Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.2.1. Preparation of MWCNTs Suspensions
2.2.2. Preparation of Heavy Metal Ions Solutions
2.2.3. Adsorption Tests
3. Results and Discussion
3.1. MWCNTs Suspensions
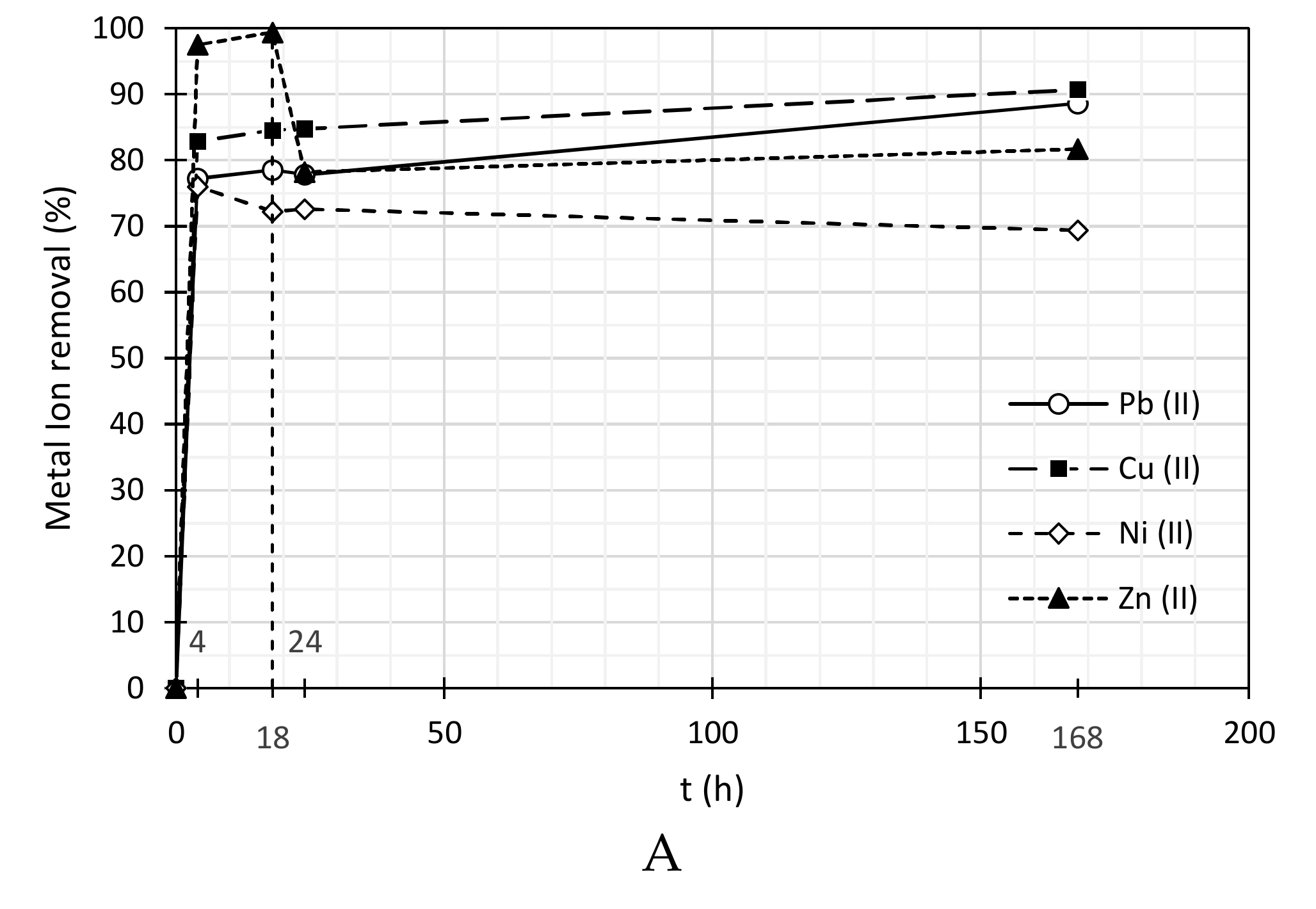
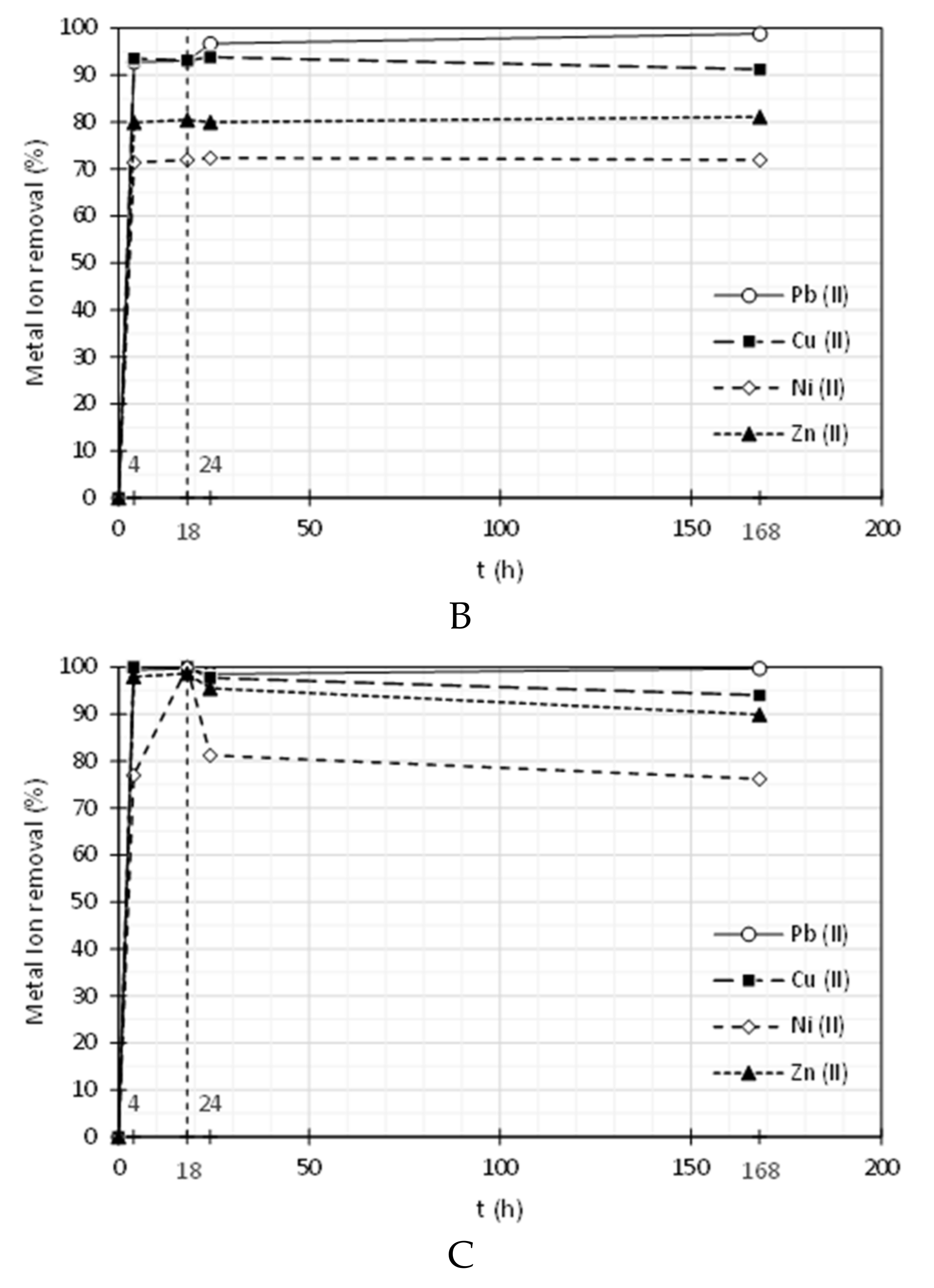
3.2. Heavy Metal Ions Removal
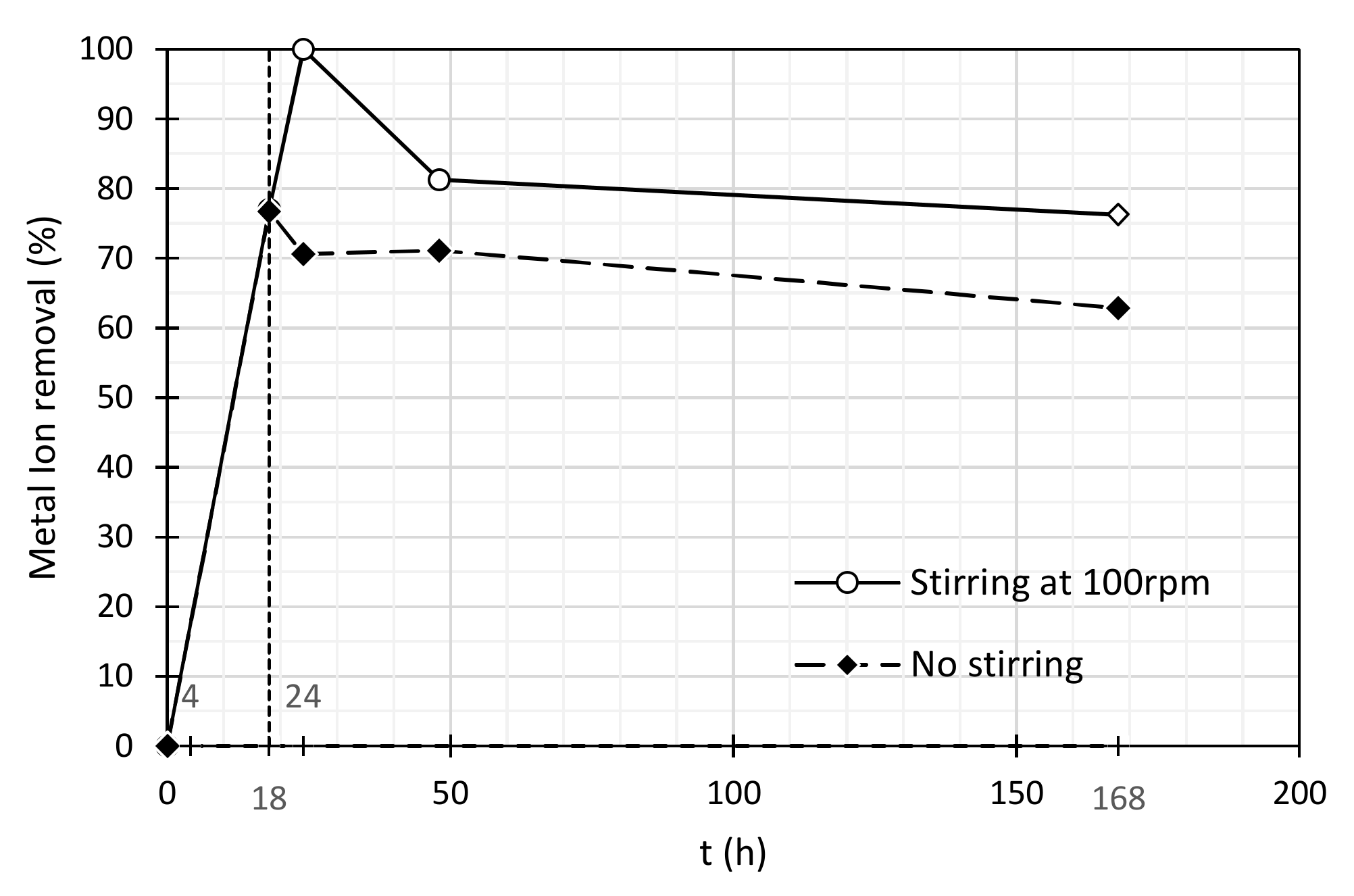
3.3. Competitive Adsorption Tests
3.4. Adsorption Tests Using a Mixture of SDBS and Pluronic F-127
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanyal, S. The End of Population Growth. Project Sindicate. 2011. Available online: https://www.project-syndicate.org/commentary/the-end-of-population-growth?barrier=true (accessed on 3 March 2016).
- United Nations. Water for a Sustainable World—The United Nations World Water Development Report 2015; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2015. [Google Scholar]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.; Horta, M.A.; Cunha, C.L. Assessment of heavy metal concentrations in sediment, water and organs of Nycticorax nycticorax (Black-crowned Night Heron) in Sepetiba Bay, Rio de Janeiro, Brazil. J. Integr. Coast. Zone Manag. 2010, 10, 229–241. [Google Scholar]
- Pryiantha, N.D.; Tennakoon, T.B.; Keethiratne, S.; Abeysinghe, T. Removal of heavy metal ions from polluted water using environmental-friendly materials. In Proceedings of the National Water Conference on Status and Future Directions of Water Research in Sri Lanka, International Irrigation Management Institute (IIMI), Colombo, Sri Lanka, 4–6 November 1998; p. 106. [Google Scholar]
- Shen, L.-C.; Nguyen, X.-T.; Hankins, N.P. Removal of heavy metal ions from dilute aqueous solutions by polymer–surfactant aggregates: A novel effluent treatment process. Sep. Purif. Technol. 2015, 152, 101–107. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Snyder, S.A. Wastewater Treatment and Reuse: Past, Present, and Future. Water 2015, 7, 4887–4895. [Google Scholar] [CrossRef] [Green Version]
- Gul, H.; Nasreen, S. Heavy metal uptake from contaminated water using carbon nanotubes: A Review. Environ. Contam. Rev. 2018, 1, 04–08. [Google Scholar] [CrossRef]
- Curiel-Esparza, J.; Cuenca-Ruiz, M.A.; Martin-Utrillas, M.; Canto-Perello, J. Selecting a Sustainable Disinfection Technique for Wastewater Reuse Projects. Water 2014, 6, 2732–2747. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Treatment in Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; pp. 166–183. [Google Scholar]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations, 3rd ed.; Taylor & Francis Group: Milton Park, UK, 2014. [Google Scholar]
- LeChevallier, M.; Au, K. Water Treatment and Pathogen Control World Health, 1st ed.; IWA Publishing: London, UK, 2004. [Google Scholar]
- Chiban, M.; Zerbet, M.; Carja, G.; Sinan, F. Application of low-cost adsorbents for arsenic removal: A review. J. Environ. Chem. Ecotoxicity 2012, 4, 91–102. [Google Scholar]
- Fu, F.; Wang, K. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 212, 317–331. [Google Scholar] [CrossRef]
- Medjo, R.E. Characterization of Carbon Nanotubes. Physical and Chemical Properties of Carbon Nanotubes; Suzuki, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 161–183. [Google Scholar]
- Upadhyayula, V.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total. Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Synder, J.W.; Mains, C.N.; E Anderson, R.; Bissonnette, G.K. Effect of point-of-use, activated carbon filters on the bacteriological quality of rural groundwater supplies. Appl. Environ. Microbiol. 1995, 61, 4291–4295. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials 2020, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.W.; Shao, D.D.; Dong, Y.H.; Li, J.X.; Wang, X.K. Adsorption and Reduction of Chromium(VI) from Aqueous Solution by Multiwalled Carbon Nanotubes. Open Environ. Pollut. Toxicol. J. 2009, 1, 66–73. [Google Scholar] [CrossRef]
- Li, Y.-H.; Luan, Z.; Xiao, X.; Zhou, X.; Xu, C.; Wu, D.; Wei, B. Removal of Cu2+ Ions from Aqueous Solutions by Carbon Nanotubes. Adsorpt. Sci. Technol. 2003, 21, 475–485. [Google Scholar] [CrossRef]
- Lu, C.; Liu, C. Removal of nickel(II) from aqueous solution by carbon nanotubes. J. Chem. Technol. Biotechnol. 2006, 81, 1932–1940. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Chen, C.; Yu, S.; Wang, X. Counterion effects of nickel and sodium dodecylbenzene sulfonate adsorption to multiwalled carbon nanotubes in aqueous solution. Carbon 2008, 46, 1741–1750. [Google Scholar] [CrossRef]
- A Kabbashi, N.; Atieh, M.; Mamun, A.; Mirghami, M.E.; Alam, M.; Yahya, N. Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J. Environ. Sci. 2009, 21, 539–544. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H.; Liu, C. Removal of Zinc (II) from Aqueous Solution by Purified Carbon Nanotubes: Kinetics and Equilibrium Studies. Ind. Eng. Chem. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Gadhave, A.; Waghmare, J. Removal of Heavy Metal Ions from Wastewater by Carbon Nanotubes (CNTs). Int. J. Chem. Sci. Appl. 2014, 5, 56–67. [Google Scholar]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Removal of Heavy Metals from Wastewater Using Carbon Nanotubes. Sep. Purif. Rev. 2014, 43, 311–338. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process. Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.; AlOmar, M.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Abolhasani, M.M.; Naebe, M. Sustainable periodically patterned carbon nanotube for environmental application: Introducing the cheetah skin structure. J. Clean. Prod. 2018, 179, 429–440. [Google Scholar] [CrossRef]
- Liné, C.; Manent, F.; Wolinski, A.; Flahaut, E.; Larue, C. Comparative study of response of four crop species exposed to carbon nanotube contamination in soil. Chemosphere 2021, 274, 129854. [Google Scholar] [CrossRef] [PubMed]
- Ncibi, M.C.; Gaspard, S.; Sillanpää, M. As-synthesized multi-walled carbon nanotubes for the removal of ionic and non-ionic surfactants. J. Hazard. Mater. 2015, 286, 195–203. [Google Scholar] [CrossRef]
- Moradi, O.; Zare, K.; Yari, M. Interaction of some heavy metal ions with single walled carbon nanotube. Environ. Prot. 2011, 1, 203–220. [Google Scholar]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of Carbon Nanotubes: Mixing, Sonication, Stabilization, and Composite Properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Kun, Y.; Zili, Y.I.; Qingfeng, J.; Renliang, Y.U.E.; Wei, J.; Daohui, L.I.N. Sonication-assisted dispersion of carbon nanotubes in aqueous solutions of the anionic surfactant SDBS: The role of sonication energy. Chin. Sci. Bull. 2013, 58, 2082–2090. [Google Scholar]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Zawisza, B.; Malicka, E. Modification of carbon nanotubes for preconcentration, separation and determination of trace-metal ions. TrAC Trends Anal. Chem. 2012, 37, 22–31. [Google Scholar] [CrossRef]
- Grossiord, N.; Yu, J.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar]
- Datsyuk, V.; Landois, P.; Fitremann, J.; Peigney, A.; Galibert, A.M.; Soula, B.; Flahaut, E. Double-walled carbon nanotube dispersion via surfactant substitution. J. Mater. Chem. 2009, 19, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M. Soils Contamination by Heavy Metals. Master’s Thesis, Universidade Lusófona de Humanidades e Tecnologias, Lisbon, Portugal, 2013. [Google Scholar]
- Machado, I.; Fernandes, G. Relatório Anual dos Serviços de Águas e Resíduos em Portugal Volume 2—Controlo da Qualidade da Água para Consumo Humano; ERSAR: Lisbon, Portugal, 2015. [Google Scholar]
- Correia, A.A.S.; Casaleiro, P.D.; Rasteiro, M.G.B. Applying Multiwall Carbon Nanotubes for Soil Stabilization. Procedia Eng. 2015, 102, 1766–1775. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, D.T.; Correia, A.A.S.; Hunkeler, D.; Rasteiro, M.G. Surfactants for dispersion of carbon nanotubes applied in soil stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Costa, P. Chemical Stabilization of Soils Using Carbon Nanotubes. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2016. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Abdulkareem, A.S.; Hamzat, W.A.; Tijani, J.O.; Bankole, M.T.; Egbosiuba, T.; Abubakre, O.K.; Mustapha, S. Comparative adsorptive removal of selected heavy metals from battery wastewater by purified and polyethylene glycol modified carbon nanotubes. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Aslam, M.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
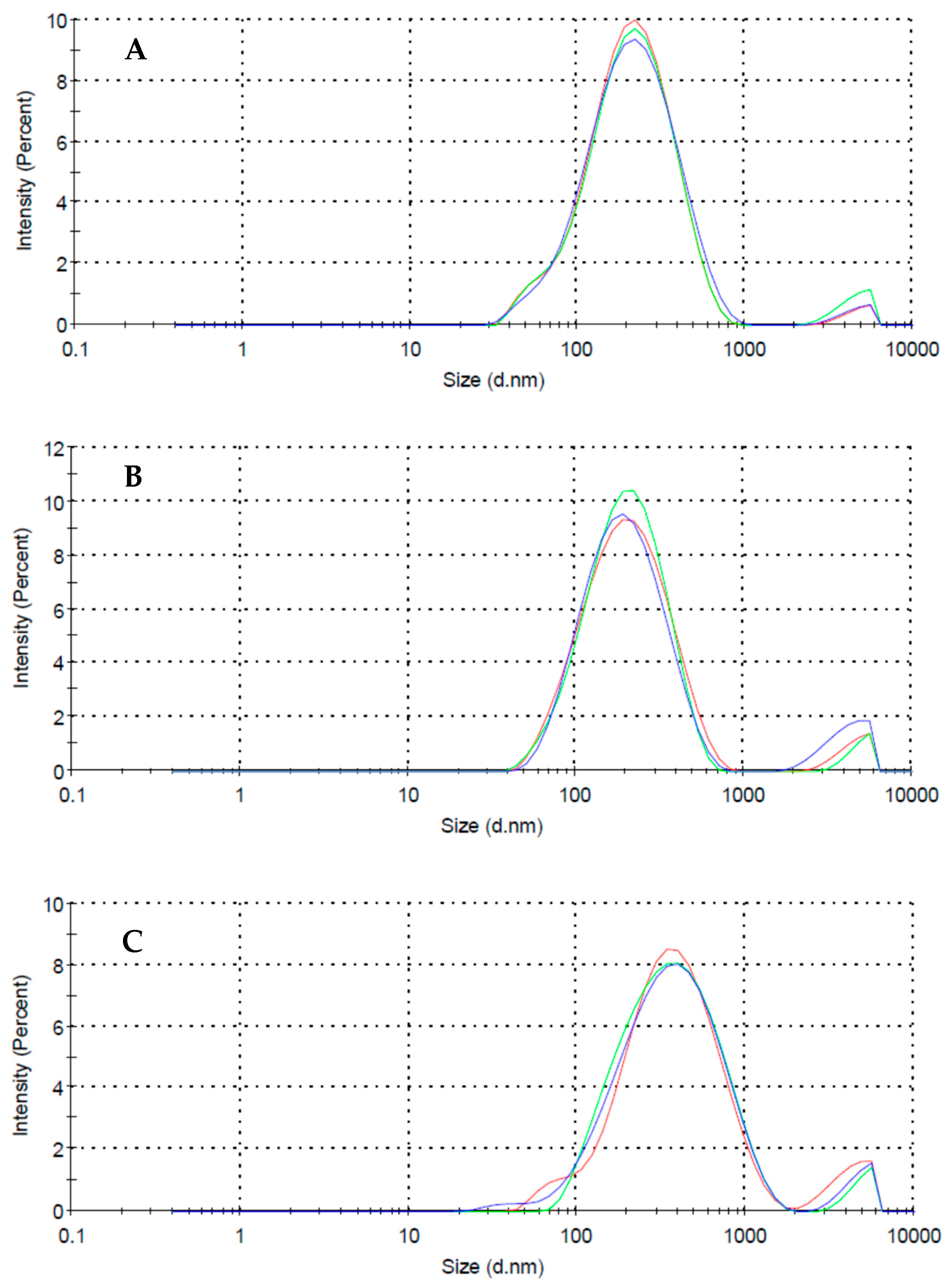
| Surfactant | Charge (−) | Chain Type | Dzav (nm) | Zav (mV) | MWav (kDa) |
|---|---|---|---|---|---|
| SDBS | Anionic | Linear | 81.02 | −66.97 | 363 |
| Pluronic F-127 | Non-ionic | Linear | 6.92 | −0.43 | 9.49 |
| PolyDADMAC MMW | Cationic | Linear | 48.58 | 69.47 | 240 |
| Surfactant | Surfactant Concentration (w/w %) | MWCNTs Concentration (w/w %) | Dzav (nm) |
|---|---|---|---|
| No-surfactant | - | 536.4 ± 4.5 | |
| SDBS | 0.03 | 0.01 | 180.5 ± 2.3 |
| Pluronic F-127 | 0.03 | 187.3 ± 3.8 | |
| PolyDADMAC MMW | 0.03 | 307.7 ± 6.3 | |
| 0.1 | 363.0 ± 23.1 |
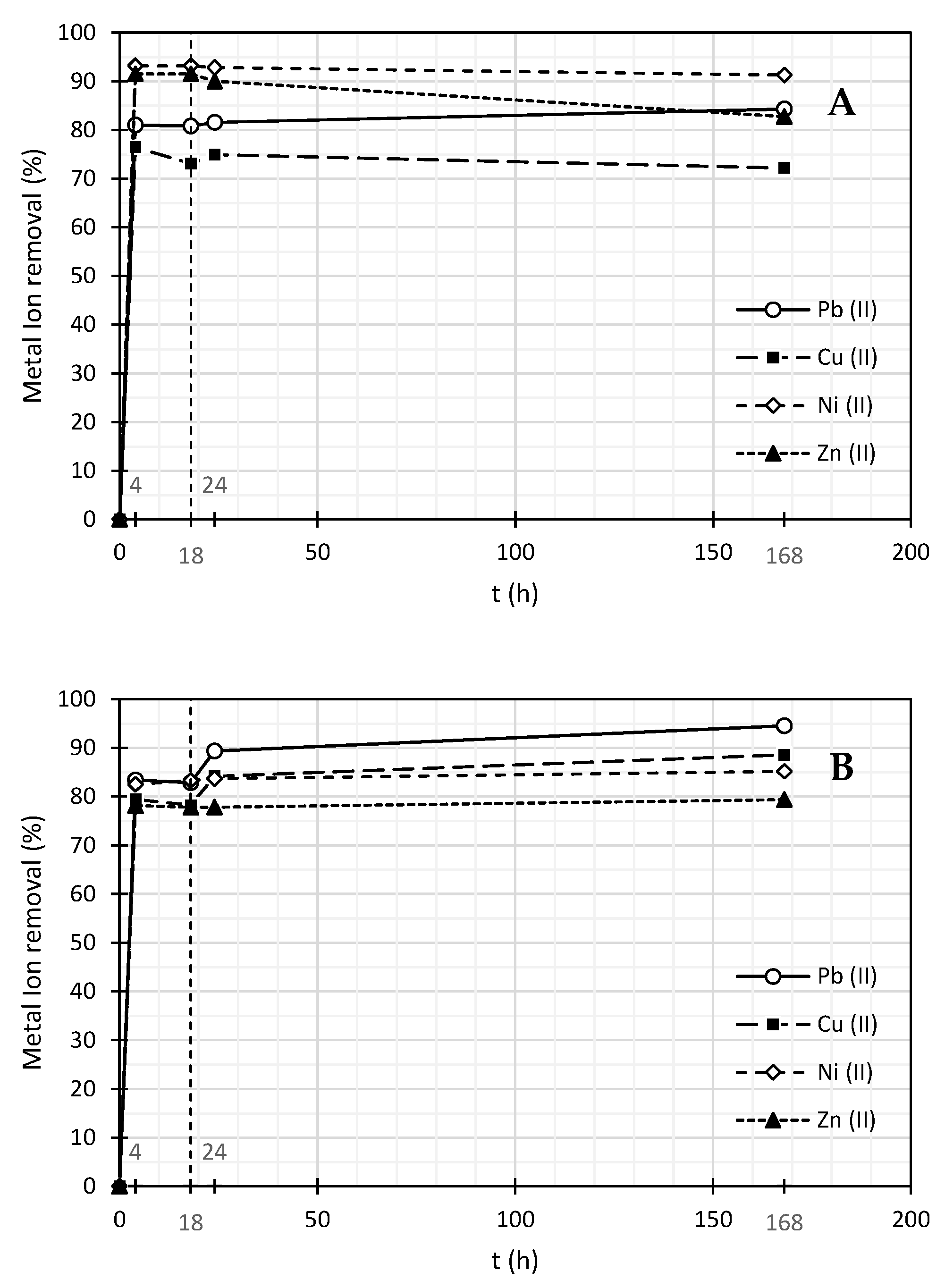
| SDBS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pb (II) | Cu (II) | Ni (II) | Zn (II) | |||||
| Time (h) | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal |
| 0 | 2.98 | 0.0 | 2.38 | 0.0 | 2.64 | 0.0 | 3.43 | 0.0 |
| 4 | 0.68 | 77.2 | 0.41 | 82.8 | 0.63 | 76.0 | 0.09 | 97.5 |
| 18 | 0.64 | 78.5 | 0.37 | 84.6 | 0.73 | 72.2 | 0.02 | 99.4 |
| 24 | 0.66 | 77.9 | 0.36 | 84.8 | 0.72 | 72.6 | 0.75 | 78.2 |
| 168 | 0.34 | 88.6 | 0.22 | 90.7 | 0.81 | 69.4 | 0.63 | 81.7 |
| Pluronic F-127 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pb (II) | Cu (II) | Ni (II) | Zn (II) | |||||
| Time (h) | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal |
| 0 | 3.33 | 0.00 | 5.41 | 0.00 | 2.64 | 0.00 | 3.43 | 0.00 |
| 4 | 0.02 | 99.4 | 0.00 | 100 | 0.61 | 77.0 | 0.07 | 98.0 |
| 18 | 0.01 | 99.7 | 0.00 | 100 | 0.00 | 100 | 0.05 | 98.6 |
| 24 | 0.05 | 98.5 | 0.12 | 97.8 | 0.50 | 81.2 | 0.15 | 95.5 |
| 168 | 0.01 | 99.7 | 0.32 | 94.0 | 0.63 | 76.2 | 0.35 | 89.9 |
| PolyDADMAC MMW | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pb (II) | Cu (II) | Ni (II) | Zn (II) | |||||
| Time (h) | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal | Concentration (mg/L) | % of Removal |
| 0 | 3.33 | 0.0 | 5.41 | 0.0 | 3.74 | 0.0 | 3.8 | 0.0 |
| 4 | 0.24 | 92.8 | 0.35 | 93.6 | 1.07 | 71.4 | 0.76 | 79.9 |
| 18 | 0.23 | 93.1 | 0.37 | 93.2 | 1.05 | 71.9 | 0.74 | 80.5 |
| 24 | 0.11 | 96.7 | 0.33 | 93.9 | 1.03 | 72.4 | 0.76 | 80.0 |
| 168 | 0.04 | 98.8 | 0.47 | 91.3 | 1.05 | 72.0 | 0.72 | 81.1 |
| Stirring at 100 rpm | No Stirring | |||
|---|---|---|---|---|
| Time (h) | Ni(II) Concentration (mg/L) | Ni (II) Removal (%) | Ni(II) Concentration (mg/L) | Ni (II) Removal (%) |
| 0 | 2.64 | 0.0 | 2.64 | 0.0 |
| 4 | 0.61 | 77.0 | 0.62 | 76.7 |
| 18 | 0.0 | 100 | 0.78 | 70.6 |
| 24 | 0.50 | 81.2 | 0.76 | 71.1 |
| 168 | 0.63 | 76.2 | 0.98 | 62.9 |
| Metal | Removal % | Reference |
|---|---|---|
| Ni (II) | 35 76.12 92.79 100 90 | Oxidized CNTs [24] Purified CNTs (HNO3/H2SO4, 1:3) [48] PEG modified CNTs [48] Present work—maximum removal obtained Chitosan modified CNTs [49] |
| Pb (II) | 90.7 97.16 99.7 | Purified CNTs (HNO3/H2SO4, 1:3) [48] PEG modified CNTs [48] Present work—maximum removal obtained |
| Cu (II) | 99.09 99.99 95 100 | Purified CNTs (HNO3/H2SO4, 1:3) [48] PEG modified CNTs [48] Chitosan modified CNTs [49] Present work—maximum removal obtained |
| Zn (II) | 95.4 90.3 98.6 | Oxidized CNTs, pH 5.4 [23] NaOCl purified CNTs [27] Present work—maximum removal obtained |
| SDBS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Pb (mg/L) | Cu (mg/L) | Ni (mg/L) | Zn (mg/L) | % Removal | |||
| Pb (%) | Cu (%) | Ni (%) | Zn (%) | |||||
| 0 | 3.69 | 6.58 | 2.64 | 3.30 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0.70 | 1.55 | 0.18 | 0.28 | 81.0 | 76.4 | 93.2 | 91.5 |
| 18 | 0.71 | 1.77 | 0.18 | 0.28 | 80.8 | 73.1 | 93.2 | 91.5 |
| 24 | 0.68 | 1.65 | 0.19 | 0.33 | 81.6 | 74.9 | 92.8 | 90.0 |
| 168 | 0.58 | 1.83 | 0.23 | 0.57 | 84.3 | 72.2 | 91.3 | 82.7 |
| Pluronic F-127 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Pb (mg/L) | Cu (mg/L) | Ni (mg/L) | Zn (mg/L) | % Removal | |||
| Pb (%) | Cu (%) | Ni (%) | Zn (%) | |||||
| 0 | 3.69 | 6.58 | 2.64 | 3.30 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0.61 | 1.35 | 0.46 | 0.72 | 83.5 | 79.5 | 82.6 | 78.2 |
| 18 | 0.63 | 1.43 | 0.44 | 0.73 | 82.9 | 78.3 | 83.3 | 77.9 |
| 24 | 0.39 | 1.04 | 0.43 | 0.73 | 89.4 | 84.2 | 83.7 | 77.9 |
| 168 | 0.20 | 0.75 | 0.39 | 0.68 | 94.6 | 88.6 | 85.2 | 79.4 |
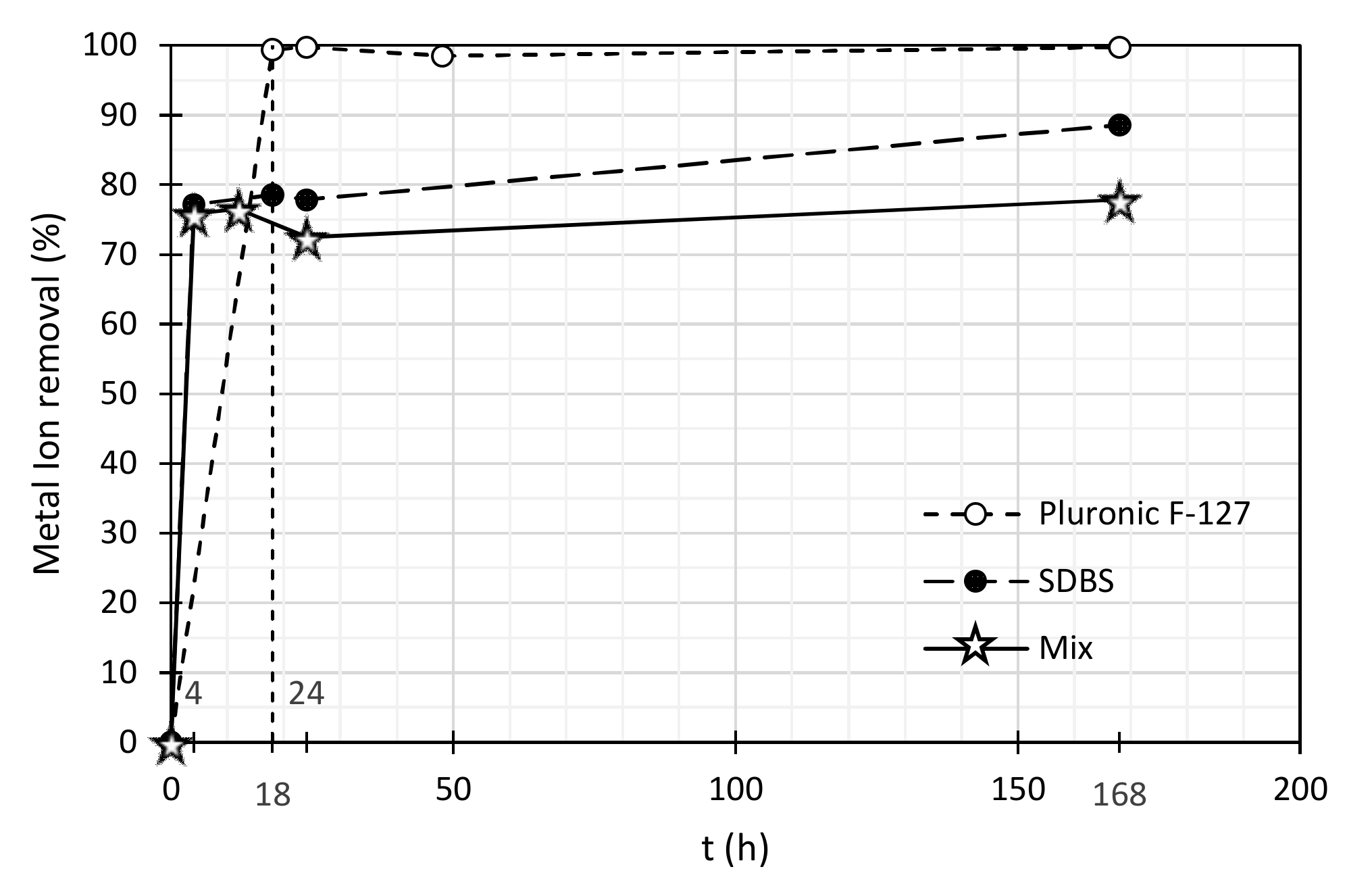
| Pb (II) Concentration (mg/L) | Pb (II) Removal (%) | |||||
|---|---|---|---|---|---|---|
| Time (h) | Mixture of Surfactants | SDBS | Pluronic F-127 | Mixture of Surfactants | SDBS | Pluronic F-127 |
| 0 | 2.98 | 2.98 | 3.33 | 0.0 | 0.0 | 0.00 |
| 4 | 0.72 | 0.68 | 0.02 | 75.8 | 77.2 | 99.4 |
| 18 | 0.70 | 0.64 | 0.01 | 76.5 | 78.5 | 99.7 |
| 24 | 0.82 | 0.66 | 0.05 | 72.5 | 77.9 | 98.5 |
| 168 | 0.66 | 0.34 | 0.01 | 77.9 | 88.6 | 99.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.R.; Correia, A.A.; Rasteiro, M.G. Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion. Nanomaterials 2021, 11, 2082. https://doi.org/10.3390/nano11082082
Oliveira AR, Correia AA, Rasteiro MG. Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion. Nanomaterials. 2021; 11(8):2082. https://doi.org/10.3390/nano11082082
Chicago/Turabian StyleOliveira, Ana Rita, António Alberto Correia, and Maria Graça Rasteiro. 2021. "Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion" Nanomaterials 11, no. 8: 2082. https://doi.org/10.3390/nano11082082
APA StyleOliveira, A. R., Correia, A. A., & Rasteiro, M. G. (2021). Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion. Nanomaterials, 11(8), 2082. https://doi.org/10.3390/nano11082082








