On-Surface Synthesis of Ligands to Elaborate Coordination Polymers on an Au(111) Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. STM Experiments
3. Results
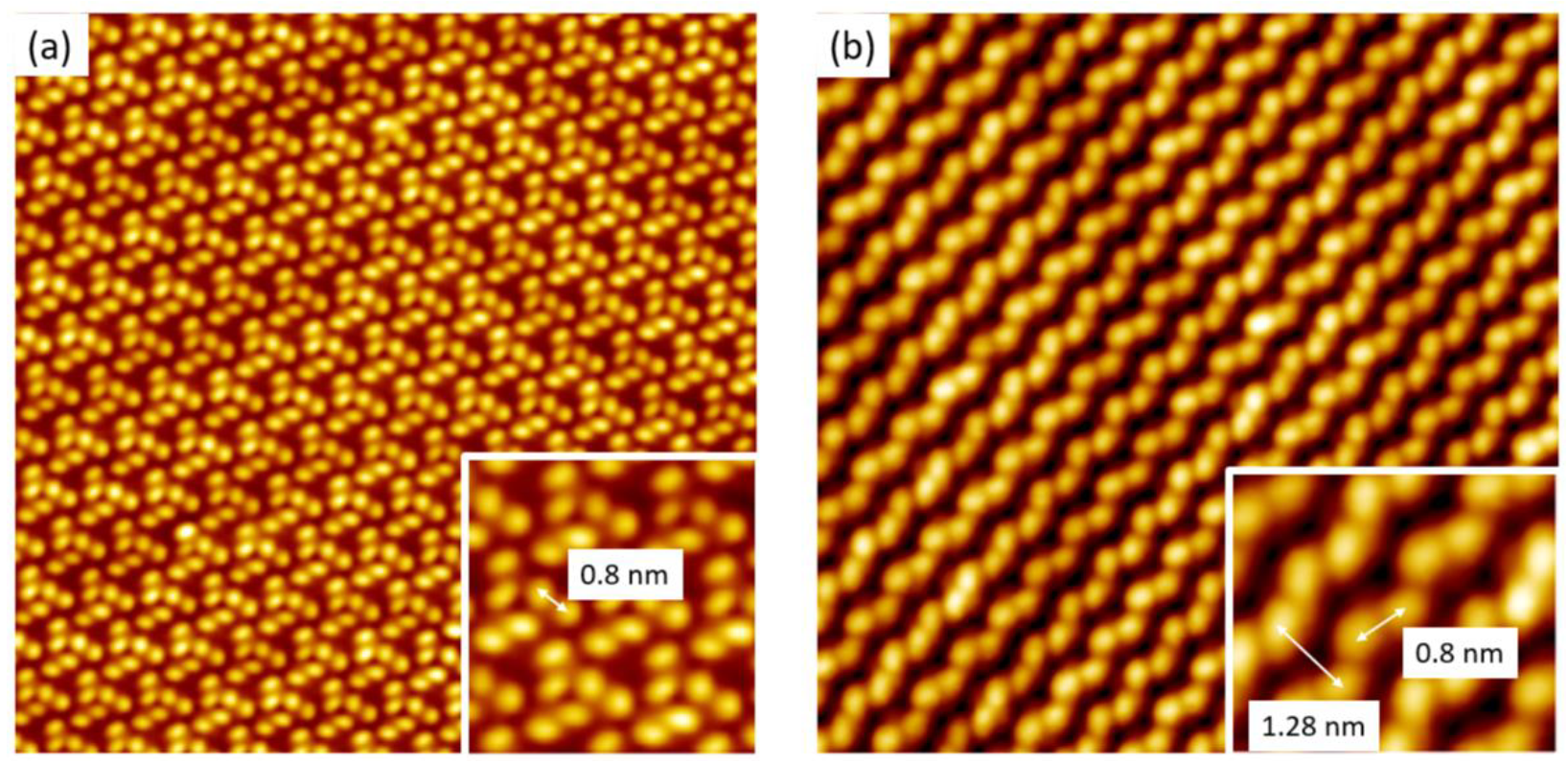
3.1. Supramolecular Self-Assembly on an Au(111) Surface
3.2. Annealing of the Supramolecular Self-Assemblies on an Au(111) Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xing, L.; Peng, Z.; Li, W.; Wu, K. On Controllability and Applicability of Surface Molecular Self-Assemblies. Acc. Chem. Res. 2019, 52, 1048–1058. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering Atomic and Molecular Nanostructures at Surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kudernac, T.; Lei, S.; Elemans, J.A.; De Feyter, S. Two-Dimensional Supramolecular Self-assembly: Nano-Porous Networks on surfaces. Chem. Soc. Rev. 2009, 38, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Vargas, L.; Kim, E.; Attias, A.-J. Beyond “Decorative” 2D Supramolecular Self-Assembly: Strategies Towards Functional Surfaces for Nanotechnology. Mater. Horiz. 2017, 4, 570–583. [Google Scholar] [CrossRef]
- Makoudi, Y.; Jeannoutot, J.; Palmino, F.; Chérioux, F.; Copie, G.; Krzeminski, C.; Cléri, F.; Grandidier, B. Supramolecular self-assembly on the B-Si(111)-(√3x√3)R30°surface: From Single Molecules to Multicomponent Networks. Surf. Sci. Rep. 2017, 72, 316–349. [Google Scholar] [CrossRef]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.V.; Hecht, S. Nano-Architectures by Covalent Assembly of Molecular Building Blocks. Nat. Nanotech. 2007, 2, 687–691. [Google Scholar] [CrossRef]
- Cai, L.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; Mullen, K.; et al. Atomically Precise Bottom-Up Fabrication of Graphene Nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Matena, M.; Riehm, T.; Stöhr, M.; Jung, T.A.; Gade, L.H. Transforming Surface Coordination Polymers into Covalent Surface Polymers: Linked Polycondensed Aromatics through Oligomerization of N-Heterocyclic Carbene Intermediates. Angew. Chem. Int. Ed. 2008, 47, 2414–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmino, F.; Loppacher, C.; Chérioux, F. Photochemistry Highlights on On-Surface Synthesis. ChemPhysChem 2019, 20, 2271–2280. [Google Scholar] [CrossRef]
- Held, P.A.; Fuchs, H.; Studer, A. Covalent-Bond Formation via On-Surface Chemistry. Chem. Eur. J. 2017, 23, 5874–5892. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Song, S.; Kojima, T.; Nakae, T. Homochiral Polymerization-Driven Selective Growth of Graphene Nanoribbons. Nat. Chem. 2017, 9, 57–63. [Google Scholar] [CrossRef]
- Pvalicek, N.; Majzik, Z.; Collazos, S.; Meyer, G.; Perez, D.; Guitian, E.; Pena, D.; Gross, L. Generation and Characterization of ameta-Aryne on Cu and NaCl Surfaces. ACS Nano 2017, 11, 10768–10773. [Google Scholar] [CrossRef]
- Para, F.; Bocquet, F.; Nony, L.; Loppacher, C.; Féron, M.; Chérioux, F.; Gao, D.Z.; Canova, F.F.; Watkins, M.B. Micrometre-Long Covalent Organic Fibres by Photoinitiated Chain-Growth Radical Polymerization on an Alkali-Halide Surface. Nat. Chem. 2018, 10, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Sang, H.; Kantorovitch, L.; Takahashi, K.; Nozaki, K.; Ito, S. An Endergonic Synthesis of Single Sondheimer–Wong Diyne by Local Probe Chemistry. Angew. Chem. Int. Ed. 2020, 59, 10842–10847. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, B.; Chi, L. On-Surface Intramolecular Reactions. ACS Nano 2020, 14, 6376–6382. [Google Scholar] [CrossRef]
- Clair, S.; de Oteyza, D.G. Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef]
- Lackinger, M. On-Surface Polymerization—A Versatile Synthetic Route to Two-Dimensional Polymers: On-surface Polymerization. Polym. Inter. 2015, 64, 1073–1078. [Google Scholar] [CrossRef]
- Geagea, E.; Jeannoutot, J.; Féron, M.; Palmino, F.; Thomas, C.M.; Rochefort, A.; Chérioux, F. Collective Radical Oligomerisation Induced by STM Tip on a Silicon Surface. Nanoscale 2021, 13, 349–351. [Google Scholar] [CrossRef]
- Grossmann, L.; King, B.T.; Reichlmaier, S.; Hartmann, N.; Rosen, J.; Heckl, W.M.; Bjork, J.; Lackinger, M. On-Surface Photopolymerization of Two-dimensional Polymers Ordered on the Mesoscale. Nat. Chem. 2021, 13, 730–736. [Google Scholar] [CrossRef]
- Grill, L.; Hecht, S. Covalent on-Surface Polymerization. Nat. Chem. 2020, 12, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Schlickhum, U.; Decker, R.; Klappenberger, F.; Zoppellaro, G.; Klyatskaya, S.; Ruben, M.; Silanes, I.; Arnau, A.; Kern, K.; Brune, H.; et al. Metal-Organic Honeycomb Nanomeshes with Tunable Cavity Size. Nano Lett. 2007, 7, 3813–3817. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Gao, Z.; Lin, N. Self-Assembly of Metal–Organic Coordination Structures on Surfaces. Prog. Surf. Sci. 2016, 91, 101–135. [Google Scholar] [CrossRef]
- Moreno-Lopez, J.C.; Perez Paz, A.; Gottardi, S.; Solianyk, L.; Li, J.; Monjas, L.; Hirsch, A.K.H.; Mowbray, D.C.; Stohr, M. Unveiling Adatoms in On-Surface Reactions: Combining Scanning Probe Microscopy with van’t Hoff Plots. J. Phys. Chem. C 2021, 125, 9847–9854. [Google Scholar] [CrossRef]
- Bartels, L. Tailoring Molecular Layers at Metal Surfaces. Nat. Chem. 2010, 2, 87–95. [Google Scholar] [CrossRef]
- Koepf, M.; Chérioux, F.; Wytko, J.A.; Weiss, J. 1D and 3D Surface-Assisted Self-Organization. Coord. Chem. Rev. 2012, 256, 2872–2892. [Google Scholar] [CrossRef]
- Natarajan, P.; Schmittel, M.J. Photoluminescence, Redox Properties, and Electrogenerated Chemiluminescence of Twisted 9,9′-Bianthryls. J. Org. Chem. 2013, 78, 10383–10394. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Geagea, E.; Jeannoutot, J.; Morgenthaler, L.; Lamare, S.; Rochefort, A.; Palmino, F.; Chérioux, F. Unravelling the Growth Mechanism of (3,1) Graphene Nanoribbons on a Cu(111) Surface. Chem. Commun. 2021, 57, 6043–6045. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; Lei, S.; De Feyter, S. Molecular and Supramolecular Networks on Surfaces: From Two-Dimensional Crystal Engineering to Reactivity. Angew. Chem. Int. Ed. 2009, 48, 7298–7332. [Google Scholar] [CrossRef]
- Mishra, B.K.; Sathyamurthy, N. π-π interaction in Pyridine. J. Phys. Chem. A 2005, 109, 6–8. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Trier, M.; Pignedoli, C.A.; Laino, T.; Rieger, R.; Mullen, K.; Passerone, D.; Fasel, F. Surface-Assisted Cyclodehydrogenation Provides a Synthetic Route Towards Easily Processable and Chemically Tailored Nanographenes. Nat. Chem. 2010, 3, 61–67. [Google Scholar] [CrossRef]
- Tarliz, L.; Ruffieux, P.; Fasel, F. On-Surface Synthesis of Atomically Precise Graphene Nanoribbons. Adv. Mater. 2016, 28, 6222–6231. [Google Scholar] [CrossRef]
- Moreno, C.; Vilas-Varela, M.; Kretz, B.; Garcia-Lekue, A.; Costache, M.; Paradinas, M.; Panighel, M.; Ceballos, G.; Valenzuela, S.O.; Pena, D.; et al. Bottom-up Synthesis of Multifunctional Nanoporous Graphene. Science 2018, 360, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, S.; Nakatsuka, S.; Hatakeyama, T.; Pawlak, R.; Meier, T.; Tracey, J.; Meyer, E.; Foster, A.S. Multiple Heteroatom Substitution to Graphene Nanoribbon. Sci. Adv. 2018, 4, eaar7181. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sanchez, C.; Dienel, T.; Deniz, O.; Ruffieux, P.; Berger, R.; Feng, X.; Mullen, K.; Fasel, R. Purely Armchair or Partially Chiral: Noncontact Atomic Force Microscopy Characterization of Dibromo-Bianthryl-Based Graphene Nanoribbons Grown on Cu(111). ACS Nano 2016, 10, 8006–8011. [Google Scholar] [CrossRef] [PubMed]
- Schulz, F.; Jacobse, P.H.; Canova, F.F.; van der Lit, J.; Gao, D.Z.; von den Hoogenband, A.; Han, P.; Kelin Gebbink, R.J.M.; Moret, M.-E.; Joensuu, P.M.; et al. Precursor Geometry Determines the Growth Mechanism in Graphene Nanoribbons. J. Phys. Chem. C 2017, 121, 2896–2904. [Google Scholar] [CrossRef]
- de Oteyza, D.G.; Garcia-Lekue, A.; Vilas-Varela, M.; Merino-Diez, N.; Carbonell-Sanroma, E.; Corso, M.; Vasseur, G.; Rogero, C.; Guitan, E.; Pascual, J.I.; et al. Substrate-Independent Growth of Atomically Precise Chiral Graphene Nanoribbons. ACS Nano 2016, 10, 9000–9008. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Dienel, T.; Di Giovannantonio, M.; Barin, B.G.; Kharche, N.; Deniz, O.; Urgl, J.I.; Widmer, R.; Stolz, S.; De Lima, L.H.; et al. Heteroatom-Doped Perihexacene from a Double Helicene Precursor: On-Surface Synthesis and Properties. J. Am. Chem. Soc. 2017, 139, 4671–4674. [Google Scholar] [CrossRef]
- Heim, D.; Ecija, D.; Seufert, K.; Auwarter, W.; Aurisicchio, C.; Fabbro, C.; Bonifazi, D.; Barth, J.V. Self-Assembly of Flexible One-Dimensional Coordination Polymers on Metal Surfaces. J. Am Chem. Soc. 2010, 132, 6783–6790. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, C.; Han, Y.; Zhu, J.; Kuttner, J.; Hilt, G.; Gottfried, J.M. Surface-Assisted Formation, Assembly, and Dynamics of Planar Organometallic Macrocycles and Zigzag Shaped Polymer Chains with C-Cu-C Bonds. ACS Nano 2014, 8, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Song, F.; Alberti, M.N.; Nguyen, M.-T.; Trapp, N.; Thilgen, C.; Diedrich, F.; Stöhr, M. Heat-induced formation of one-dimensional coordination polymers on Au(111): An STM study. Chem. Commun. 2014, 51, 14473. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geagea, E.; Jeannoutot, J.; Morgenthaler, L.; Lamare, S.; Palmino, F.; Chérioux, F. On-Surface Synthesis of Ligands to Elaborate Coordination Polymers on an Au(111) Surface. Nanomaterials 2021, 11, 2102. https://doi.org/10.3390/nano11082102
Geagea E, Jeannoutot J, Morgenthaler L, Lamare S, Palmino F, Chérioux F. On-Surface Synthesis of Ligands to Elaborate Coordination Polymers on an Au(111) Surface. Nanomaterials. 2021; 11(8):2102. https://doi.org/10.3390/nano11082102
Chicago/Turabian StyleGeagea, Elie, Judicael Jeannoutot, Louise Morgenthaler, Simon Lamare, Frank Palmino, and Frédéric Chérioux. 2021. "On-Surface Synthesis of Ligands to Elaborate Coordination Polymers on an Au(111) Surface" Nanomaterials 11, no. 8: 2102. https://doi.org/10.3390/nano11082102





