The Contribution of NMR Spectroscopy in Understanding Perovskite Stabilization Phenomena
Abstract
:1. Introduction
2. Bulk Perovskites
2.1. Solution NMR
2.1.1. Perovskite-Polymer Interactions
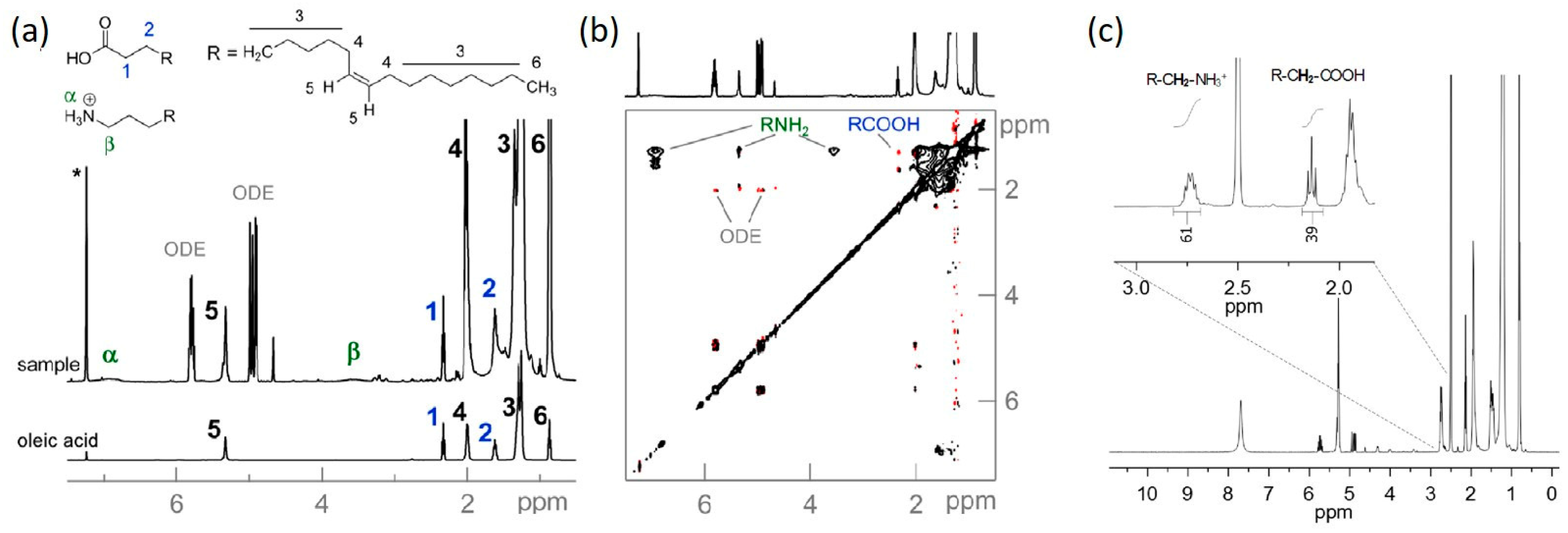
2.1.2. Solubility Enhancers for Perovskite Precursors
2.1.3. Stability of Mixed Cation Perovskite Solutions

2.2. Solid-State NMR
2.2.1. Organic Molecules for Improving Formamidinium Perovskite Stability
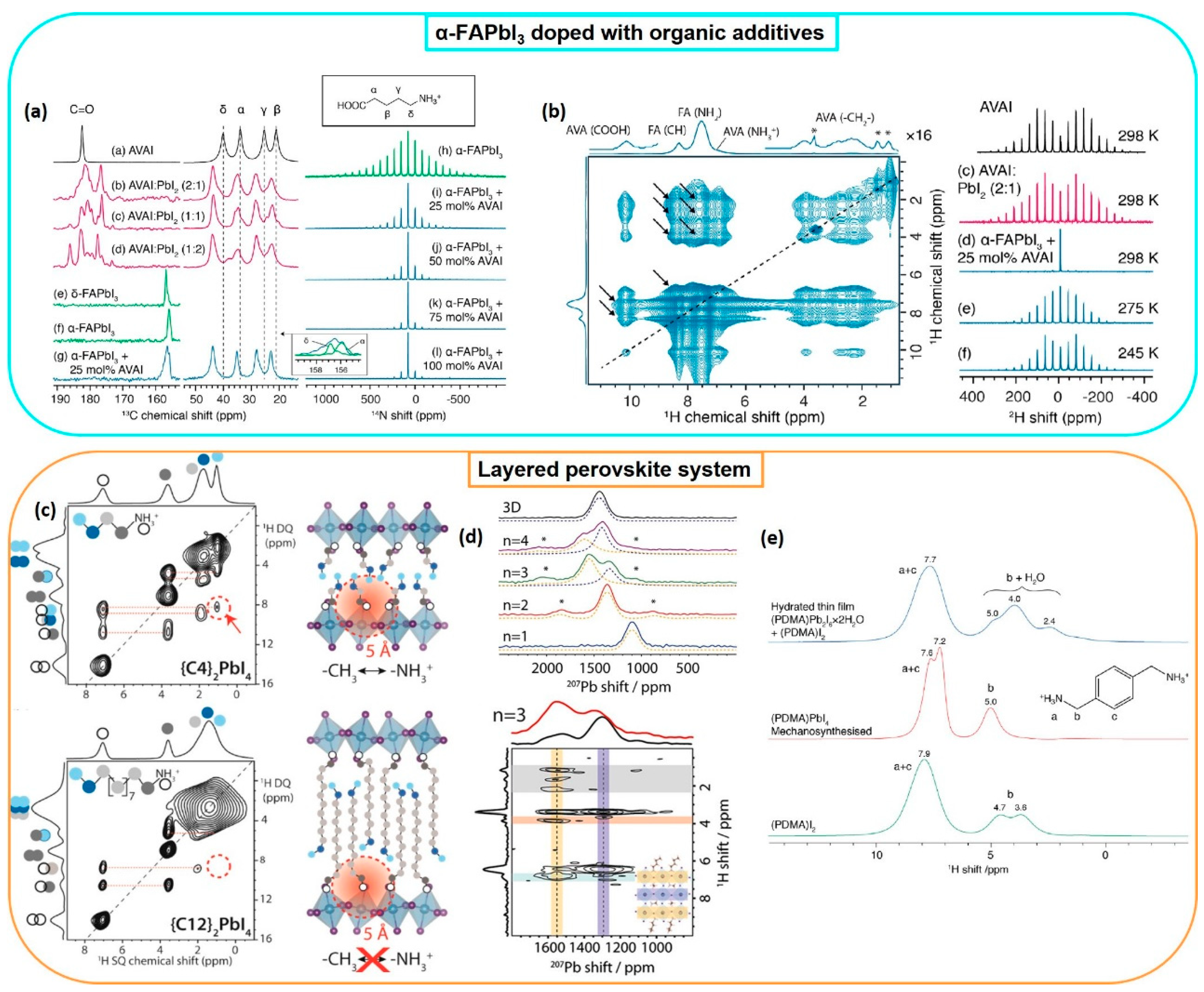
2.2.2. Layered Systems
2.2.3. Cesium-Based Perovskites
3. Nanocrystal Perovskite
3.1. Solution NMR
3.2. Solid-State NMR
4. Concluding Remarks and Future Perspectives
- (1)
- The interaction between perovskite precursors and additives, particularly with polar functional groups. The strength of the interaction is proved and measured by NMR in solution, via investigation of signal shifts in the 1H spectra and changes in relaxation measurements (T1 and T2) and/or in the diffusion coefficients (D). Analysis of 13C CPMAS, 14N MAS and 1H-1H spin diffusion measurements in solid samples corroborates the different environments or a more symmetric distribution of the perovskite components in the case of additives. Moreover, 13C experiments are a sensitive indicator for phase composition (i.e., yellow/black phase of FAPbI3, without/with an additive, respectively).
- (2)
- Use of big organic cations as spacers in the 2D/3D composition. 207Pb-1H correlations, detected via HETCOR experiments, discriminate between the outer and the inner lead signal in 2D/3D perovskites. In general, the increase in linewidth observed for 1H, 13C and 14N nuclei is expected, if multiple environments are surrounding the metal (i.e., Cs+, Pb2+, Sn2+). However, the introduction of a long alkyl chain cation or an external organic additive could negatively affect the charge transport. Thus, in order to foresee the optimum method and optimum organic additive/cation for the stabilization of the perovskite phase, characterization with isotopic enrichment (2H) is needed to clearly define the orientation and the localization of organic cations in the bulk with respect to Pb, and the nature of the interaction with other perovskite elements.
- (3)
- Controlled compositional engineering. 1H NMR has an excellent reliability in quantifying the cations in solution, allowing for the identification of their exact molar ratio. Moreover, reactions and side products are detected, and long-term stability is analyzed by exploiting homo- (1H-1H) and heteronuclear (1H-13C) 1D and 2D experiments. Despite the great progress with ssNMR for detecting the phase transitions in single-cation perovskites (i.e., the transition from the cubic to the monoclinic phase for CsSnCl3), it is crucial to also distinguish the different crystal phases in mixed cation-halide perovskites, in order to explore potential black phases for optoelectronic applications. For this purpose, 207Pb experiments provide a tool for investigating the lead-halogen interaction and PbX6 symmetry, while the analysis of the cation reorientation with respect to Pb is still difficult to probe quantitatively and needs costly isotopic enrichment (15N or 2H experiments).
- (4)
- Efficient ligand coordination with the inorganic core of PNCs. 1H, NOESY and DOSY experiments provide information about the ligands in free and bound states, providing insights into the ligands ratio and the nature of the chemical bond of the ligand coordinated to the surface, which positively passivate defects and affect the colloidal stability of the perovskite. The introduction of various ligands stabilizes perovskite NCs. However, the mechanism is not completely understood, and more efforts are needed to characterize, at the atomic level, these systems, along with the long-term stability in different solvents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best Research-Cell Efficiencie. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev210726.pdf (accessed on 7 August 2021).
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2020, 20, 10–21. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; San Martin, J.; Sun, Y.; Zhu, D.; Yan, Y. Lead halide perovskites for photocatalytic organic synthesis. Nat. Commun. 2019, 10, 2843. [Google Scholar] [CrossRef] [PubMed]
- Etgar, L. The merit of perovskite’s dimensionality; can this replace the 3D halide perovskite? Energy Environ. Sci. 2018, 11, 234–242. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2019, 8, 27–54. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; de Miguel, G.; Nazeeruddin, M.K. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Yun, J.S.; Kim, J.; Soufiani, A.M.; Chen, S.; Cho, Y.; Deng, X.; Seidel, J.; Lim, S.; Huang, S.; et al. Passivation of Grain Boundaries by Phenethylammonium in Formamidinium-Methylammonium Lead Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 647–654. [Google Scholar] [CrossRef]
- Xue, J.; Wang, R.; Chen, X.; Yao, C.; Jin, X.; Wang, K.-L.; Huang, W.; Huang, T.; Zhao, Y.; Zhai, Y.; et al. Reconfiguring the band-edge states of photovoltaic perovskites by conjugated organic cations. Science 2021, 371, 636–640. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; Sanchez-Diaz, J.; Echeverria-Arrondo, C.; Masi, S.; Esparza, D.; Barea, E.M.; Mora-Sero, I. Widening the 2D/3D Perovskite Family for Efficient and Thermal-Resistant Solar Cells by the Use of Secondary Ammonium Cations. ACS Energy Lett. 2020, 5, 1013–1021. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Gualdron-Reyes, A.F.; Mora-Sero, I. Stabilization of Black Perovskite Phase in FAPbI(3) and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards commercialization: The operational stability of perovskite solar cells. Chem. Soc. Rev. 2020, 49, 8235–8286. [Google Scholar] [CrossRef] [PubMed]
- Phung, N.; Abate, A. Chapter 9—Stability of materials and complete devices. In Characterization Techniques for Perovskite Solar Cell Materials; Pazoki, M., Hagfeldt, A., Edvinsson, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–215. [Google Scholar]
- Masi, S.; Rizzo, A.; Aiello, F.; Balzano, F.; Uccello-Barretta, G.; Listorti, A.; Gigli, G.; Colella, S. Multiscale morphology design of hybrid halide perovskites through a polymeric template. Nanoscale 2015, 7, 18956–18963. [Google Scholar] [CrossRef] [PubMed]
- Hassanabadi, E.; Latifi, M.; Gualdron-Reyes, A.F.; Masi, S.; Yoon, S.J.; Poyatos, M.; Julian-Lopez, B.; Mora-Sero, I. Ligand & band gap engineering: Tailoring the protocol synthesis for achieving high-quality CsPbI(3)quantum dots. Nanoscale 2020, 12, 14194–14203. [Google Scholar]
- Levchuk, I.; Hou, Y.; Gruber, M.; Brandl, M.; Herre, P.; Tang, X.; Hoegl, F.; Batentschuk, M.; Osvet, A.; Hock, R.; et al. Deciphering the Role of Impurities in Methylammonium Iodide and Their Impact on the Performance of Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1600593. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Masi, S.; Aiello, F.; Listorti, A.; Balzano, F.; Altamura, D.; Giannini, C.; Caliandro, R.; Uccello-Barretta, G.; Rizzo, A.; Colella, S. Connecting the solution chemistry of PbI2 and MAI: A cyclodextrin-based supramolecular approach to the formation of hybrid halide perovskites. Chem. Sci. 2018, 9, 3200–3208. [Google Scholar] [CrossRef] [Green Version]
- Avram, L.; Cohen, Y. Diffusion NMR of molecular cages and capsules. Chem. Soc. Rev. 2015, 44, 586–602. [Google Scholar] [CrossRef]
- Grisorio, R.; Di Clemente, M.E.; Fanizza, E.; Allegretta, I.; Altamura, D.; Striccoli, M.; Terzano, R.; Giannini, C.; Irimia-Vladu, M.; Suranna, G.P. Exploring the surface chemistry of cesium lead halide perovskite nanocrystals. Nanoscale 2019, 11, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Ferrara, C.; Quadrelli, P.; Guizzetti, G.; Patrini, M.; Milanese, C.; Tealdi, C.; Malavasi, L. The FA1–xMAxPbI3 System: Correlations among Stoichiometry Control, Crystal Structure, Optical Properties, and Phase Stability. J. Phys. Chem. C 2017, 121, 8746–8751. [Google Scholar] [CrossRef]
- Askar, A.M.; Bernard, G.M.; Wiltshire, B.; Shankar, K.; Michaelis, V.K. Multinuclear Magnetic Resonance Tracking of Hydro, Thermal, and Hydrothermal Decomposition of CH3NH3PbI3. J. Phys. Chem. C 2017, 121, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Hope, M.A.; Nakamura, T.; Ahlawat, P.; Mishra, A.; Cordova, M.; Jahanbakhshi, F.; Mladenović, M.; Runjhun, R.; Merten, L.; Hinderhofer, A.; et al. Nanoscale Phase Segregation in Supramolecular π-Templating for Hybrid Perovskite Photovoltaics from NMR Crystallography. J. Am. Chem. Soc. 2021, 143, 1529–1538. [Google Scholar] [CrossRef]
- Fabini, D.H.; Siaw, T.A.; Stoumpos, C.C.; Laurita, G.; Olds, D.; Page, K.; Hu, J.G.; Kanatzidis, M.G.; Han, S.; Seshadri, R. Universal Dynamics of Molecular Reorientation in Hybrid Lead Iodide Perovskites. J. Am. Chem. Soc. 2017, 139, 16875–16884. [Google Scholar] [CrossRef] [Green Version]
- Milić, J.V.; Im, J.-H.; Kubicki, D.J.; Ummadisingu, A.; Seo, J.-Y.; Li, Y.; Ruiz-Preciado, M.A.; Dar, M.I.; Zakeeruddin, S.M.; Emsley, L.; et al. Supramolecular Engineering for Formamidinium-Based Layered 2D Perovskite Solar Cells: Structural Complexity and Dynamics Revealed by Solid-State NMR Spectroscopy. Adv. Energy Mater. 2019, 9, 1900284. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Prochowicz, D.; Hofstetter, A.; Péchy, P.; Zakeeruddin, S.M.; Grätzel, M.; Emsley, L. Cation Dynamics in Mixed-Cation (MA)x(FA)1–xPbI3 Hybrid Perovskites from Solid-State NMR. J. Am. Chem. Soc. 2017, 139, 10055–10061. [Google Scholar] [CrossRef] [Green Version]
- Mączka, M.; Ptak, M.; Vasconcelos, D.L.M.; Giriunas, L.; Freire, P.T.C.; Bertmer, M.; Banys, J.; Simenas, M. NMR and Raman Scattering Studies of Temperature- and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. [Google Scholar] [CrossRef]
- Baikie, T.; Barrow, N.S.; Fang, Y.; Keenan, P.J.; Slater, P.R.; Piltz, R.O.; Gutmann, M.; Mhaisalkar, S.G.; White, T.J. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 2015, 3, 9298–9307. [Google Scholar] [CrossRef]
- Qiao, W.-C.; Wu, J.; Zhang, R.; Ou-Yang, W.; Chen, X.; Yang, G.; Chen, Q.; Wang, X.L.; Wang, H.F.; Yao, Y.-F. In situ NMR Investigation of the Photoresponse of Perovskite Crystal. Matter 2020, 3, 2042–2054. [Google Scholar] [CrossRef]
- Wasylishen, R.E.; Knop, O.; Macdonald, J.B. Cation rotation in methylammonium lead halides. Solid State Commun. 1985, 56, 581–582. [Google Scholar] [CrossRef]
- Bernard, G.M.; Wasylishen, R.E.; Ratcliffe, C.I.; Terskikh, V.; Wu, Q.; Buriak, J.M.; Hauger, T. Methylammonium Cation Dynamics in Methylammonium Lead Halide Perovskites: A Solid-State NMR Perspective. J. Phys. Chem. A 2018, 122, 1560–1573. [Google Scholar] [CrossRef] [Green Version]
- Colella, S.; Todaro, M.; Masi, S.; Listorti, A.; Altamura, D.; Caliandro, R.; Giannini, C.; Carignani, E.; Geppi, M.; Meggiolaro, D.; et al. Light-Induced Formation of Pb3+ Paramagnetic Species in Lead Halide Perovskites. ACS Energy Lett. 2018, 3, 1840–1847. [Google Scholar] [CrossRef]
- Rosales, B.A.; Men, L.; Cady, S.D.; Hanrahan, M.P.; Rossini, A.J.; Vela, J. Persistent Dopants and Phase Segregation in Organolead Mixed-Halide Perovskites. Chem. Mater. 2016, 28, 6848–6859. [Google Scholar] [CrossRef] [Green Version]
- Aebli, M.; Piveteau, L.; Nazarenko, O.; Benin, B.M.; Krieg, F.; Verel, R.; Kovalenko, M.V. Lead-Halide Scalar Couplings in 207Pb NMR of APbX3 Perovskites (A = Cs, Methylammonium, Formamidinium; X = Cl, Br, I). Sci. Rep. 2020, 10, 8229. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Hidalgo, J.; Perini, C.A.R.; Castro-Méndez, A.-F.; Vagott, J.N.; Bairley, K.; Wang, S.; Li, X.; Correa-Baena, J.-P. Structural Stability of Formamidinium- and Cesium-Based Halide Perovskites. ACS Energy Lett. 2021, 6, 1942–1969. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Additive engineering for highly efficient organic–inorganic halide perovskite solar cells: Recent advances and perspectives. J. Mater. Chem. A 2017, 5, 12602–12652. [Google Scholar] [CrossRef]
- Hsiao, K.-C.; Jao, M.-H.; Tian, K.-Y.; Lin, T.-H.; Tran, D.-P.; Liao, H.-C.; Hou, C.-H.; Shyue, J.-J.; Wu, M.-C.; Su, W.-F. Acetamidinium Cation to Confer Ion Immobilization and Structure Stabilization of Organometal Halide Perovskite toward Long Life and High-Efficiency p-i-n Planar Solar Cell via Air-Processable Method. Solar Rrl 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Lee, H.; Gaiaschi, S.; Chapon, P.; Tondelier, D.; Bourée, J.-E.; Bonnassieux, Y.; Derycke, V.; Geffroy, B. Effect of Halide Ion Migration on the Electrical Properties of Methylammonium Lead Tri-Iodide Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 17728–17734. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S.; Lee, S.-W.; Cho, K.; Lee, K.D.; Kim, H.; Park, S.; Kwon, G.; Ahn, S.-W.; Lee, H.-M.; et al. Relationship between ion migration and interfacial degradation of CH3NH3PbI3 perovskite solar cells under thermal conditions. Sci. Rep. 2017, 7, 1200. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Pineda De La O, E.; Alhazmi, N.; Ebbens, S.J.; Dunbar, A.D.F. Influence of Additives on the In Situ Crystallization Dynamics of Methyl Ammonium Lead Halide Perovskites. ACS Appl. Energy Mater. 2021, 4, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzo, A.; Munir, R.; Listorti, A.; Giuri, A.; Corcione, C.E.; Treat, N.D.; Gigli, G.; Amassian, A.; Stingelin, N.; et al. Organic Gelators as Growth Control Agents for Stable and Reproducible Hybrid Perovskite-Based Solar Cells. Adv. Energy Mater. 2017, 7, 1602600–1602610. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Mabrouk, S.; Wu, F.; Chen, K.; Yang, S.; Qiao, Q. A strategic review on processing routes towards highly efficient perovskite solar cells. J. Mater. Chem. A 2018, 6, 2406–2431. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Long, M.; Zhang, T.; Wei, Z.; Chen, H.; Yang, S.; Xu, J. Hybrid Halide Perovskite Solar Cell Precursors: Colloidal Chemistry and Coordination Engineering behind Device Processing for High Efficiency. J. Am. Chem. Soc. 2015, 137, 4460–4468. [Google Scholar] [CrossRef]
- Fairfield, D.J.; Sai, H.; Narayanan, A.; Passarelli, J.V.; Chen, M.; Palasz, J.; Palmer, L.C.; Wasielewski, M.R.; Stupp, S.I. Structure and chemical stability in perovskite–polymer hybrid photovoltaic materials. J. Mater. Chem. A 2019, 7, 1687–1699. [Google Scholar] [CrossRef]
- Zuo, L.; Guo, H.; deQuilettes, D.W.; Jariwala, S.; De Marco, N.; Dong, S.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M.; et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, e1700106. [Google Scholar] [CrossRef] [Green Version]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular self-healing materials via host-guest strategy between cyclodextrin and specific types of guest molecules. Coord. Chem. Rev. 2021, 432, 213711. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Belenguer-Sapiña, C.; Pellicer-Castell, E.; Mauri-Aucejo, A.R.; Simó-Alfonso, E.F.; Amorós, P. Cyclodextrins as a Key Piece in Nanostructured Materials: Quantitation and Remediation of Pollutants. Nanomaterials 2021, 11, 7. [Google Scholar] [CrossRef]
- Weber, O.J.; Charles, B.; Weller, M.T. Phase behaviour and composition in the formamidinium–methylammonium hybrid lead iodide perovskite solid solution. J. Mater. Chem. A 2016, 4, 15375–15382. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, K.; Guo, D.Y.; Wang, S.L.; Li, P.G. Cations substitution tuning phase stability in hybrid perovskite single crystals by strain relaxation. RSC Adv. 2018, 8, 2900–2905. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, B.; Koh, T.M.; Febriansyah, B.; Bruno, A.; Mathews, N.; Mhaisalkar, S.G.; Soci, C. Mixed-Dimensional Naphthylmethylammonium-Methylammonium Lead Iodide Perovskites with Improved Thermal Stability. Sci. Rep. 2020, 10, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Park, N.-G. A thin film (<200 nm) perovskite solar cell with 18% efficiency. J. Mater. Chem. A 2020, 8, 17420–17428. [Google Scholar] [CrossRef]
- Van Gompel, W.T.M.; Herckens, R.; Reekmans, G.; Ruttens, B.; D’Haen, J.; Adriaensens, P.; Lutsen, L.; Vanderzande, D. Degradation of the Formamidinium Cation and the Quantification of the Formamidinium–Methylammonium Ratio in Lead Iodide Hybrid Perovskites by Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. C 2018, 122, 4117–4124. [Google Scholar] [CrossRef]
- Valenzano, V.; Cesari, A.; Balzano, F.; Milella, A.; Fracassi, F.; Listorti, A.; Gigli, G.; Rizzo, A.; Uccello-Barretta, G.; Colella, S. Methylammonium-formamidinium reactivity in aged organometal halide perovskite inks. Cell Rep. Phys. Sci. 2021, 2, 100432. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Wang, L.; Chen, C.; Li, Z.; Liu, R.; Meng, H.; Shao, Z.; Du, X.; Zhang, H.; et al. Perovskite Solution Aging: What Happened and How to Inhibit? Chem 2020, 6, 1369–1378. [Google Scholar] [CrossRef]
- Furukawa, Y.; Nakamura, D. Cationic Dynamics in the Crystalline Phases of (CH3NH3)PbX3 (X: Cl, Br) as Studied by Proton Magnetic Resonance Techniques. Z. Naturforsch. A 1989, 44, 1122–1126. [Google Scholar] [CrossRef]
- Knop, O.; Wasylishen, R.E.; White, M.A.; Cameron, T.S.; Oort, M.J.M.V. Alkylammonium lead halides. Part 2. CH3NH3PbX3 (X = Cl, Br, I) perovskites: Cuboctahedral halide cages with isotropic cation reorientation. Can. J. Chem. 1990, 68, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Eguchi, T.; Nakayama, H.; Nakamura, N.; Kishita, M. Molecular Motions and Phase Transitions in Solid CH3NH3PbX3 (X = C1, Br, I) as Studied by NMR and NQR. Z. Naturforsch. A 1991, 46, 240–246. [Google Scholar] [CrossRef]
- Franssen, W.M.J.; Kentgens, A.P.M. Solid–state NMR of hybrid halide perovskites. Solid State Nucl. Magn. Reson. 2019, 100, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Piveteau, L.; Morad, V.; Kovalenko, M.V. Solid-State NMR and NQR Spectroscopy of Lead-Halide Perovskite Materials. J. Am. Chem. Soc. 2020, 142, 19413–19437. [Google Scholar] [CrossRef]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Blancon, J.C.; Stier, A.V.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Traoré, B.; Pedesseau, L.; Kepenekian, M.; Katsutani, F.; Noe, G.T.; et al. Scaling law for excitons in 2D perovskite quantum wells. Nat. Commun. 2018, 9, 2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Yang, Z.; Liu, S. Two dimensional metal halide perovskites: Promising candidates for light-emitting diodes. J. Energy Chem. 2019, 37, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Turren-Cruz, S.-H.; Hagfeldt, A.; Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 2018, 362, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Echeverría-Arrondo, C.; Salim, K.M.M.; Ngo, T.T.; Mendez, P.F.; López-Fraguas, E.; Macias-Pinilla, D.F.; Planelles, J.; Climente, J.I.; Mora-Seró, I. Chemi-Structural Stabilization of Formamidinium Lead Iodide Perovskite by Using Embedded Quantum Dots. ACS Energy Lett. 2020, 5, 418–427. [Google Scholar] [CrossRef]
- Alanazi, A.Q.; Kubicki, D.J.; Prochowicz, D.; Alharbi, E.A.; Bouduban, M.E.F.; Jahanbakhshi, F.; Mladenović, M.; Milić, J.V.; Giordano, F.; Ren, D.; et al. Atomic-Level Microstructure of Efficient Formamidinium-Based Perovskite Solar Cells Stabilized by 5-Ammonium Valeric Acid Iodide Revealed by Multinuclear and Two-Dimensional Solid-State NMR. J. Am. Chem. Soc. 2019, 141, 17659–17669. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tress, W.; Milić, J.V.; Kubicki, D.; Emsley, L.; Grätzel, M. Addition of adamantylammonium iodide to hole transport layers enables highly efficient and electroluminescent perovskite solar cells. Energy Environ. Sci. 2018, 11, 3310–3320. [Google Scholar] [CrossRef]
- Alanazi, A.Q.; Almalki, M.H.; Mishra, A.; Kubicki, D.J.; Wang, Z.; Merten, L.; Eickemeyer, F.T.; Zhang, H.; Ren, D.; Alyamani, A.Y.; et al. Benzylammonium-Mediated Formamidinium Lead Iodide Perovskite Phase Stabilization for Photovoltaics. Adv. Funct. Mater. 2021, 31, 2101163. [Google Scholar] [CrossRef]
- Bi, D.; Li, X.; Milić, J.V.; Kubicki, D.J.; Pellet, N.; Luo, J.; LaGrange, T.; Mettraux, P.; Emsley, L.; Zakeeruddin, S.M.; et al. Multifunctional molecular modulators for perovskite solar cells with over 20% efficiency and high operational stability. Nat. Commun. 2018, 9, 4482. [Google Scholar] [CrossRef]
- Sanchez-Godoy, H.E.; Erazo, E.A.; Gualdron-Reyes, A.F.; Khan, A.H.; Agouram, S.; Barea, E.M.; Rodriguez, R.A.; Zarazua, I.; Ortiz, P.; Cortes, M.T.; et al. Preferred Growth Direction by PbS Nanoplatelets Preserves Perovskite Infrared Light Harvesting for Stable, Reproducible, and Efficient Solar Cells. Adv. Energy Mater. 2020, 10, 2002422–2002430. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, C.J.; Kennard, R.M.; Paluch, P.; Venkatesan, N.R.; Chabinyc, M.L.; Manjunatha Reddy, G.N. Dynamic Motion of Organic Spacer Cations in Ruddlesden–Popper Lead Iodide Perovskites Probed by Solid-State NMR Spectroscopy. Chem. Mater. 2021, 33, 642–656. [Google Scholar] [CrossRef]
- Lee, J.; Lee, W.; Kang, K.; Lee, T.; Lee, S.K. Layer-by-Layer Structural Identification of 2D Ruddlesden–Popper Hybrid Lead Iodide Perovskites by Solid-State NMR Spectroscopy. Chem. Mater. 2021, 33, 370–377. [Google Scholar] [CrossRef]
- Dučinskas, A.; Kim, G.Y.; Moia, D.; Senocrate, A.; Wang, Y.-R.; Hope, M.A.; Mishra, A.; Kubicki, D.J.; Siczek, M.; Bury, W.; et al. Unravelling the Behavior of Dion–Jacobson Layered Hybrid Perovskites in Humid Environments. ACS Energy Lett. 2021, 6, 337–344. [Google Scholar] [CrossRef]
- Zhang, H.; Eickemeyer, F.T.; Zhou, Z.; Mladenović, M.; Jahanbakhshi, F.; Merten, L.; Hinderhofer, A.; Hope, M.A.; Ouellette, O.; Mishra, A.; et al. Multimodal host–guest complexation for efficient and stable perovskite photovoltaics. Nat. Commun. 2021, 12, 3383. [Google Scholar] [CrossRef]
- Karmakar, A.; Bhattacharya, A.; Sarkar, D.; Bernard, G.M.; Mar, A.; Michaelis, V.K. Influence of hidden halogen mobility on local structure of CsSn(Cl1−xBrx)3 mixed-halide perovskites by solid-state NMR. Chem. Sci. 2021, 12, 3253–3263. [Google Scholar] [CrossRef]
- Karmakar, A.; Bhattacharya, A.; Bernard, G.M.; Mar, A.; Michaelis, V.K. Revealing the Local Sn and Pb Arrangements in CsSnxPb1–xBr3 Perovskites with Solid-State NMR Spectroscopy. ACS Mater. Lett. 2021, 3, 261–267. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Yu, D.; Cao, F.; Gao, Y.; Xiong, Y.; Zeng, H. Room-Temperature Ion-Exchange-Mediated Self-Assembly toward Formamidinium Perovskite Nanoplates with Finely Tunable, Ultrapure Green Emissions for Achieving Rec. 2020 Displays. Adv. Funct. Mater. 2018, 28, 1800248. [Google Scholar] [CrossRef]
- Perovskite QD Films Get Closer to Market-Avantama Qualifies Its Green pQD Display Film. Available online: https://www.perovskite-info.com/perovskite-qd-films-get-closer-market-avantama-qualifies-its-green-pqd-display (accessed on 7 August 2021).
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [Green Version]
- Hens, Z.; Martins, J.C. A Solution NMR Toolbox for Characterizing the Surface Chemistry of Colloidal Nanocrystals. Chem. Mater. 2013, 25, 1211–1221. [Google Scholar] [CrossRef]
- Brown, A.A.M.; Vashishtha, P.; Hooper, T.J.N.; Ng, Y.F.; Nutan, G.V.; Fang, Y.; Giovanni, D.; Tey, J.N.; Jiang, L.; Damodaran, B.; et al. Precise Control of CsPbBr3 Perovskite Nanocrystal Growth at Room Temperature: Size Tunability and Synthetic Insights. Chem. Mater. 2021, 33, 2387–2397. [Google Scholar] [CrossRef]
- Yang, H.S.; Noh, S.H.; Suh, E.H.; Jung, J.; Oh, J.G.; Lee, K.H.; Jang, J. Enhanced Stabilities and Production Yields of MAPbBr3 Quantum Dots and Their Applications as Stretchable and Self-Healable Color Filters. ACS Appl. Mater. Interfaces 2021, 13, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.L.; Kessler, M.L.; Dempsey, J.L. Molecular-Level Insight into Semiconductor Nanocrystal Surfaces. J. Am. Chem. Soc. 2021, 143, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.K.; Santra, P.K.; Joshi, N.; Chugh, J.; Singh, S.K.; Rensmo, H.; Ghosh, P.; Nag, A. Origin of the Substitution Mechanism for the Binding of Organic Ligands on the Surface of CsPbBr3 Perovskite Nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. [Google Scholar] [CrossRef]
- Baranov, D.; Caputo, G.; Goldoni, L.; Dang, Z.; Scarfiello, R.; De Trizio, L.; Portone, A.; Fabbri, F.; Camposeo, A.; Pisignano, D.; et al. Transforming colloidal Cs4PbBr6 nanocrystals with poly(maleic anhydride-alt-1-octadecene) into stable CsPbBr3 perovskite emitters through intermediate heterostructures. Chem. Sci. 2020, 11, 3986–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grisorio, R.; Fanizza, E.; Allegretta, I.; Altamura, D.; Striccoli, M.; Terzano, R.; Giannini, C.; Vergaro, V.; Ciccarella, G.; Margiotta, N.; et al. Insights into the role of the lead/surfactant ratio in the formation and passivation of cesium lead bromide perovskite nanocrystals. Nanoscale 2020, 12, 623–637. [Google Scholar] [CrossRef]
- Ko, J.; Ma, K.; Joung, J.F.; Park, S.; Bang, J. Ligand-Assisted Direct Photolithography of Perovskite Nanocrystals Encapsulated with Multifunctional Polymer Ligands for Stable, Full-Colored, High-Resolution Displays. Nano Lett. 2021, 21, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Fang, Y.-H.; Chen, L.-G.; Liang, F.-C.; Yan, Z.-L.; Ebe, H.; Takahashi, Y.; Chiba, T.; Kido, J.; Kuo, C.-C. High luminescence and external quantum efficiency in perovskite quantum-dots light-emitting diodes featuring bilateral affinity to silver and short alkyl ligands. Chem. Eng. J. 2021, 414, 128866. [Google Scholar] [CrossRef]
- Zhong, K.; Lu, S.; Guo, W.; Su, J.; Sun, S.; Hai, J.; Chen, F.; Wang, A.; Wang, B. Embedding CsPbBr3 quantum dots into a pillar[5]arene-based supramolecular self-assembly for an efficient photocatalytic cross-coupling hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 10180–10185. [Google Scholar] [CrossRef]
- Smock, S.R.; Williams, T.J.; Brutchey, R.L. Quantifying the Thermodynamics of Ligand Binding to CsPbBr3 Quantum Dots. Angew. Chem. Int. Ed. 2018, 57, 11711–11715. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.A.M.; Hooper, T.J.N.; Veldhuis, S.A.; Chin, X.Y.; Bruno, A.; Vashishtha, P.; Tey, J.N.; Jiang, L.; Damodaran, B.; Pu, S.H.; et al. Self-assembly of a robust hydrogen-bonded octylphosphonate network on cesium lead bromide perovskite nanocrystals for light-emitting diodes. Nanoscale 2019, 11, 12370–12380. [Google Scholar] [CrossRef]
- Chen, Y.; Smock, S.R.; Flintgruber, A.H.; Perras, F.A.; Brutchey, R.L.; Rossini, A.J. Surface Termination of CsPbBr3 Perovskite Quantum Dots Determined by Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2020, 142, 6117–6127. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Nucleus | Spin | NMR Experiment | Information Obtained |
|---|---|---|---|
| 1H | ½ | 1D spectrum | Quantitative determination of the organic cations ratio Detection of strong and weak interactions with additives Characterization of free and bound organic ligands for nanocrystals Analysis of stability |
| T1 and T2 measurements | Changes in molecular motions due to intermolecular dipolar interactions | ||
| DOSY | Changes in hydrodynamic radius due to complexation/interaction Detection of ligand-nanocrystal surface interaction | ||
| 2D experiments (1H-1H and 1H-X) | Detection of intra- and intermolecular dipolar interactions Characterization of impurities Characterization of ligands Detection of ligand-nanocrystal surface interaction | ||
| 2H | 1 | 1D spectrum | Detection of phase transitions via analysis of cation dynamics |
| 13C | ½ | CP MAS spectrum | Detection of interactions with additives Analysis of phase composition Evaluation of ligand exchange in nanocrystals |
| 14N | 1 | 1D spectrum | Analysis of spinning sidebands for elucidating cation reorientation dynamics |
| 207Pb | ½ | 1D spectrum | Analysis of sample composition in mixed systems Changes at the metal environment (symmetry) |
| 133Cs | ½ | 1D spectrum | Changes at the metal environment (symmetry) Detection of phase transitions Detection of impurities Analysis of sample composition in mixed systems |
| 119Sn | ½ | 1D spectrum | Detection of impurities Analysis of sample composition in mixed systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, F.; Masi, S. The Contribution of NMR Spectroscopy in Understanding Perovskite Stabilization Phenomena. Nanomaterials 2021, 11, 2024. https://doi.org/10.3390/nano11082024
Aiello F, Masi S. The Contribution of NMR Spectroscopy in Understanding Perovskite Stabilization Phenomena. Nanomaterials. 2021; 11(8):2024. https://doi.org/10.3390/nano11082024
Chicago/Turabian StyleAiello, Federica, and Sofia Masi. 2021. "The Contribution of NMR Spectroscopy in Understanding Perovskite Stabilization Phenomena" Nanomaterials 11, no. 8: 2024. https://doi.org/10.3390/nano11082024
APA StyleAiello, F., & Masi, S. (2021). The Contribution of NMR Spectroscopy in Understanding Perovskite Stabilization Phenomena. Nanomaterials, 11(8), 2024. https://doi.org/10.3390/nano11082024






