Physicochemical and Electrochemical Characterization of Electropolymerized Polydopamine Films: Influence of the Deposition Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
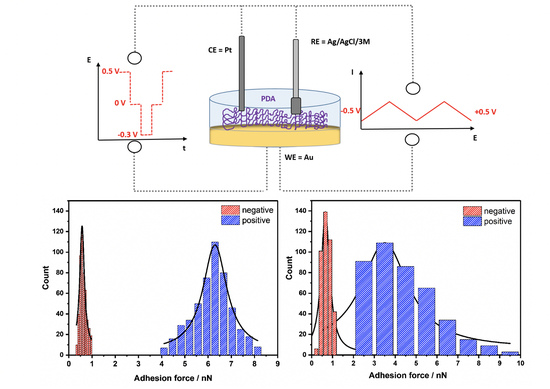
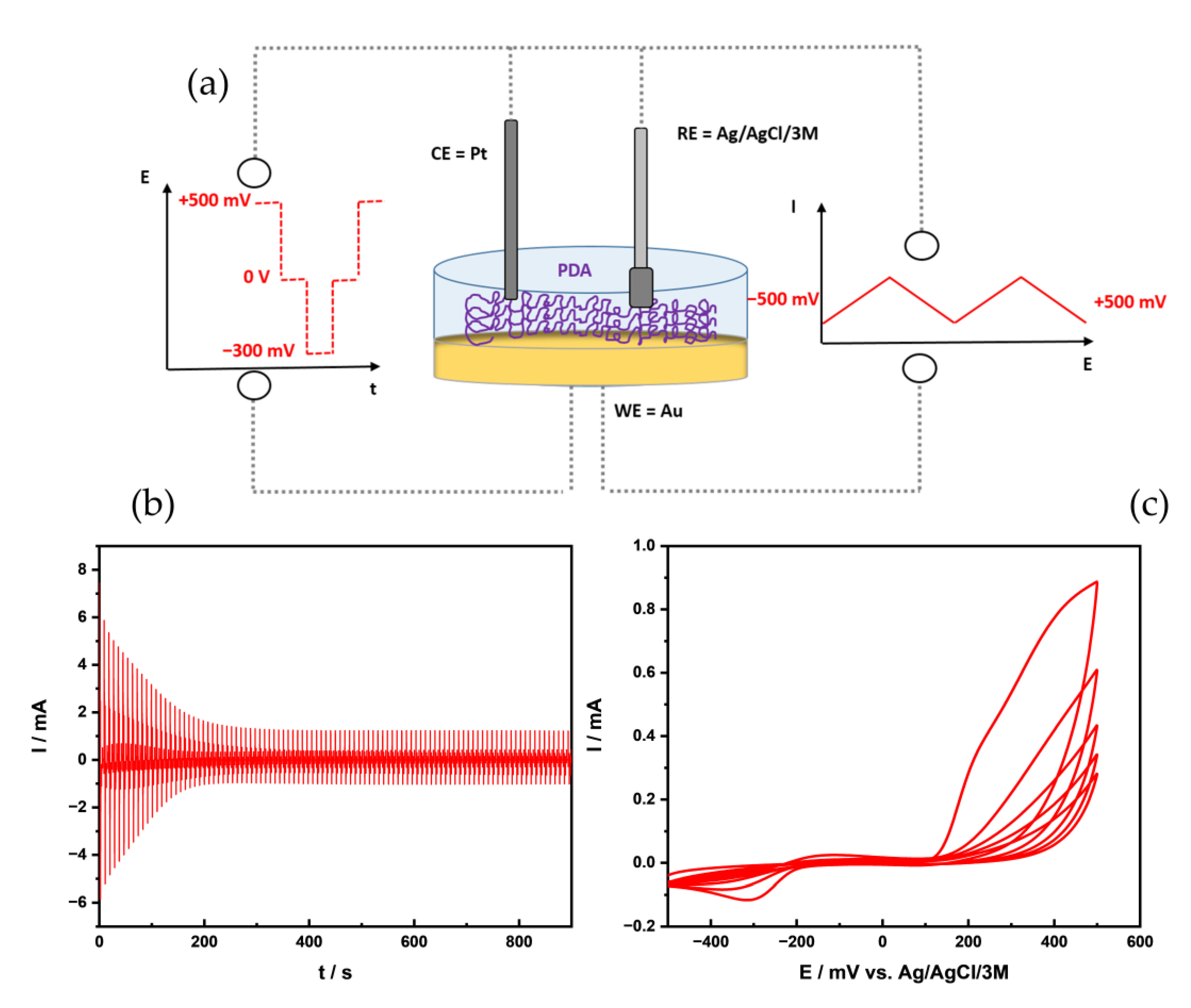
2.2. PDA Deposition
2.3. SECM Approach Curves
2.4. AFM Measurements
2.5. Scanning Electron Microscopy (SEM)
2.6. EIS Measurements
2.7. EQCM
2.8. Strain-Induced Elastic Buckling Instability for Mechanical Measurements (SIEBIMM)
3. Results and Discussion
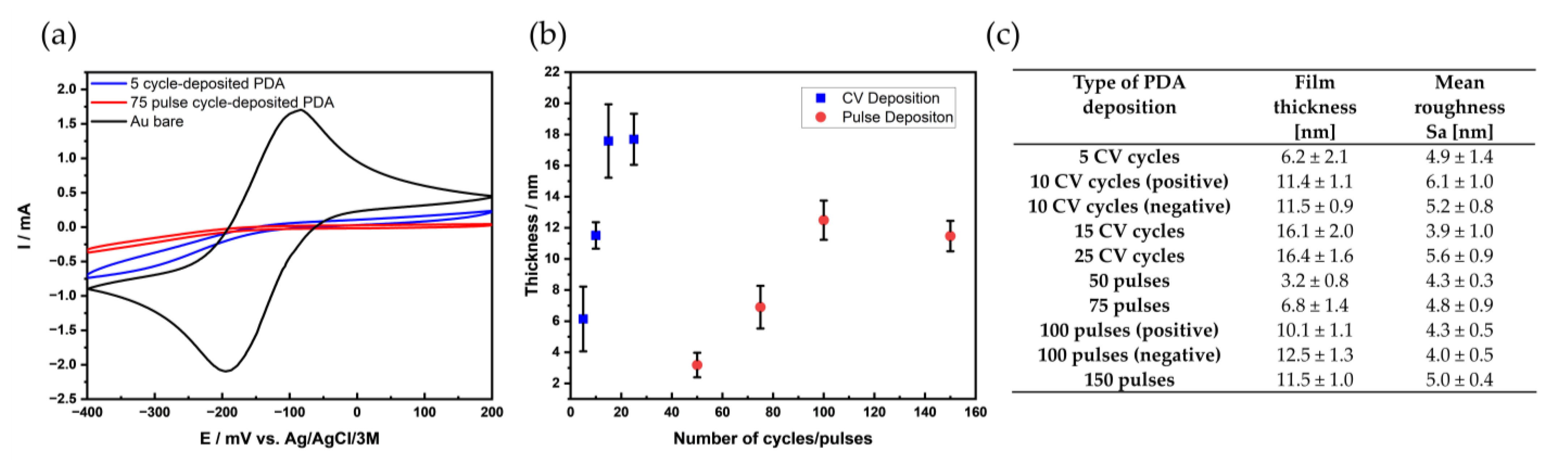
3.1. Deposition Methods
3.2. Film Thickness and Morphology
3.3. SECM Kinetic Studies
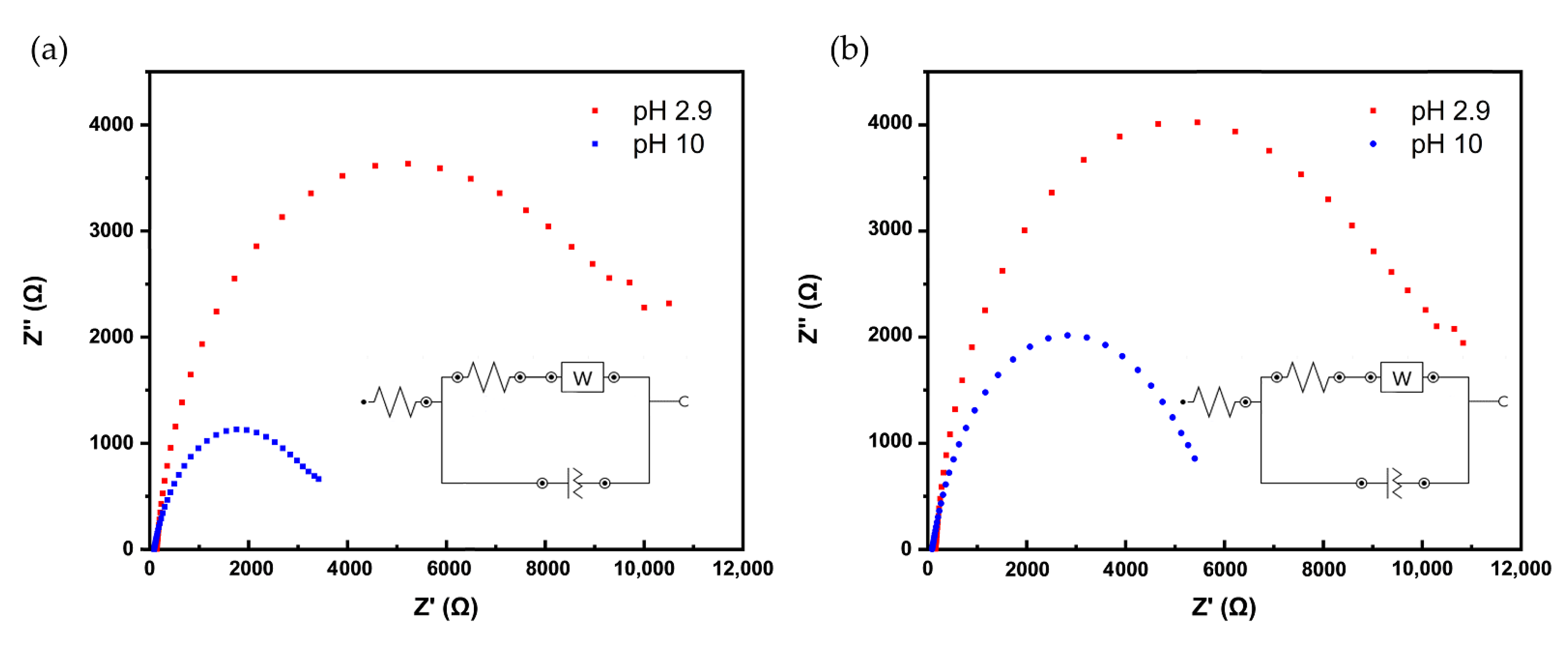
3.4. Electrochemical Impedance Spectroscopy (EIS) Studies
3.5. Electrochemical Quartz Microbalance (EQCM)
3.6. AFM Force Spectroscopy
3.7. SIEBIMM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaowana, R.; Bunkoed, O. A Nanocomposite Probe of Polydopamine/Molecularly Imprinted Polymer/Quantum Dots for Trace Sarafloxacin Detection in Chicken Meat. Anal. Bioanal. Chem. 2019, 411, 6081–6090. [Google Scholar] [CrossRef]
- Wu, N.; Yu, A.; Zhang, L.; Liu, W.; Gao, J.; Zhang, C.; Zheng, Y. Biocompatible Nanoplatform Based on Mussel Adhesive Chemistry: Effective Assembly, Dual Mode Sensing, and Cellular Imaging Performance. Adv. Mater. Interfaces 2019, 6, 1900732. [Google Scholar] [CrossRef]
- Lynge, M.E.; Van Der Westen, R.; Postma, A.; Stadler, B. Polydopamine—A Nature-Inspired Polymer Coating for Biomedical Science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Gao, B.; Su, L.; Tong, Y.; Guan, M.; Zhang, X. Ion Permeability of Polydopamine Films Revealed Using a Prussian Blue-Based Electrochemical Method. J. Phys. Chem. B 2014, 118, 12781–12787. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Liu, Y.; Wu, Y.; Ouyang, K.; Zhou, L.; Yu, P.; Liao, J.; Ning, C. Electrically Reversible Redox-Switchable Polydopamine Films for Regulating Cell Behavior. Electrochim. Acta 2017, 228, 343–350. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine-Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Delparastan, P.; Malollari, K.G.; Lee, H.; Messersmith, P.B. Direct Evidence for the Polymeric Nature of Polydopamine. Angew. Chem. Int. Ed. 2019, 58, 1077–1082. [Google Scholar] [CrossRef]
- Stöckle, B.; Ng, D.; Meier, C.; Paust, T.; Bischoff, F.; Diemant, T.; Behm, R.J.; Gottschalk, K.-E.; Ziener, U.; Weil, T. Precise Control of Polydopamine Film Formation by Electropolymerization. Macromol. Symp. 2014, 346, 73–81. [Google Scholar] [CrossRef]
- Schindler, S.; Aguayo, N.A.; Dornbierer, U.; Bechtold, T. Anodic Coating of 1.4622 Stainless Steel with Polydopamine by Repetitive Cyclic Voltammetry and Galvanostatic Deposition. Ind. Eng. Chem. Res. 2019, 59, 236–244. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Wang, K.; Yuan, H.; Fang, L.; Zheng, X.; Chan, C.; Ren, F.; Zhao, C. Pulse Electrochemical Driven Rapid Layer-by-Layer Assembly of Polydopamine and Hydroxyapatite Nanofilms via Alternative Redox in Situ Synthesis for Bone Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 920–928. [Google Scholar] [CrossRef]
- Lin, J.; Daboss, S.; Blaimer, D.; Kranz, C. Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition. Nanomaterials 2019, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Daboss, S.; Lin, J.; Godejohann, M.; Kranz, C. Redox Switchable Polydopamine-Modified AFM-SECM Probes: A Probe for Electrochemical Force Spectroscopy. Anal. Chem. 2020, 92, 8404–8413. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic Insights into the Electrochemical Oxidation of Dopamine by Cyclic Voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Xiang, C.; Xie, Q.; Yao, S. Electrochemical Quartz Crystal Microbalance Study on Growth and Property of the Polymer Deposit at Gold Electrodes during Oxidation of Dopamine in Aqueous Solutions. Thin Solid Films 2006, 497, 270–278. [Google Scholar] [CrossRef]
- Bacil, R.P.; Chen, L.; Serrano, S.H.P.; Compton, R.G. Dopamine Oxidation at Gold Electrodes: Mechanism and Kinetics Near Neutral pH. Phys. Chem. Chem. Phys. 2020, 22, 607–614. [Google Scholar] [CrossRef]
- Lin, C.; Chen, L.; Tanner, E.E.L.; Compton, R.G. Electroanalytical Study of Dopamine Oxidation on Carbon Electrodes: From the Macro- to the Micro-Scale. Phys. Chem. Chem. Phys. 2018, 20, 148–157. [Google Scholar] [CrossRef]
- Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the Conductivity and Adhesion of Polypyrrole Hydrogels with Electropolymerized Polydopamine. Chem. Mater. 2020, 32, 234–244. [Google Scholar] [CrossRef]
- Janshoff, A.; Neitzert, M.; Oberdörfer, Y.; Fuchs, H. Force Spectroscopy of Molecular Systems—Single Molecule Spectroscopy of Polymers and Biomolecules. Angew. Chem. Int. Ed. 2000, 39, 3212–3237. [Google Scholar] [CrossRef]
- Florin, E.; Moy, V.; Gaub, H. Adhesion Forces between Individual Ligand-Receptor Pairs. Science 1994, 264, 415–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, H.; Young, M.; Xu, F.; Cheng, G.; Cong, H. Properties of Electropolymerized Dopamine and Its Analogues. Langmuir 2018, 35, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.L.; Maddalena, P.; Napolitano, A.; Ball, V.; D’Ischia, M. Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue. ACS Appl. Mater. Interfaces 2017, 10, 7670–7680. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Toniazzo, V.; Ruch, D. Kinetics of Polydopamine Film Deposition as a Function of pH and Dopamine Concentration: Insights in the Polydopamine Deposition Mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Peltola, E.; Sainio, S.; Holt, K.B.; Palomäki, T.; Koskinen, J.; Laurila, T. Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem. 2018, 90, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Bernsmann, F.; Voegel, J.-C.; Ball, V. Different Synthesis Methods Allow to Tune the Permeability and Permselectivity of Dopamine–Melanin Films to Electrochemical Probes. Electrochim. Acta 2011, 56, 3914–3919. [Google Scholar] [CrossRef]
- Hecht, E.; Liedert, A.; Ignatius, A.; Mizaikoff, B.; Kranz, C. Local Detection of Mechanically Induced ATP Release from Bone Cells with ATP Microbiosensors. Biosens. Bioelectron. 2013, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cornut, R.; Lefrou, C. New Analytical Approximation of Feedback Approach Curves with a Microdisk SECM Tip and Irreversible Kinetic Reaction at the Substrate. J. Electroanal. Chem. 2008, 621, 178–184. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-Force Microscope Tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Marchesi D’Alvise, T.; Harvey, S.; Hueske, L.; Szelwicka, J.; Veith, L.; Knowles, T.P.J.; Kubiczek, D.; Flaig, C.; Port, F.; Gottschalk, K.; et al. Ultrathin Polydopamine Films with Phospholipid Nanodiscs Containing a Glycophorin A Domain. Adv. Funct. Mater. 2020, 30, 2000378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z.-K. Deposition and Adhesion of Polydopamine on the Surfaces of Varying Wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Tsehaye, M.T.; Li, J.; Dong, G.; Yuan, S.; Li, X.; Zhang, Y.; Liu, J.; Van der Bruggen, B. High Flux Electroneutral loose Nanofiltration Membranes Based on Rapid Deposition of Polydopamine/Polyethyleneimine. J. Mater. Chem. A 2017, 5, 14847–14857. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Grácio, J.; Toniazzo, V.; Ruch, D. Dopamine−Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef]
- Singh, N.; Nayak, J.; Patel, K.; Sahoo, S.K.; Kumar, R.; Nayak, J.M. Electrochemical Impedance Spectroscopy Reveals a New Mechanism Based on Competitive Binding between Tris and Protein on a Conductive Biomimetic Polydopamine Surface. Phys. Chem. Chem. Phys. 2018, 20, 25812–25821. [Google Scholar] [CrossRef]
- Turco, A.; Corvaglia, S.; Mazzotta, E.; Pompa, P.P.; Malitesta, C. Preparation and Characterization of Molecularly Imprinted Mussel Inspired Film as Antifouling and Selective Layer for Electrochemical Detection of Sulfamethoxazole. Sensors Actuators B Chem. 2018, 255, 3374–3383. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, L.; Weng, W.-J.; Cheng, K. Redox State of PDA Directs Cellular Responses through Preadsorbed Protein. ACS Biomater. Sci. Eng. 2018, 5, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Cornut, R.; Griveau, S.; Lefrou, C. Accuracy Study on Fitting Procedure of Kinetics SECM Feedback Experiments. J. Electroanal. Chem. 2010, 650, 55–61. [Google Scholar] [CrossRef]
- Luo, R.; Tang, L.; Wang, J.; Zhao, Y.; Tu, Q.; Weng, Y.; Shen, R.; Huang, N. Improved Immobilization of Biomolecules to Quinone-Rich Polydopamine for Efficient Surface Functionalization. Coll. Surf. B Biointerfaces 2013, 106, 66–73. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.; Liu, S.; Zhou, F. Pdop Layer Exhibiting Zwitterionicity: A Simple Electrochemical Interface for Governing ion Permeability. Chem. Commun. 2010, 46, 5900–5902. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ren, J.; Han, B.; Xu, L.; Han, L.; Jia, L. Stability of Polydopamine and poly(DOPA) Melanin-Like Films on the Surface of Polymer Membranes under Strongly Acidic and Alkaline Conditions. Coll. Surf. B Biointerfaces 2013, 110, 22–28. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kim, Y.J.; Klosterman, L.; Forssell, M.; Fedder, G.K.; Bettinger, C.J. In Vitro Electrochemical Characterization of Polydopamine Melanin as a Tissue Stimulating Electrode Material. J. Mater. Chem. B 2016, 4, 3031–3036. [Google Scholar] [CrossRef]
- Yang, J.; Niu, L.; Zhang, Z.; Zhao, J.; Chou, L. Electrochemical Behavior of a Polydopamine Nanofilm. Anal. Lett. 2015, 48, 2031–2039. [Google Scholar] [CrossRef]
- Poseu, J.S.; Mancebo-Aracil, J.; Nador, F.; Busque, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Malollari, K.G.; Delparastan, P.; Sobek, C.; Vachhani, S.J.; Fink, T.; Zha, R.H.; Messersmith, P.B. Mechanical Enhancement of Bioinspired Polydopamine Nanocoatings. ACS Appl. Mater. Interfaces 2019, 11, 43599–43607. [Google Scholar] [CrossRef]
- Mezzasalma, S.; Baldovino, D. Characterization of Silicon Nitride Surface in Water and Acid Environment: A General Approach to the Colloidal Suspensions. J. Colloid Interface Sci. 1996, 180, 413–420. [Google Scholar] [CrossRef]
- Bao, Y.; Luo, Z.; Cui, S. Environment-Dependent Single-Chain Mechanics of Synthetic Polymers and Biomacromolecules by Atomic Force Microscopy-Based Single-Molecule Force Spectroscopy and the Implications for Advanced Polymer Materials. Chem. Soc. Rev. 2020, 49, 2799–2827. [Google Scholar] [CrossRef]
- Grebikova, L.; Maroni, P.; Muresan, L.; Zhang, B.; Schlüter, A.D.; Borkovec, M. Interactions between Individual Charged Dendronized Polymers and Surfaces. Macromolecules 2013, 46, 3603–3610. [Google Scholar] [CrossRef]
- Wang, M. Nanoindentation of Polymeric Thin Films with an Interfacial Force Microscope. J. Mech. Phys. Solids 2004, 52, 2329–2354. [Google Scholar] [CrossRef]
- Stafford, C.; Harrison, C.; Beers, K.L.; Karim, A.; Amis, E.J.; VanLandingham, M.R.; Kim, H.-C.; Volksen, W.; Miller, R.D.; Simonyi, E.E. A Buckling-Based Metrology for Measuring the Elastic Moduli of Polymeric Thin Films. Nat. Mater. 2004, 3, 545–550. [Google Scholar] [CrossRef]
- Klosterman, L.; Ahmad, Z.; Viswanathan, V.; Bettinger, C.J. Synthesis and Measurement of Cohesive Mechanics in Polydopamine Nanomembranes. Adv. Mater. Interfaces 2017, 4, 1700041. [Google Scholar] [CrossRef]
- Tahk, D.; Lee, H.H.; Khang, D.-Y. Elastic Moduli of Organic Electronic Materials by the Buckling Method. Macromolecules 2009, 42, 7079–7083. [Google Scholar] [CrossRef]
- Nolte, A.J.; Cohen, R.E.; Rubner, M.F. A Two-Plate Buckling Technique for Thin Film Modulus Measurements: Applications to Polyelectrolyte Multilayers. Macromolecules 2006, 39, 4841–4847. [Google Scholar] [CrossRef]




| Type of PDA Deposition | Substrate Potential vs. Ag/AgCl: Redox Mediator: [Ru(NH3)6]3+ | Redox Mediator: [Ru(NH3)6]3+ in Different pH | ||
|---|---|---|---|---|
| −100 mV | +500 mV | pH 3 | pH 10 | |
| 5 cycles | 0.0027 ± 0.0015 | 0.0139 ± 0.0078 | 2.95 × 10−10 ± 5.67 × 10−10 | 0.0074 ± 0.0041 |
| 10 cycles | 0.0025 ± 0.0014 | 0.0090 ± 0.0018 | 4.37 × 10−10 ± 1.15 × 10−10 | 0.0075 ± 0.0025 |
| 100 pulse cycles | 1.44 × 10−9 ± 4.03 × 10−10 | 0.0162 ± 0.0121 | 1.22 × 10−9 ± 1.01 × 10−10 | 0.0040 ± 0.0036 |
| pH | Rct (kΩ) [Ru(NH3)6]3+ | |
|---|---|---|
| 10 CV cycles | 100 pulse cycles | |
| 2.9 | 7.3 ± 1.1 | 7.6 ± 1.6 |
| 10.0 | 3.7 ± 0.45 | 3.6 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kund, J.; Daboss, S.; D’Alvise, T.M.; Harvey, S.; Synatschke, C.V.; Weil, T.; Kranz, C. Physicochemical and Electrochemical Characterization of Electropolymerized Polydopamine Films: Influence of the Deposition Process. Nanomaterials 2021, 11, 1964. https://doi.org/10.3390/nano11081964
Kund J, Daboss S, D’Alvise TM, Harvey S, Synatschke CV, Weil T, Kranz C. Physicochemical and Electrochemical Characterization of Electropolymerized Polydopamine Films: Influence of the Deposition Process. Nanomaterials. 2021; 11(8):1964. https://doi.org/10.3390/nano11081964
Chicago/Turabian StyleKund, Julian, Sven Daboss, Tommaso Marchesi D’Alvise, Sean Harvey, Christopher V. Synatschke, Tanja Weil, and Christine Kranz. 2021. "Physicochemical and Electrochemical Characterization of Electropolymerized Polydopamine Films: Influence of the Deposition Process" Nanomaterials 11, no. 8: 1964. https://doi.org/10.3390/nano11081964
APA StyleKund, J., Daboss, S., D’Alvise, T. M., Harvey, S., Synatschke, C. V., Weil, T., & Kranz, C. (2021). Physicochemical and Electrochemical Characterization of Electropolymerized Polydopamine Films: Influence of the Deposition Process. Nanomaterials, 11(8), 1964. https://doi.org/10.3390/nano11081964