e−  Pd Pd | 0.95 |
Ag+ + e−  Ag Ag | 0.80 |
Rh3+ + 3e−  Rh Rh | 0.76 |
Ru3+ + 3e−  Ru Ru | 0.45 |
Cu2+ + 2e−  Cu Cu | 0.34 |
Ni2+ + 2e−  Ni Ni | −0.25 |
Co2+ + 2e−  Co Co | −0.28 |
Fe2+ + 2e−  Fe Fe | −0.44 |
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Co-reduction | (i) Simple synthesis process (ii) Easily control composition (iii) High yield and low cost | (i) The overuse reducing agent pollutes environment |
| Seed-mediated growth | (i) Tunable size, shape, and composition (ii) Facile and wide applicability | (i) Complicated synthesis process (ii) Time- and cost-consuming |
| Thermal decomposition | (i) Facile, time-saving and highly efficient (ii) controllable composition, size and morphology | Limited metal precursors (only some organometallic precursors) |
| Galvanic replacement reaction | (i) Easily prepare hollow bimetallic nanostructure (ii) controllable size and morphology of Ag seed | (i) Complicated synthesis process (ii) Time- and cost-consuming |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhang, W.; Luo, N.; Xue, Z.; Hu, Q.; Zeng, W.; Xu, J. Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing. Nanomaterials 2021, 11, 1926. https://doi.org/10.3390/nano11081926
Li G, Zhang W, Luo N, Xue Z, Hu Q, Zeng W, Xu J. Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing. Nanomaterials. 2021; 11(8):1926. https://doi.org/10.3390/nano11081926
Chicago/Turabian StyleLi, Gaojie, Wenshuang Zhang, Na Luo, Zhenggang Xue, Qingmin Hu, Wen Zeng, and Jiaqiang Xu. 2021. "Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing" Nanomaterials 11, no. 8: 1926. https://doi.org/10.3390/nano11081926
APA StyleLi, G., Zhang, W., Luo, N., Xue, Z., Hu, Q., Zeng, W., & Xu, J. (2021). Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing. Nanomaterials, 11(8), 1926. https://doi.org/10.3390/nano11081926