Nickel-Doped ZnO Nanowalls with Enhanced Electron Transport Ability for Electrochemical Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
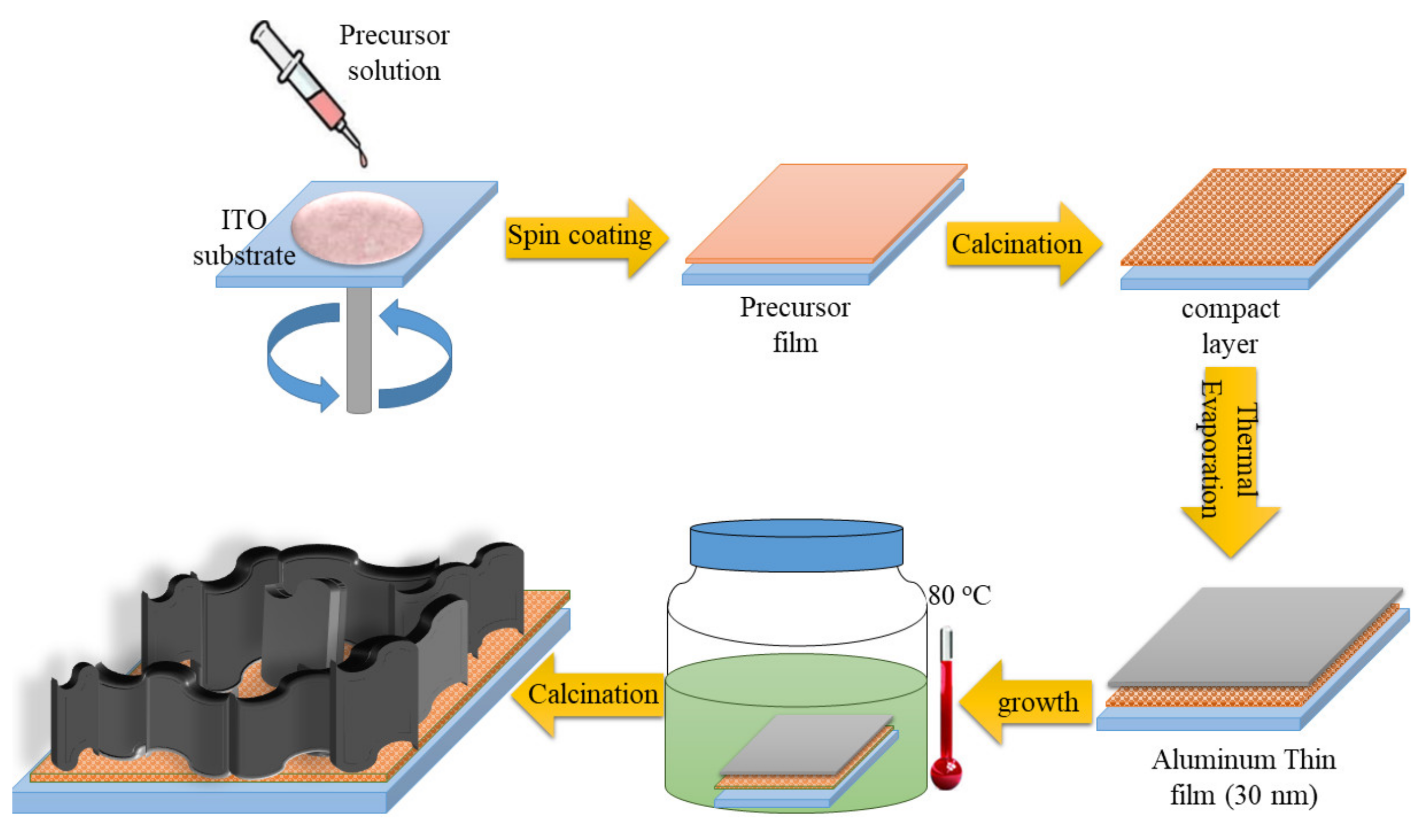
2.2. Preparation of ZnO NWLs
2.3. Characterization Methods
3. Results and Discussion
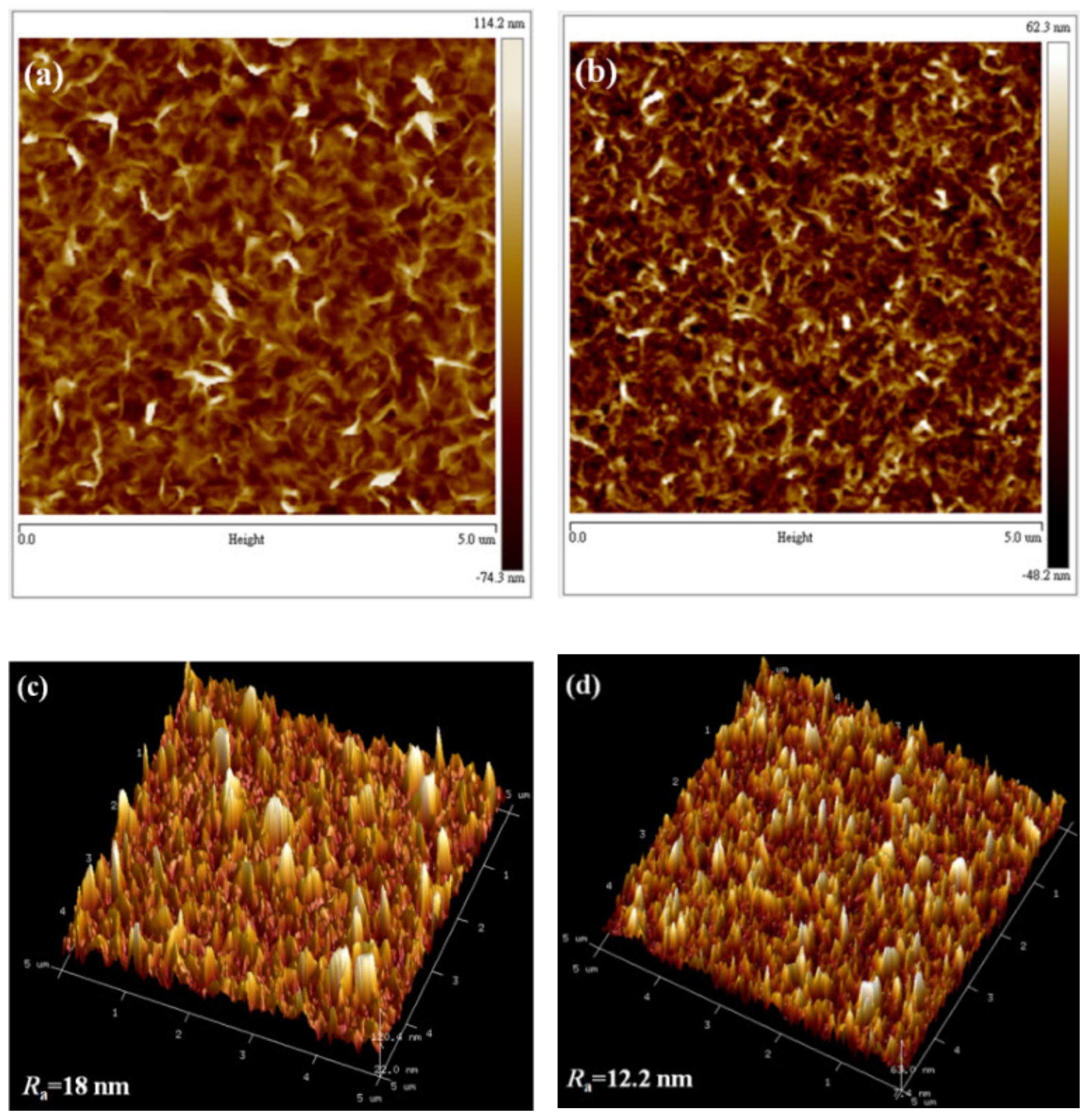
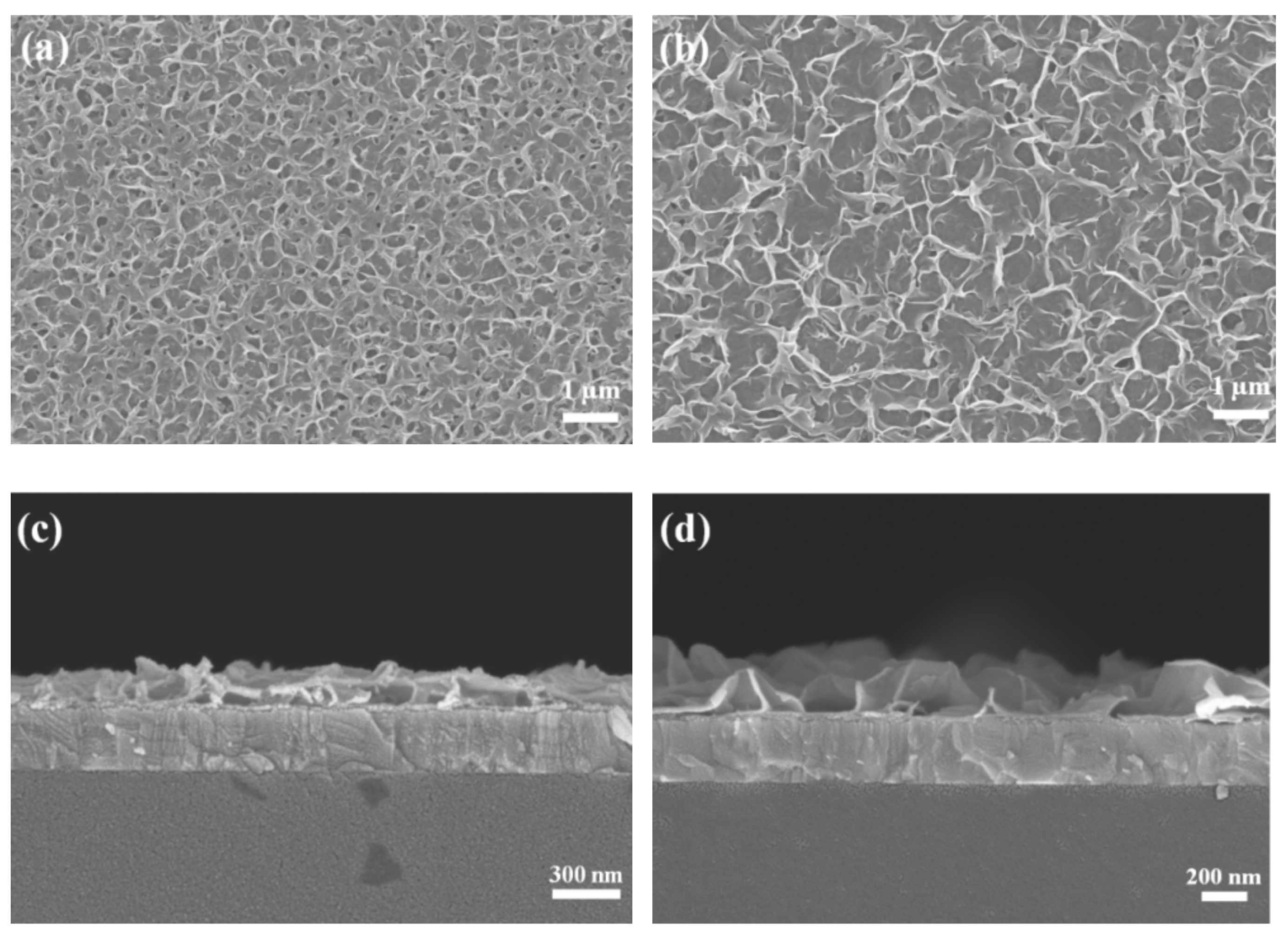
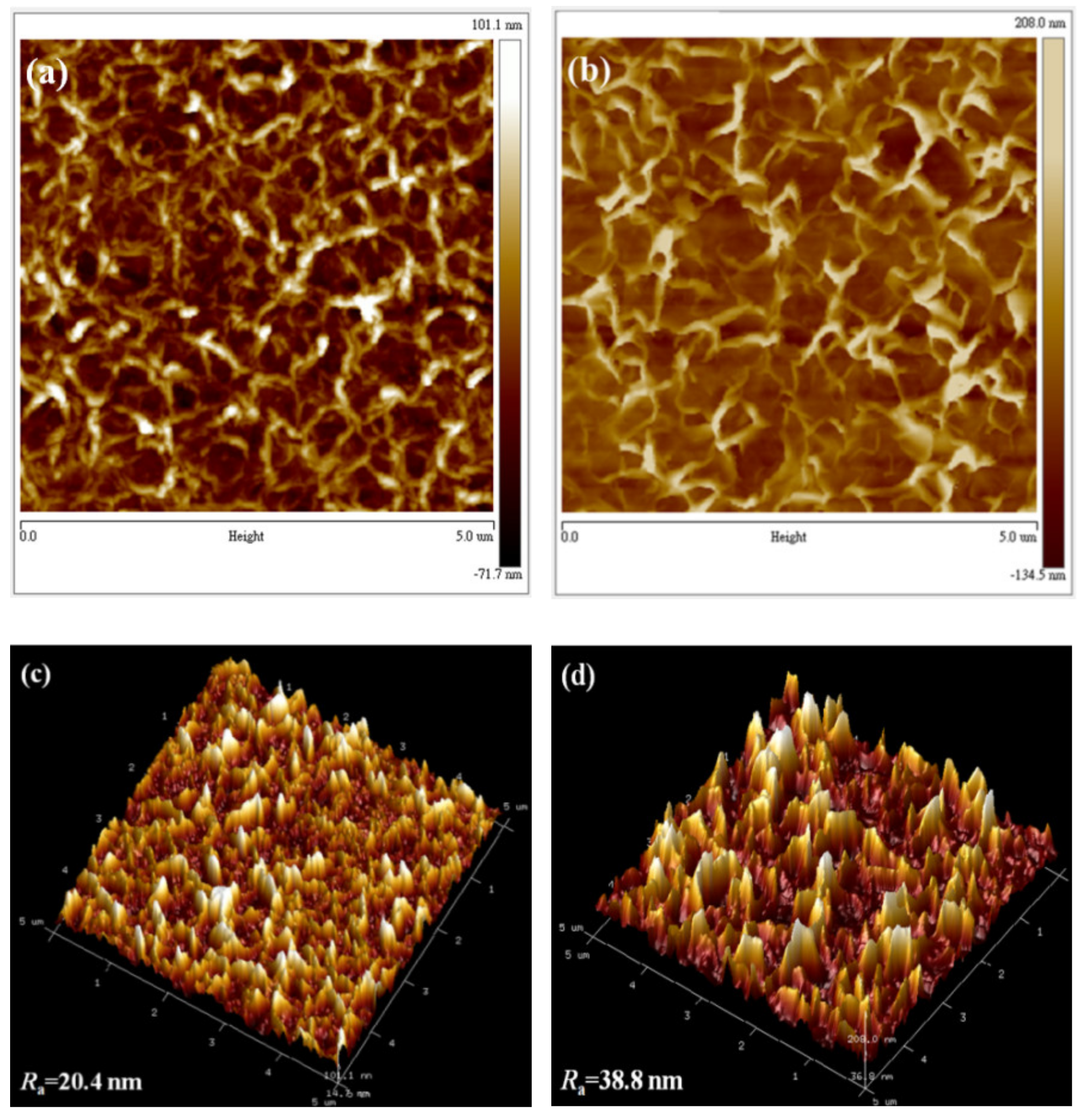
3.1. Morphological Investigation
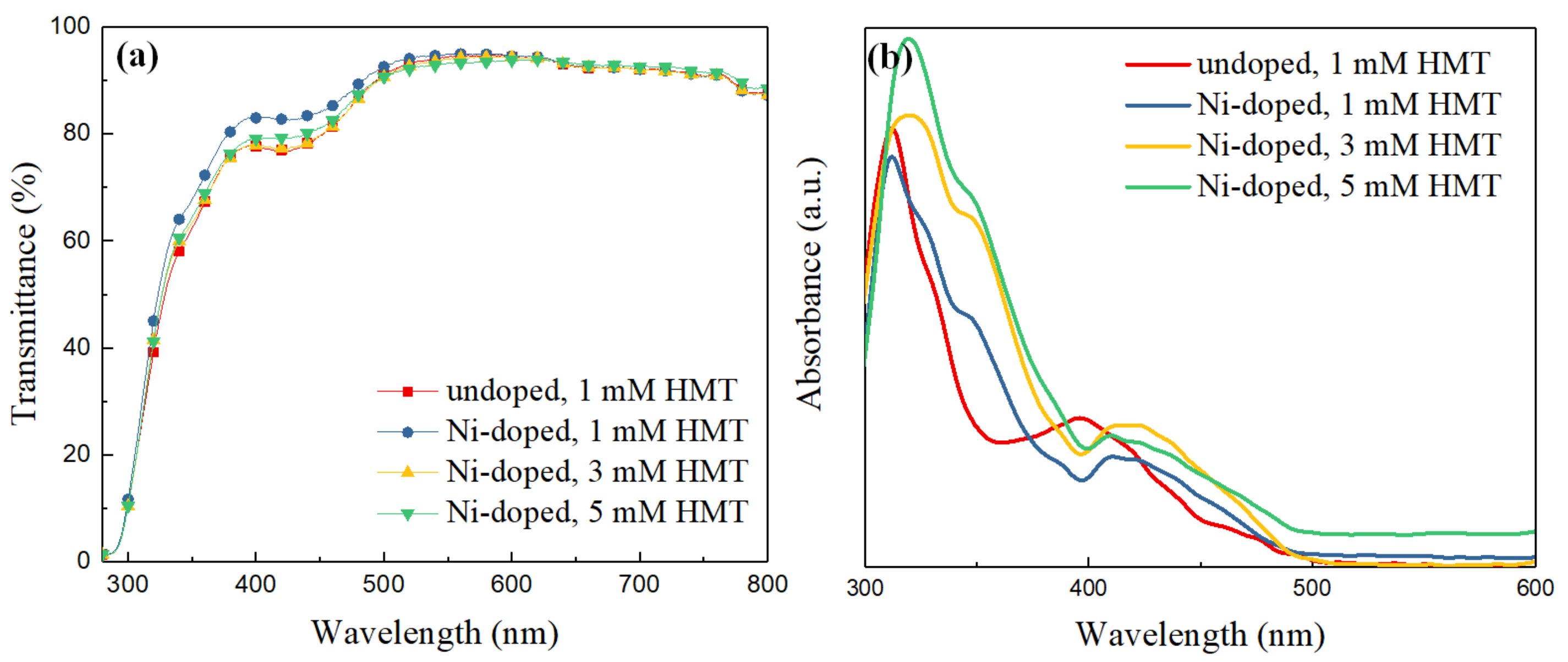
3.2. Optical Measurements
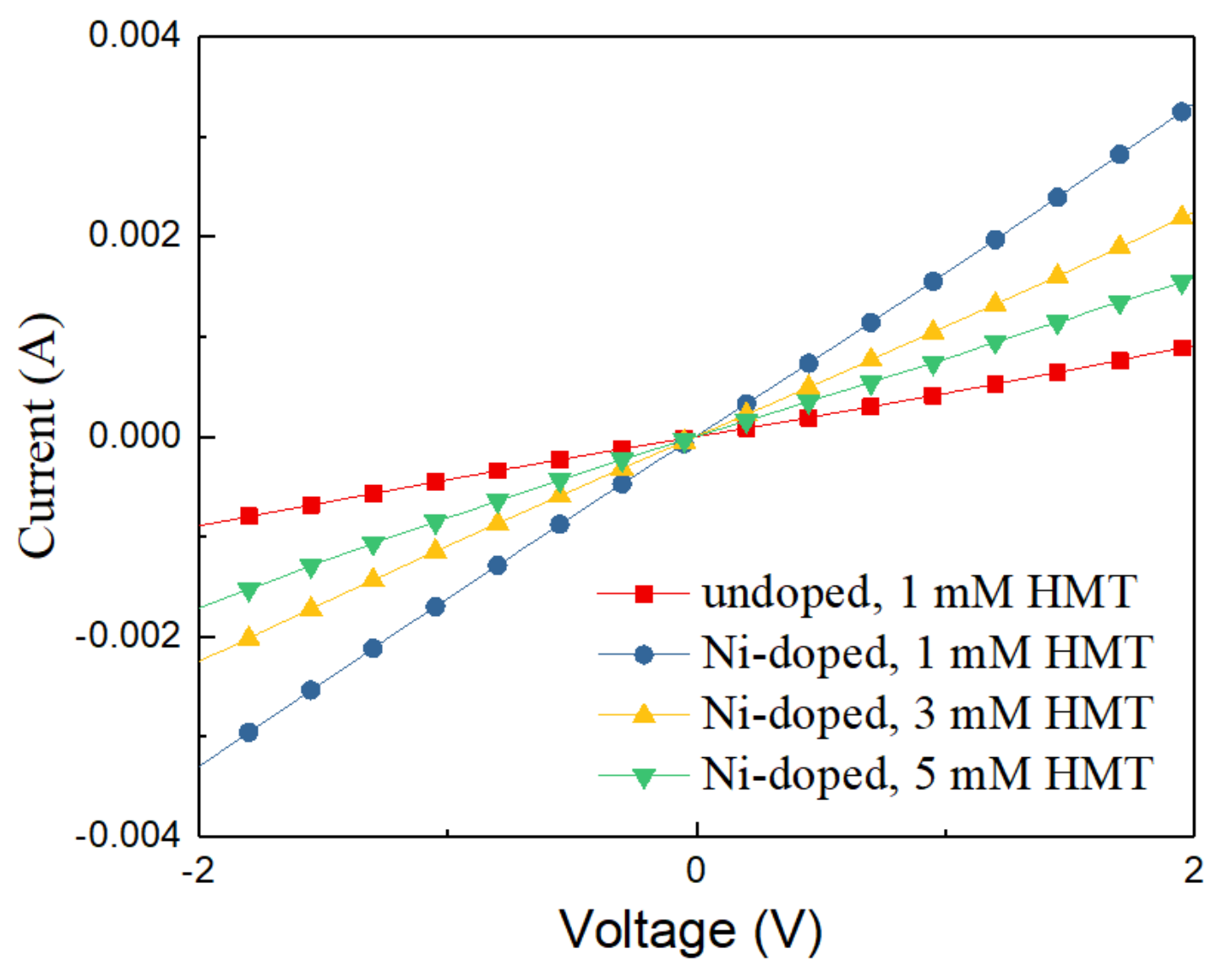
3.3. Electrical Measurements
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Dahiya, A.S.; Sporea, R.A.; Poulin-Vittrant, G.; Alquier, D. Stability evaluation of ZnO nanosheet based source-gated transistors. Sci. Rep. 2019, 9, 2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röder, R.; Geburt, S.; Zapf, M.; Franke, D.; Lorke, M.; Frauenheim, T.; da Rosa, A.L.; Ronning, C. Transition metal and rare earth element doped zinc oxide nanowires for optoelectronics. Phys. Status Solidi B 2019, 256, 1800604. [Google Scholar] [CrossRef] [Green Version]
- Godlewski, M.; Guziewicz, E.; Kopalko, K.; Łuka, G.; Łukasiewicz, M.I.; Krajewski, T.; Witkowski, B.S.; Gierałtowska, S. Zinc oxide for electronic, photovoltaic and optoelectronic applications. Low Temp. Phys. 2011, 37, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.F.; Tseng, Z.L.; Chen, L.C.; Chu, S.Y. ZnO nanowalls grown at low-temperature for electron collection in high-efficiency perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 154, 18–22. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO and materials devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.M.; Lin, Z.K.; Huang, W.J.; Yang, S.H. WO3Nanoparticles or Nanorods Incorporating Cs2CO3/PCBM Buffer Bilayer as Carriers Transporting Materials for Perovskite Solar Cells. Nanoscale Res. Lett. 2016, 11, 464. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.Y.; Yan, P.R.; Yang, S.H. Solution-Processed Hybrid Light-Emitting Devices Comprising TiO2 Nanorods and WO3 Layers as Carrier-Transporting Layers. Nanoscale Res. Lett. 2016, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.L.; Chen, C.M.; Lin, Z.K.; Yang, S.H. Efficiency enhancement of regular-type perovskite solar cells based on Al-doped ZnO nanorods as electron transporting layers. Superlattices Microstruct. 2017, 102, 94–102. [Google Scholar] [CrossRef]
- Pellegrino, D.; Franzò, G.; Strano, V.; Mirabella, S.; Bruno, E. Improved synthesis of ZnO nanowalls effects of chemical bath deposition time and annealing temperature. Chemosensors 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Y.; Yang, S.H. Improved efficiency of perovskite solar cells based on Ni-doped ZnO nanorod arrays and Li salt-doped P3HT layer for charge collection. Opt. Mater. Express 2016, 6, 3651–3669. [Google Scholar] [CrossRef]
- Feng, Z.; Rafique, S.; Cai, Y.; Han, L.; Huang, M.C.; Zhao, H. ZnO nanowall networks for sensor devices: From hydrothermal synthesis to device demonstration. ECS J. Solid State Sci. Technol. 2018, 7, Q3114–Q3119. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, S.H.; Han, S.W. Growth of homoepitaxial ZnO film on ZnO nanorods and light emitting diode applications. Nanotechnology 2007, 18, 055608. [Google Scholar] [CrossRef]
- Lupan, O.; Chai, G.; Chow, L.; Emelchenko, G.A.; Heinrich, H.; Ursaki, V.V.; Gruzintsev, A.N.; Tiginyanu, I.M.; Redkin, A.N. Ultraviolet photoconductive sensor based on single ZnO nanowire. Phys. Status Solidi A 2010, 207, 1735–1740. [Google Scholar] [CrossRef]
- Geng, W.; Kostcheev, S.; Sartel, C.; Sallet, V.; Molinari, M.; Simonetti, O.; Lérondel, G.; Giraudet, L.; Couteau, C. Ohmic contact on single ZnO nanowires grown by MOCVD. Phys. Status Solidi C 2013, 10, 1292–1296. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Chen, M.; Yu, X.; Chang, Y.; Chen, A.; Zhu, H.; Tang, Z. Growth of aligned ZnO nanowires via modified atmospheric pressure chemical vapor deposition. Phys. Lett. A 2016, 380, 3993–3997. [Google Scholar] [CrossRef]
- Zhang, N.; Yi, R.; Shi, R.; Gao, G.; Chen, G.; Liu, X. Novel rose-like ZnO nanoflowers synthesized by chemical vapor deposition. Mater. Lett. 2009, 63, 496–499. [Google Scholar] [CrossRef]
- Maeng, J.; Jo, G.; Choe, M.; Park, W.; Kwon, M.K.; Park, S.J.; Lee, T. Structural and photoluminescence characterization of ZnO nanowalls grown by metal organic chemical vapor deposition. Thin Solid Films 2009, 518, 865–869. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Mehran, M.T. Nanostructured ZnO electron transporting materials for hysteresis-free perovskite solar cells. Sol. Energy 2018, 173, 496–503. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Zhao, E.F.; Meng, F.L.; Lai, X.S.; Dong, X.M.; Wu, J.J.; Tao, X. Iodine-doped ZnO nanopillar arrays for perovskite solar cells with high efficiency up to 18.24%. J. Mater. Chem. A 2017, 5, 12416–12425. [Google Scholar] [CrossRef]
- Narayanan, G.N.; Ganesh, R.S.; Karthigeyan, A. Effect of annealing temperature on structural, optical and electricalproperties of hydrothermal assisted zinc oxide nanorods. Thin Solid Films 2016, 598, 39–45. [Google Scholar] [CrossRef]
- Ji, Y. One-step method for growing of large scale ZnO nanowires on zinc foil. Mater. Lett. 2015, 138, 92–95. [Google Scholar] [CrossRef]
- Tian, J.H.; Hu, J.; Li, S.S.; Zhang, F.; Liu, J.; Shi, J.; Li, X.; Tian, Z.Q.; Chen, Y. Improved seedless hydrothermal synthesis of dense and ultralong ZnO nanowires. Nanotechnology 2011, 22, 245601. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Cen, J.; Zhang, W.; Orlov, A.; Liu, M. Hydrothermal growth of ZnO nanowire arrays: Fine tuning by precursor supersaturation. CrystEngComm 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Tang, J.F.; Su, H.H.; Lu, Y.M.; Chu, S.Y. Controlled growth of ZnO nanoflowers on nanowall and nanorod networks via a hydrothermal method. CrystEngComm 2015, 17, 592–597. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, R.; Lan, J.L.; Wiranwetchayan, O.; Zhang, Q.; Li, C.; Cao, G. Growth of vertically aligned ZnO nanowalls for inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 117, 34–40. [Google Scholar] [CrossRef]
- Tang, J.F.; Chu, S.Y.; Lu, Y.M.; Tseng, Z.L. Using an Al reaction layer to control the morphology and optical properties of ZnO nanorods and nanowalls. Mater. Lett. 2016, 171, 195–199. [Google Scholar] [CrossRef]
- Iwu, K.O.; Strano, V.; Mauro, A.D.; Impellizzeri, G.; Mirabella, S. Enhanced quality, growth kinetics, and photocatalysis of ZnO nanowalls prepared by chemical bath deposition. Cryst. Growth Des. 2015, 15, 4206–4212. [Google Scholar] [CrossRef]
- Shin, K.S.; Park, H.J.; Kumar, B.; Kim, K.H.; Kim, S.H.; Kim, S.W. Inverted organic solar cells with ZnO nanowalls prepared using wet chemical etching in a KOH solution. J. Nanosci. Nanotechnol. 2012, 12, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.N.; Sreedhar, A.; Reddy, C.V.; Cho, M.; Kim, D.; Shim, J. Nickel-doped ZnO structures for efficient water splitting under visible light. Mater. Res. Express 2019, 6, 055517. [Google Scholar] [CrossRef]
- Khan, H.R.; Akram, B.; Aamir, M.; Malik, M.A.; Tahir, A.A.; Choudhary, M.A.; Akhtar, J. Fabrication of Ni2+ incorporated ZnO photoanode for efficient overall water splitting. Appl. Surf. Sci. 2019, 490, 302–308. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guana, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Du, R.; Zhou, J.; Huang, M.Y.; Liu, R.; Li, J.; Xie, Z.; Wu, L.Z.; Liu, Z.; et al. Direct synthesis of graphdiyne nanowalls on arbitrary substrates and its application for photoelectrochemical water splitting cell. Adv. Mater. 2017, 29, 1605308. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Kang, Z.; Li, Y.; Shen, Y.; Sun, Y.; Wang, L.; Zhang, Y. Synergistic Effect of Surface Plasmonic Particles and Surface Passivation Layer on ZnO Nanorod Array for Improved Photoelectrochemical Water Splitting. Sci. Rep. 2016, 6, 29907. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.-C.; Yang, S.-H. Nickel-Doped ZnO Nanowalls with Enhanced Electron Transport Ability for Electrochemical Water Splitting. Nanomaterials 2021, 11, 1980. https://doi.org/10.3390/nano11081980
Jiang B-C, Yang S-H. Nickel-Doped ZnO Nanowalls with Enhanced Electron Transport Ability for Electrochemical Water Splitting. Nanomaterials. 2021; 11(8):1980. https://doi.org/10.3390/nano11081980
Chicago/Turabian StyleJiang, Bing-Chang, and Sheng-Hsiung Yang. 2021. "Nickel-Doped ZnO Nanowalls with Enhanced Electron Transport Ability for Electrochemical Water Splitting" Nanomaterials 11, no. 8: 1980. https://doi.org/10.3390/nano11081980
APA StyleJiang, B.-C., & Yang, S.-H. (2021). Nickel-Doped ZnO Nanowalls with Enhanced Electron Transport Ability for Electrochemical Water Splitting. Nanomaterials, 11(8), 1980. https://doi.org/10.3390/nano11081980






