Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engineered Nanomaterials: Synthetized TiO2NPs and Commercial HNTs
2.2. Morphological and Compositional Characterization of TiO2NPs and HNTs
2.3. Fabrication of PMMA Based Samples
2.4. Color Analysis
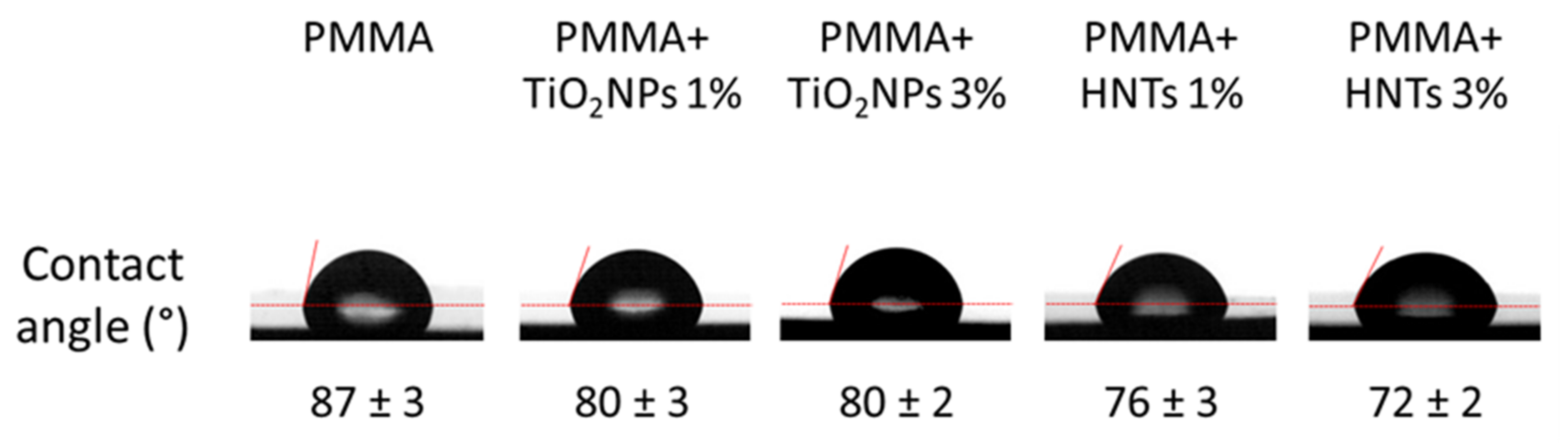
2.5. Wettability Characterization
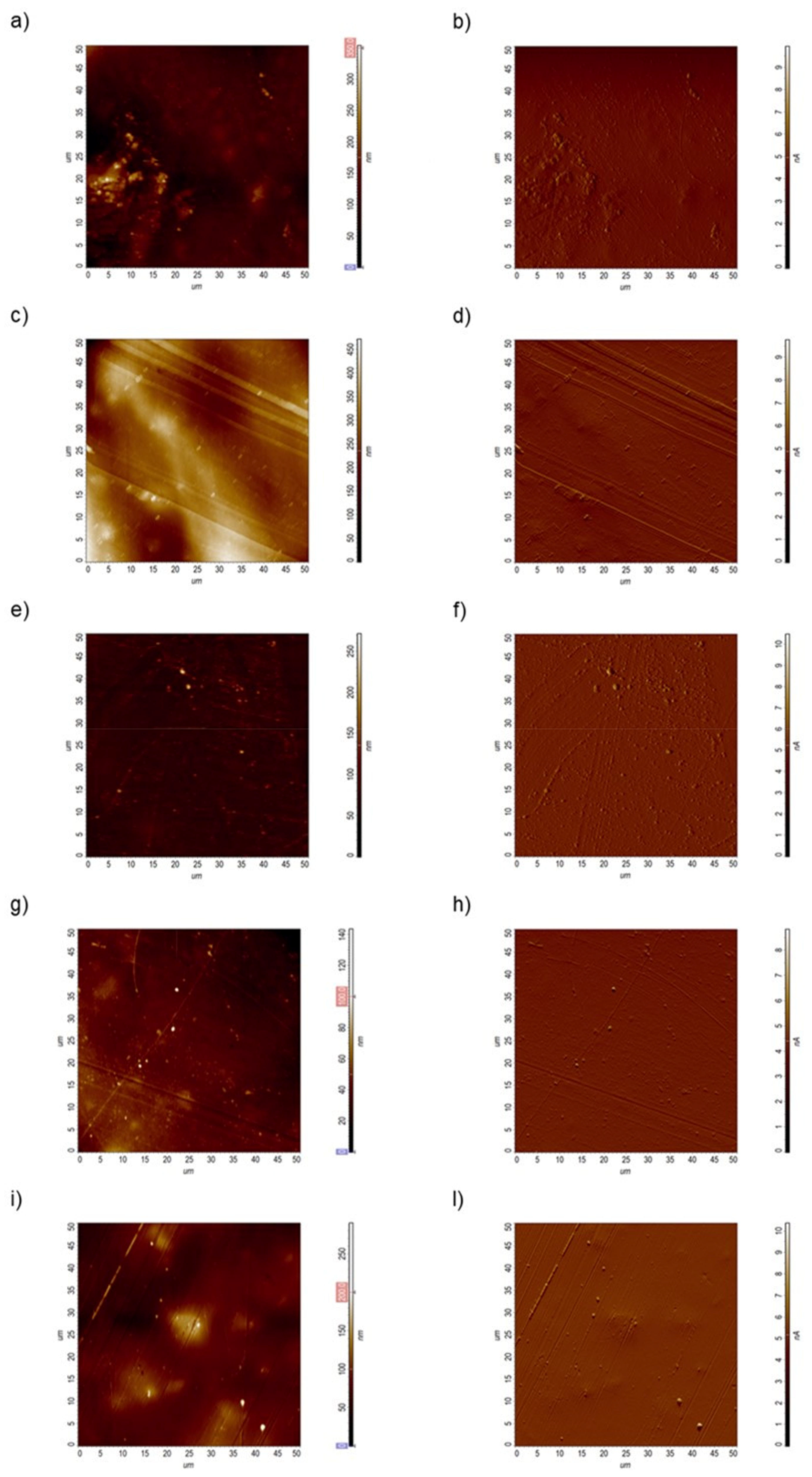
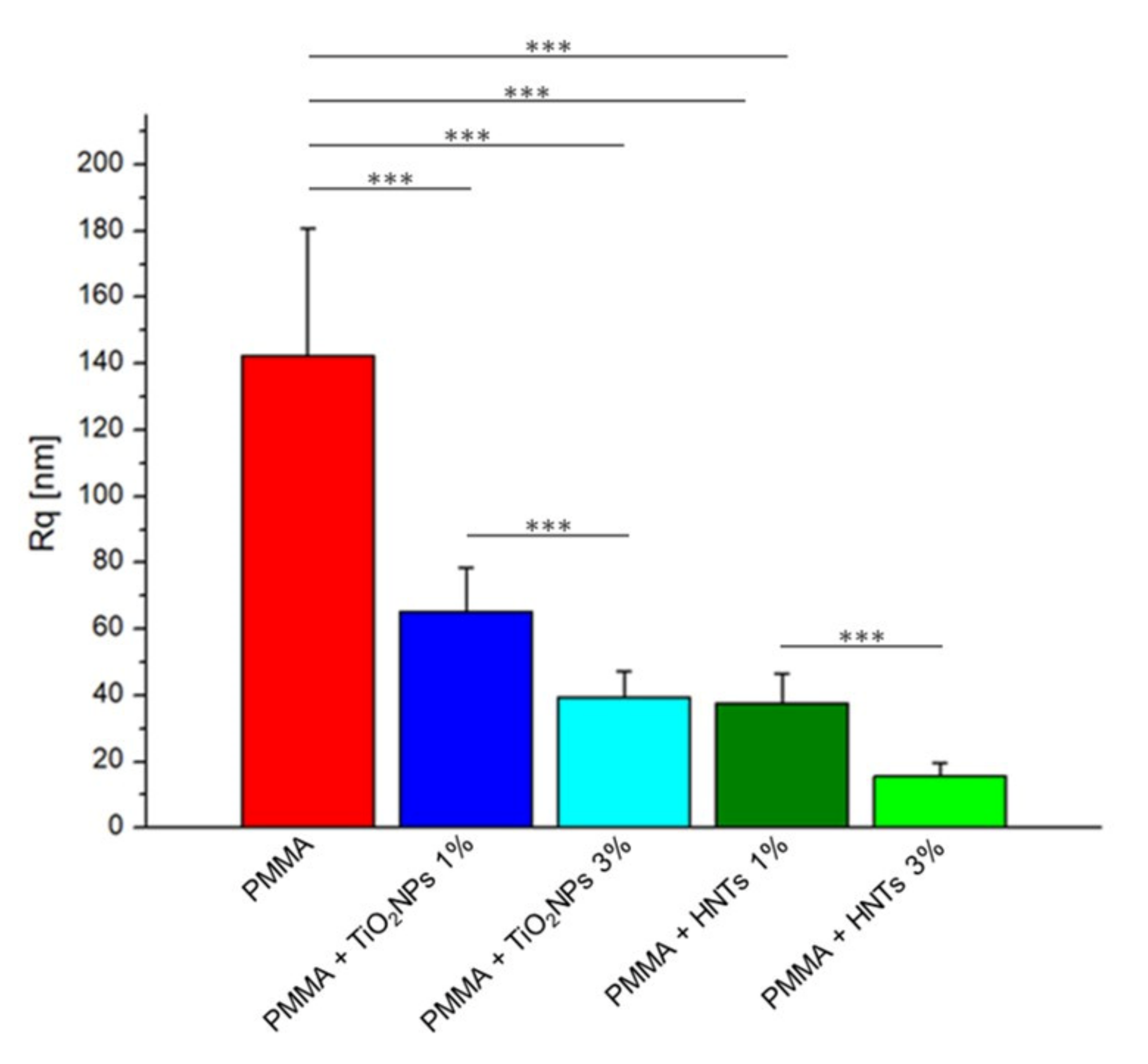
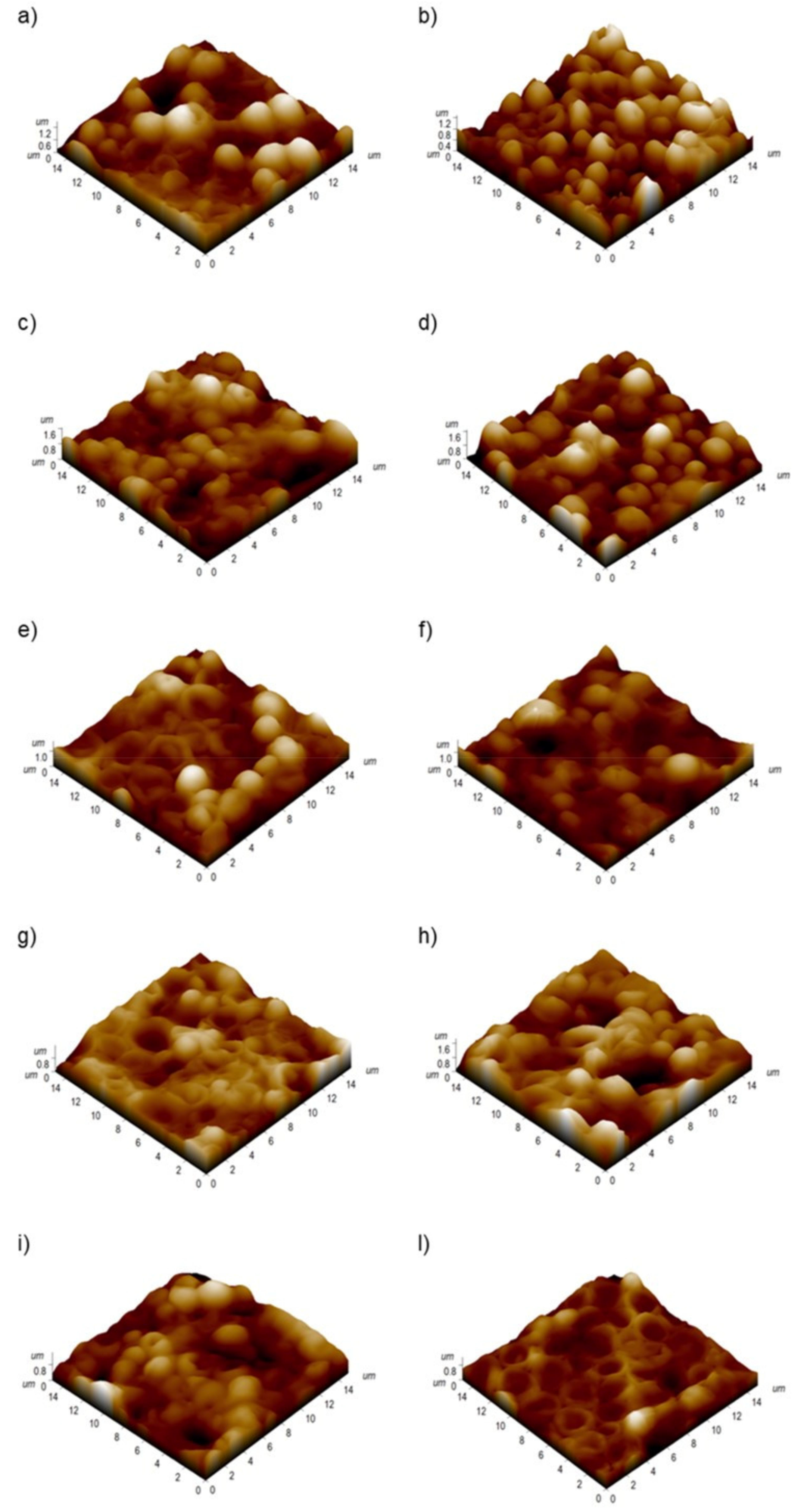
2.6. Atomic Force Microscopy (AFM) Analysis
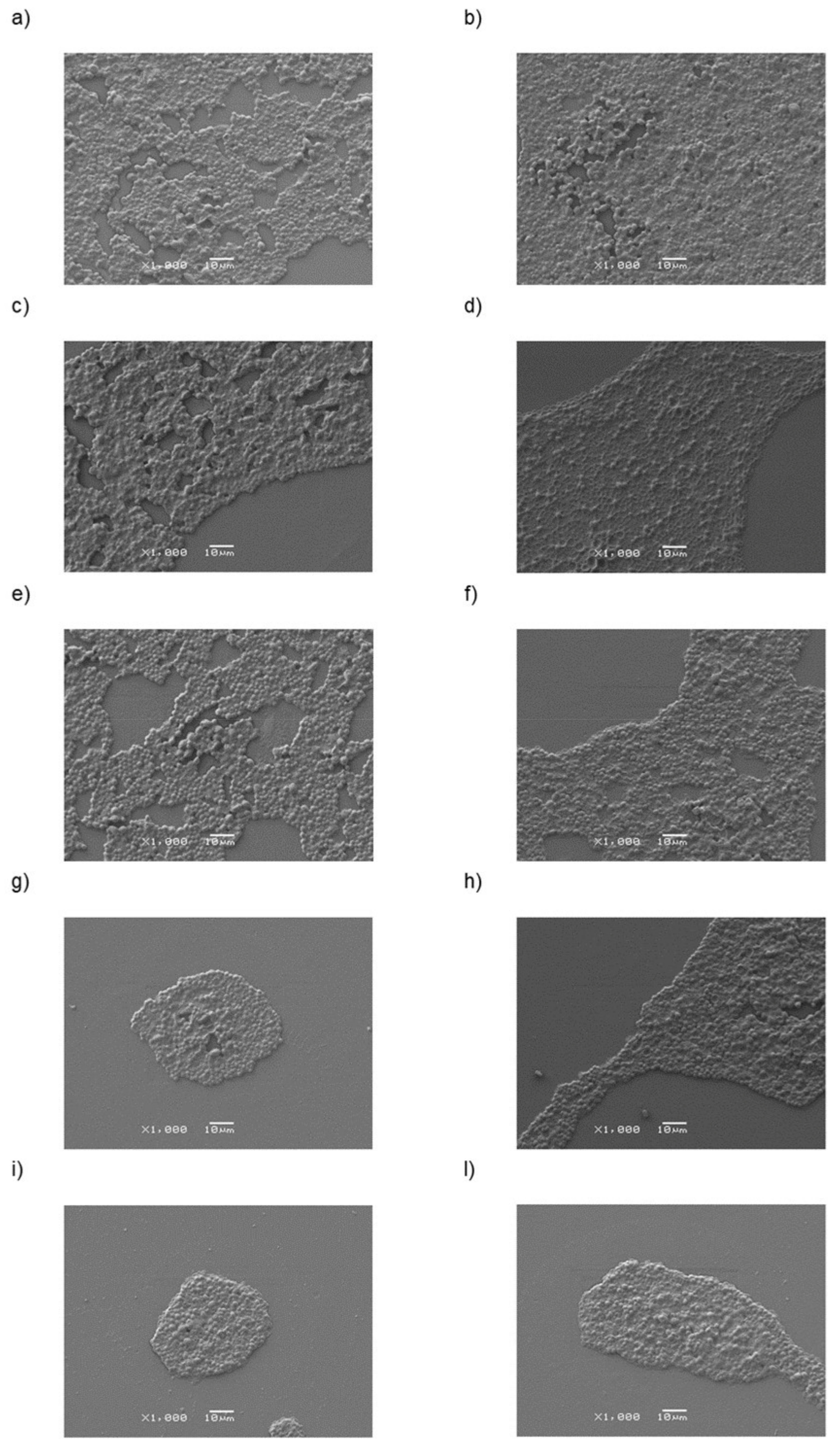
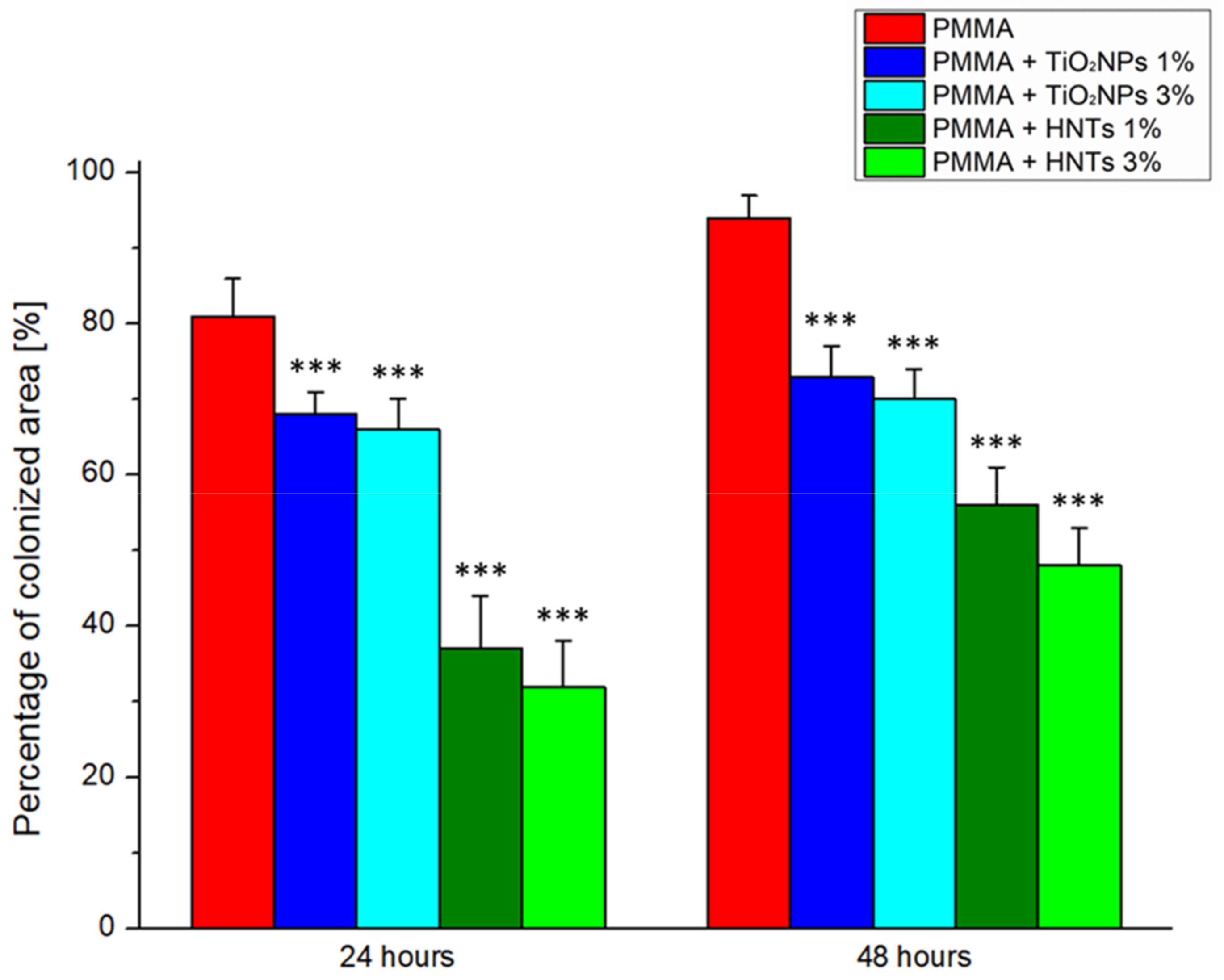
2.7. C. albicans Colonization Analyses
2.8. Statistical Analysis
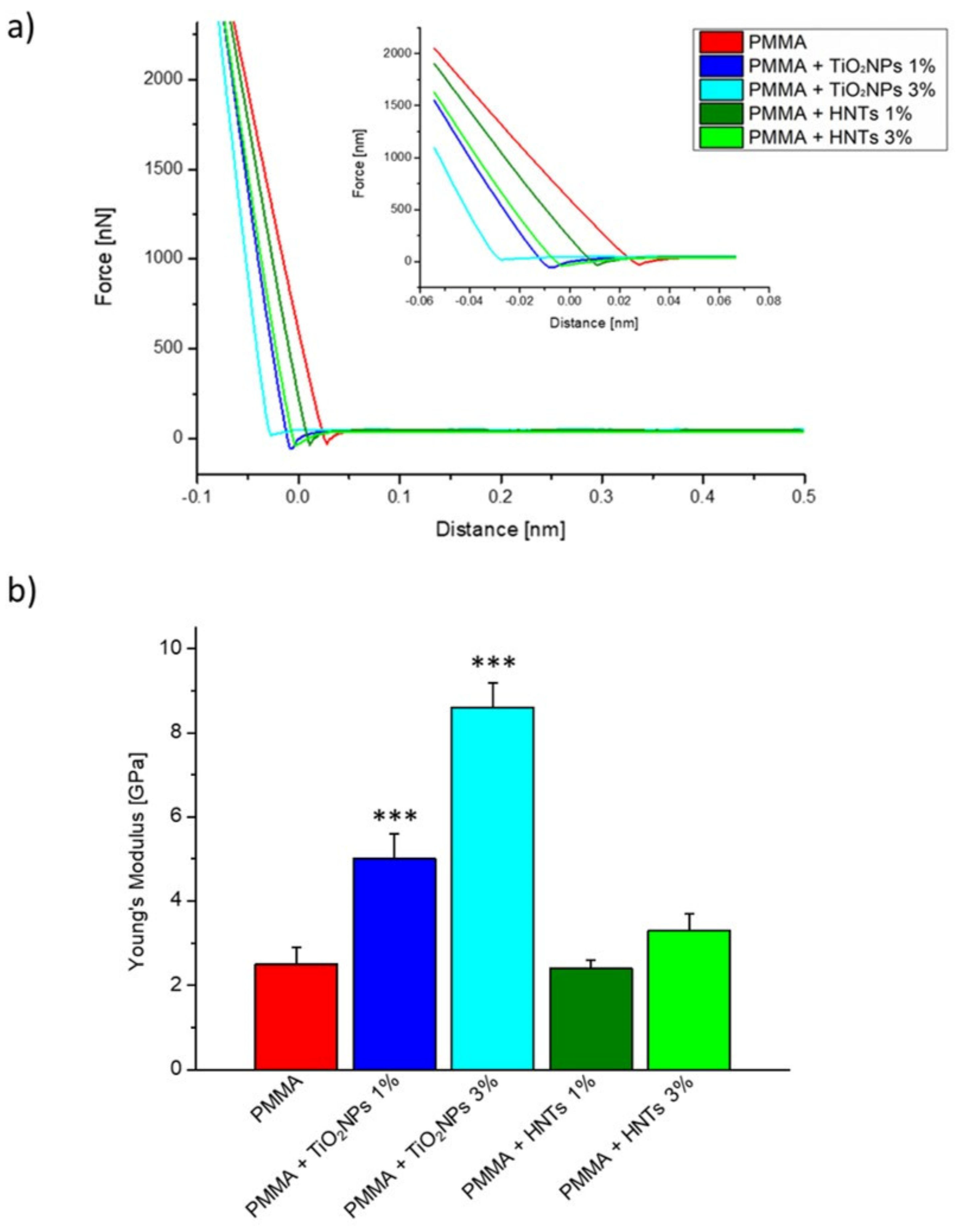
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cramer, N.B.; Stansbury, J.W.; Bowman, C. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alla, R.K.; Swamy, K.N.; Vyas, R.; Konakanchi, A. Conventional and Contemporary Polymers for the Fabrication of Denture Prosthesis: Part I-Overview, Composition and Properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Papadiochou, S.; Pissiotis, A.L. Marginal Adaptation and CAD-CAM Technology: A Systematic Review of Restorative Material and Fabrication Techniques. J. Prosthet. Dent. 2018, 119, 545–551. [Google Scholar] [CrossRef]
- Ladha, K.; Shah, D. An In-Vitro Evaluation of the Flexural Strength of Heat-Polymerized Poly (Methyl Methacrylate) Denture Resin Reinforced with Fibers. J. Indian Prosthodont. Soc. 2011, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Kasina, S.P.; Ajaz, T.; Attili, S.; Surapaneni, H.; Cherukuri, M.; Srinath, H.P. To evaluate and compare the porosities in the acrylic mandibular denture bases processed by two different polymerization techniques, using two different brands of commercially available denture base resins—An in vitro study. J. Int. Oral Health 2014, 6, 72–77. [Google Scholar]
- Sakaguchi, R.; Power, J. Craig’s Restorative Dental Materials, 13th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; ISBN 9780323081085. [Google Scholar]
- Ayaz, E.A.; Durkan, R.; Koroglu, A.; Bagis, B. Comparative Effect of Different Polymerization Techniques on Residual Monomer and Hardness Properties of PMMA-Based Denture Resins. J. Appl. Biomater. Funct. Mater. 2014, 12, 228–233. [Google Scholar] [CrossRef]
- Arenas-Arrocena, M.C.; Argueta-Figueroa, L.; García-Contreras, R.; Martínez-Arenas, O.; Camacho-Flores, B.; del Rodriguez-Torres, M.P.; de la Fuente-Hernández, J.; Acosta-Torres, L.S. New Trends for the Processing of Poly(Methyl Methacrylate) Biomaterial for Dental Prosthodontics. In Acrylic Polymers in Healthcare; InTech: London, UK, 2017. [Google Scholar]
- Moura, J.S.; Jose Da Silva, W.; Pereira, T.; Del, A.A.; Cury, B.; Cunha, R.; Rodrigues Garcia, M. Influence of Acrylic Resin Polymerization Methods and Saliva on the Adherence of Four Candida Species. J. Prosthet. Dent. 2006, 96, 205–211. [Google Scholar] [CrossRef]
- Mehendale, A. 47. Adherence of Candida Albicans on Polyamides in Comparison with Conventional Acrylic Surfaces—A Short Study. J. Indian Prosthodont. Soc. 2018, 18, 71. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; Cury, A.A.D.B.; Cenci, M.S.; Rodrigues-Garcia, R.C.M. In Vitro Candida Colonization on Acrylic Resins and Denture Liners: Influence of Surface Free Energy, Roughness, Saliva, and Adhering Bacteria. Int. J. Prosthodont. 2007, 20, 308–310. [Google Scholar] [PubMed]
- Al-Bakri, I.; Harty, D.; Al-Omari, W.; Swain, M.; Chrzanowski, W.; Ellakwa, A. Surface Characteristics and Microbial Adherence Ability of Modified Polymethylmethacrylate by Fluoridated Glass Fillers. Aust. Dent. J. 2014, 59, 482–489. [Google Scholar] [CrossRef]
- Aeran, H.; Kumar, V.; Uniyal, S.; Tanwer, P. Nanodentistry: Is just a Fiction or Future. J. Oral Biol. Craniofac. Res. 2015, 5, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asar, N.V.; Albayrak, H.; Korkmaz, T.; Turkyilmaz, I. Influence of Various Metal Oxides on Mechanical and Physical Properties of Heat-Cured Polymethyl Methacrylate Denture Base Resins. J. Adv. Prosthodont. 2013, 5, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Sabri, B.; Satgunam, M.; Abreeza, N.; Abed, A. A Review on Enhancements of PMMA Denture Base Material with Different Nano-Fillers. Cogent Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Wang, W.; Liao, S.; Zhu, Y.; Liu, M.; Zhao, Q.; Fu, Y. Recent Applications of Nanomaterials in Prosthodontics. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; de Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, M.M.; Al-Thobity, A.M.; Rahoma, A.; Abualsaud, R.; Al-Harbi, F.A.; Akhtar, S. Reinforcement of PMMA Denture Base Material with a Mixture of ZrO 2 Nanoparticles and Glass Fibers. Int. J. Dent. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA Denture Base Material Enhancement: A Review of Fiber, Filler, and Nanofiller Addition. Int. J. Nanomed. 2017, 12, 3801–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangal, U.; Kim, J.-Y.; Seo, J.-Y.; Kwon, J.-S.; Choi, S.-H. Novel Poly(Methyl Methacrylate) Containing Nanodiamond to Improve the Mechanical Properties and Fungal Resistance. Materials 2019, 12, 3438. [Google Scholar] [CrossRef] [Green Version]
- Karbushev, V.V.; Konstantinov, I.I.; Parsamyan, I.L.; Kulichikhin, V.G.; Popov, V.A.; George, T.F. Preparation of Polymer-Nanodiamond Composites with Improved Properties. Adv. Mater. Res. 2008, 59, 275–278. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Al-Thobity, A.M.; Baba, N.Z.; Al-Harbi, F.A. Influence of Addition of Different Nanoparticles on the Surface Properties of Poly(Methylmethacrylate) Denture Base Material. J. Prosthodont. 2020, 29, 422–428. [Google Scholar] [CrossRef]
- Turagam, N.; Prasad Mudrakola, D. Effect of Micro-Additions of Carbon Nanotubes to Polymethylmethacrylate on Reduction in Polymerization Shrinkage. J. Prosthodont. 2013, 22, 105–111. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Hosseini Fouladi, M.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of Carbon Nanotubes: A Review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, L.; Shirkavand, S.; Akbari, F.; Sabzikari, N. Tensile Strength and Impact Strength of Color Modified Acrylic Resin Reinforced with Titanium Dioxide Nanoparticles. J. Clin. Exp. Dent. 2017, 9, e661–e665. [Google Scholar] [CrossRef] [Green Version]
- Youssef, F.; Farghaly, U.; Abd El-Baky, R.M.; Waly, N. Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on MDR Pseudomonas aeruginosa Strains. Int. J. Nanomed. 2020, 15, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Properties Improvement of PMMA Using Nano TiO2. J. Appl. Polym. Sci. 2010, 118, 2890–2897. [Google Scholar] [CrossRef]
- Guimarães, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, Electronic, and Mechanical Properties of Single-Walled Halloysite Nanotube Models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]
- Leporatti, S.; Cascione, M.; de Matteis, V.; Rinaldi, R. Design of Nano-Clays for Drug Delivery and Bio-Imaging: Can Toxicity Be an Issue? Nanomedicine 2020, 15, 2429–2432. [Google Scholar] [CrossRef]
- Leena, M.; Srinivasan, S. Synthesis and Ultrasonic Investigations of Titanium Oxide Nanofluids. J. Mol. Liq. 2015, 206, 103–109. [Google Scholar] [CrossRef]
- Cascione, M.; de Matteis, V.; Mandriota, G.; Leporatti, S.; Rinaldi, R. Acute Cytotoxic Effects on Morphology and Mechanical Behavior in MCF-7 Induced by TiO2NPs Exposure. Int. J. Mol. Sci. 2019, 20, 3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Pellegrino, P.; Rizzello, L.; Rinaldi, R. Tailoring Cell Morphomechanical Perturbations Through Metal Oxide Nanoparticles. Nanoscale Res. Lett. 2019, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-Bond Axisymmetric Drop Shape Analysis for Surface Tension and Contact Angle Measurements of Sessile Drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Chini, S.F.; Amirfazli, A. A Method for Measuring Contact Angle of Asymmetric and Symmetric Drops. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 29–37. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force Measurements with the Atomic Force Microscope: Technique, Interpretation and Applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Cierech, M.; Szerszeń, M.; Wojnarowicz, J.; Łojkowski, W.; Kostrzewa-Janicka, J.; Mierzwińska-Nastalska, E. Preparation and Characterisation of Poly(methyl metacrylate)-Titanium Dioxide Nanocomposites for Denture Bases. Polymers 2020, 12, 2655. [Google Scholar] [CrossRef]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 Nanoparticles on Tensile Strength of Dental Acrylic Resins. Dent. Clin. Dent. Prospects 2014, 8, 197–203. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Qin, X.X.; Liu, Z.; Qin, D.Z. Electrospinning Preparation and Mechanical Properties of PVA/HNTs Composite Nanofibers. Polym. Adv. Technol. 2017, 28, 768–774. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Abdullayev, E.; Hollister, A.; Mills, D.; Lvov, Y.M. Clay Nanotube/Poly(Methyl Methacrylate) Bone Cement Composites with Sustained Antibiotic Release. Macromol. Mater. Eng. 2012, 297, 645–653. [Google Scholar] [CrossRef]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A. Cell Biology of Candida Albicans–Host Interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Altarawneh, S.; Bencharit, S.; Mendoza, L.; Curran, A.; Barrow, D.; Barros, S.; Preisser, J.; Loewy, Z.G.; Gendreau, L.; Offenbacher, S. Clinical and Histological Findings of Denture Stomatitis as Related to Intraoral Colonization Patterns of Candida Albicans, Salivary Flow, and Dry Mouth. J. Prosthodont. 2013, 22, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshijima, Y.; Murakami, K.; Kayama, S.; Liu, D.; Hirota, K.; Ichikawa, T.; Miyake, Y. Effect of Substrate Surface Hydrophobicity on the Adherence of Yeast and Hyphal Candida. Mycoses 2010, 53, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Chaffin, W.L. Oral Colonization by Candida Albicans. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Morphomechanical and Organelle Perturbation Induced by Silver Nanoparticle Exposure. J. Nanopart. Res. 2018, 20, 273. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; de Jong, W.H. A Review on Mammalian Toxicity of ZnO Nanoparticles. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of Copper Oxide Nanoparticles: A Review Study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascione, M.; De Matteis, V.; Pellegrino, P.; Albanese, G.; De Giorgi, M.L.; Paladini, F.; Corsalini, M.; Rinaldi, R. Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes. Nanomaterials 2021, 11, 2027. https://doi.org/10.3390/nano11082027
Cascione M, De Matteis V, Pellegrino P, Albanese G, De Giorgi ML, Paladini F, Corsalini M, Rinaldi R. Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes. Nanomaterials. 2021; 11(8):2027. https://doi.org/10.3390/nano11082027
Chicago/Turabian StyleCascione, Mariafrancesca, Valeria De Matteis, Paolo Pellegrino, Giovanni Albanese, Maria Luisa De Giorgi, Fabio Paladini, Massimo Corsalini, and Rosaria Rinaldi. 2021. "Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes" Nanomaterials 11, no. 8: 2027. https://doi.org/10.3390/nano11082027








