Recent Advances in Synthesis, Medical Applications and Challenges for Gold-Coated Iron Oxide: Comprehensive Study
Abstract
:1. Introduction
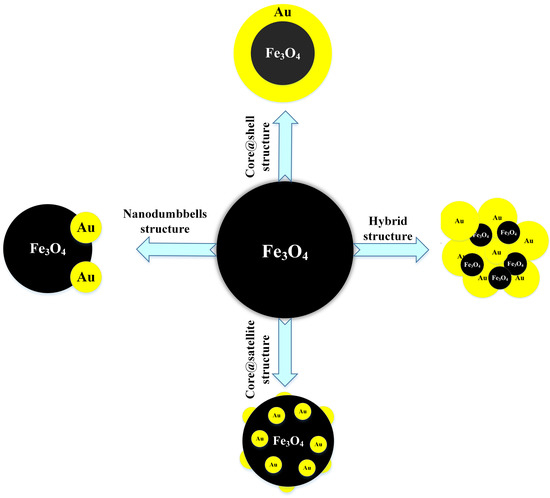
2. Synthesis of Fe3O4@Au
2.1. Core@Shell Structure of Fe3O4@Au
2.2. The Hybrid Structure of Fe3O4@Au (HNPs)
2.3. Core@Satellite Structures
2.4. Fe3O4@Au Nanodumbbells
3. Medical Application of Fe3O4@Au NPs
4. Conclusions and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Khaniabadi, P.M.; Mehrdel, B. Mechanisms of effective gold shell on Fe3O4 core nanoparticles formation using sonochemistry method. Ultrason. Sonochem. 2020, 64, 104865. [Google Scholar] [CrossRef]
- Nasrabadi, H.T.; Abbasi, E.; Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cell Nanomed. B. 2016, 44, 376–380. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.Q.; Nejad, M.S.; Varma, R.S. Core@shell Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Abu Noqta, O.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, X.; He, N.; Deng, Y.; Lu, G.; Li, K. Preparation and biomedical applications of gold-coated magnetic nanocomposites. J. Nanosci. Nanotechnol. 2013, 13, 1617–1625. [Google Scholar] [CrossRef]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Shete, P.; Patil, R.; Tiwale, B.; Pawar, S. Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Sousa, J.B.; Ramos-Jesus, J.; Silva, L.; Pereira, C.; De-Los-Santos-Álvarez, N.; Fonseca, R.A.; Miranda-Castro, R.; Delerue-Matos, C.; Júnior, J.R.S.; Barroso, M.F. Fe3O4@Au nanoparticles-based magnetoplatform for the HMGA maize endogenous gene electrochemical genosensing. Talanta 2020, 206, 120220. [Google Scholar] [CrossRef]
- Smith, M.; McKeague, M.; DeRosa, M.C. Synthesis, transfer, and characterization of core-shell gold-coated magnetic nanoparticles. MethodsX 2019, 6, 333–354. [Google Scholar] [CrossRef]
- Shiji, R.; Joseph, M.M.; Unnikrishnan, B.; Preethi, G.; Sreelekha, T. Fluorescent gold nanoclusters as a powerful tool for sensing applications in cancer management. In Advances in Biomaterials for Biomedical Applications; Springer: Singapore, 2017; pp. 385–428. [Google Scholar]
- Rabeea, M.A.; Owaid, M.N.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A. Mycosynthesis of gold nanoparticles using the extract of Flammulina velutipes, Physalacriaceae, and their efficacy for decolorization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 103841. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Owaid, M.N.; Rabeea, M.A.; Aziz, A.A.; Jameel, M.S. Mycosynthesis of gold nanoparticles by the Portabello mushroom extract, Agaricaceae, and their efficacy for decolorization of Azo dye. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100312. [Google Scholar] [CrossRef]
- Owaid, M.N.; Rabeea, M.A.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A. Mushroom-assisted synthesis of triangle gold nanoparticles using the aqueous extract of fresh Lentinula edodes (shiitake), Omphalotaceae. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100270. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Theranostics Based on Iron Oxide and Gold Nanoparticles for Imaging-Guided Photothermal and Photodynamic Therapy of Cancer. Curr. Top. Med. Chem. 2017, 17, 1858–1871. [Google Scholar] [CrossRef]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent advances in the synthesis of Fe3O4@Au core/shell nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Sabale, S.; Kandesar, P.; Jadhav, V.; Komorek, R.; Motkuri, R.K.; Yu, X.-Y. Recent developments in the synthesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater. Sci. 2017, 5, 2212–2225. [Google Scholar] [CrossRef]
- Sun, S.-N.; Wei, C.; Zhu, Z.-Z.; Hou, Y.-L.; Venkatraman, S.S.; Xu, Z.-C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Pu, H.; Huang, L. Bridging Fe3O4@Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B. Food Chem. 2020, 324, 126832. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, T.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Rapid SERS detection of acid orange II and brilliant blue in food by using Fe3O4@Au core–shell substrate. Food Chem. 2019, 270, 173–180. [Google Scholar] [CrossRef]
- Singh, N.; Nayak, J.; Sahoo, S.K.; Kumar, R. Glutathione conjugated superparamagnetic Fe3O4-Au core shell nanoparticles for pH controlled release of DOX. Mater. Sci. Eng. C 2019, 100, 453–465. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S. Synthesis and optimization of the sonochemical method for functionalizing gold shell on Fe3O4 core nanoparticles using response surface methodology. Surf. Interfaces 2020, 21, 100647. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S. Potential of a sonochemical approach to generate MRI-PPT theranostic agents for breast cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102177. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.; Suslick, K. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Ahmed, N.M.; Ali, A.T. Distinct advantages of using sonochemical over laser ablation methods for a rapid-high quality gold nanoparticles production. Mater. Res. Express 2021, 8, 015009. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Zandavar, H.; Yousefi, M.; Rajabi, H.R.; Pourmortazavi, S.M. Green-photodegradation of model pharmaceutical contaminations over biogenic Fe3O4/Au nanocomposite and antimicrobial activity. J. Environ. Manag. 2020, 270, 110831. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, E.; Basirun, W.J.; Johan, M.R.; Rezayi, M.; Darroudi, M.; Shameli, K.; Shanavaz, Z.; Akbarzadeh, O.; Izadiyan, Z. Facile and greener hydrothermal honey-based synthesis of Fe3O4/Au core/shell nanoparticles for drug delivery applications. J. Cell. Biochem. 2019, 120, 6624–6631. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, T.; Ulu, A.; Sariçam, M.; Çulha, M.; Ates, B. Maltose functionalized magnetic core/shell Fe3O4@Au nanoparticles for an efficient L-asparaginase immobilization. Int. J. Biol. Macromol. 2020, 142, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Butmee, P.; Tumcharern, G.; Thouand, G.; Kalcher, K.; Samphao, A. An ultrasensitive immunosensor based on manganese dioxide-graphene nanoplatelets and core shell Fe3O4@Au nanoparticles for label-free detection of carcinoembryonic antigen. Bioelectrochemistry 2020, 132, 107452. [Google Scholar] [CrossRef]
- Kou, Y.; Wu, T.; Xing, G.; Huang, X.; Han, D.; Yang, S.; Guo, C.; Gao, W.; Yang, J.; Liu, Y.; et al. Highly efficient and recyclable catalyst: Porous Fe3O4–Au magnetic nanocomposites with tailored synthesis. Nanotechnology 2020, 31, 225701. [Google Scholar] [CrossRef]
- Al-Sherbini, A.-S.A.; El-Ghannam, G.; Yehya, H.; Nassef, O.A. Optical and Magnetic Studies of Fe3O4/Au Core/Shell Nanocomposites. Int. J. Nanosci. 2019, 18, 1850033. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Shao, H.; Zhan, S.; Hou, P.; Zhang, X.; Chai, Y.; Liu, H. Bi-phase dispersible Fe3O4@Au core–shell multifunctional nanoparticles: Synthesis, characterization and properties. Compos. Interfaces 2019, 26, 537–549. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Giorgetti, E. Spectroscopic and Microscopic Analyses of Fe3O4/Au Nanoparticles Obtained by Laser Ablation in Water. Nanomaterials 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Ángeles-Pascual, A.; Piñón-Hernández, J.; Estevez-González, M.; Pal, U.; Velumani, S.; Pérez, R.; Esparza, R. Structure, magnetic and cytotoxic behaviour of solvothermally grown Fe3O4@Au core-shell nanoparticles. Mater. Charact. 2018, 142, 237–244. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Brullot, W.; Koeckelberghs, G.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Phys. B Condens. Matter 2019, 560, 85–90. [Google Scholar] [CrossRef]
- Park, S.-I.; Chung, S.-H.; Kim, H.-C.; Lee, S.G.; Lee, S.J.; Kim, H.; Kim, H.; Jeong, S.W. Prolonged heating of Fe3O4–Au hybrid nanoparticles in a radiofrequency solenoid coil. Colloids Surf. A 2018, 538, 304–309. [Google Scholar] [CrossRef]
- Xu, K.; Wu, J.; Fang, Q.; Bai, L.; Duan, J.; Li, J.; Xu, H.; Hui, A.; Hao, L.; Xuan, S. Magnetically separable h-Fe3O4@Au/polydopamine nanosphere with a hollow interior: A versatile candidate for nanocatalysis and metal ion adsorption. Chem. Eng. J. 2020, 398, 125571. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, S.; Zhong, M.; Tian, D.; Wang, C.; Lu, X. Constructing magnetic Fe3O4-Au@CeO2 hybrid nanofibers for selective catalytic degradation of organic dyes. Appl. Organomet. Chem. 2019, 33, e5253. [Google Scholar] [CrossRef]
- Efremova, M.V.; Nalench, Y.A.; Myrovali, E.; Garanina, A.; Grebennikov, I.S.; Gifer, P.K.; Abakumov, M.; Spasova, M.; Angelakeris, M.; Savchenko, A.G.; et al. Size-selected Fe3O4–Au hybrid nanoparticles for improved magnetism-based theranostics. Beilstein J. Nanotechnol. 2018, 9, 2684–2699. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Hao, C.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Spiky Fe3O4@Au Supraparticles for Multimodal In Vivo Imaging. Adv. Funct. Mater. 2018, 28, 1800310. [Google Scholar] [CrossRef]
- Li, N.; Zhong, T.; Liu, J.-L.; Zheng, J.; Deng, H.-C.; Zhou, W.; Li, M.; Feng, M.-S.; Liu, Q.-J.; Li, C.-Y.; et al. Precise Identification and Analysis of Micro/Nano-Sized Pore Structure in Shale with Fe3O4/Au Hybrid Nanocomposite. Anal. Chem. 2018, 90, 12706–12713. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Kou, Q.; Wang, D.; Han, D.; Lu, Z.; Chen, Y.; Chen, L.; Wang, Y.; Yang, J.; et al. Fe3O4/Au binary nanocrystals: Facile synthesis with diverse structure evolution and highly efficient catalytic reduction with cyclability characteristics in 4-nitrophenol. Powder Technol. 2018, 338, 26–35. [Google Scholar] [CrossRef]
- Song, R.-B.; Zhou, S.; Guo, D.; Li, P.; Jiang, L.-P.; Zhang, J.-R.; Wu, X.; Zhu, J.-J. Core/Satellite Structured Fe3O4/Au Nanocomposites Incorporated with Three-Dimensional Macroporous Graphene Foam as a High-Performance Anode for Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2019, 8, 1311–1318. [Google Scholar] [CrossRef]
- Klein, S.; Hübner, J.; Menter, C.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. A Facile One-Pot Synthesis of Water-Soluble, Patchy Fe3O4-Au Nanoparticles for Application in Radiation Therapy. Appl. Sci. 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Kostevsek, N.; Locatelli, E.; Garrovo, C.; Arena, F.; Monaco, I.; Nikolov, I.P.; Sturm, S.; Rozman, K.Z.; Lorusso, V.; Giustetto, P.; et al. The one-step synthesis and surface functionalization of dumbbell-like gold–iron oxide nanoparticles: A chitosan-based nanotheranostic system. Chem. Commun. 2016, 52, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Xuan, S.; Zhu, X.; Wang, D.; Chak, C.-P.; Lee, S.-F.; Ho, W.K.-W.; Chung, B.C.-T. Gold and iron oxide hybrid nanocomposite materials. Chem. Soc. Rev. 2012, 41, 1911–1928. [Google Scholar] [CrossRef]
- Huang, Q.; Li, W.; Lin, Q.; Pi, D.; Hu, C.; Shao, C.; Zhang, H. A review of significant factors in the synthesis of hetero-structured dumbbell-like nanoparticles. Chin. J. Catal. 2016, 37, 681–691. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhang, H.-L.; Yang, Z.; Li, T.; Wang, B.; Huo, X.; Wang, R.; Chen, H. A multifunctional nanoprobe based on Au–Fe3O4 nanoparticles for multimodal and ultrasensitive detection of cancer cells. Chem. Commun. 2013, 49, 4938. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.; Zeng, H.; Sun, S. Recent Progress in Syntheses and Applications of Dumbbell-like Nanoparticles. Adv. Mater. 2009, 21, 3045–3052. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, L.; Hosmane, N.; Gong, Y.; Wu, A. Cancer cell detection and imaging: MRI-SERS bimodal splat-shaped Fe3O4/Au nanocomposites. Chin. Chem. Lett. 2019, 30, 87–89. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy. Colloids Surf. B 2019, 174, 252–259. [Google Scholar]
- Sarno, M.; Iuliano, M. Highly active and stable Fe3O4/Au nanoparticles supporting lipase catalyst for biodiesel production from waste tomato. Appl. Surf. Sci. 2019, 474, 135–146. [Google Scholar] [CrossRef]
- Ge, Y.; Zhong, Y.; Ji, G.; Lu, Q.; Dai, X.; Guo, Z.; Zhang, P.; Peng, G.; Zhang, K.; Li, Y. Preparation and characterization of Fe3O4@Au-C225 composite targeted nanoparticles for MRI of human glioma. PLoS ONE 2018, 13, e0195703. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Wang, R.; Hu, Y.; Xu, F.; Zhang, N.; Cao, X.; Wang, X.; Shi, X.; Guo, R. Folic acid-modified Laponite®-stabilized Fe3O4 nanoparticles for targeted T2-weighted MR imaging of tumor. Appl. Clay Sci. 2020, 186, 105447. [Google Scholar] [CrossRef]
- Karami, C.; Taher, M.A. A catechol biosensor based on immobilizing laccase to Fe3O4@Au core-shell nanoparticles. Int. J. Biol. Macromol. 2019, 129, 84–90. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.-Y.; Peh, S.-C.; Mohamad, S.E.; Taib, S.H.M. Green fabrication of biologically active magnetic core-shell Fe3O4/Au nanoparticles and their potential anticancer effect. Mater. Sci. Eng. C 2019, 96, 51–57. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, S.; He, J.; Pan, C.C.; Li, H.; Zhou, Z.Y.; Ding, Y.; Huo, D.; Hu, Y. Synthesis and application of strawberry-like Fe3O4-Au nanoparticles as CT-MR dual-modality contrast agents in accurate detection of the progressive liver disease. Biomaterials 2015, 51, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Abu Noqta, O.; Khaniabadi, P.M.; Mehrdel, B. Excellent relaxivity and X-ray attenuation combo properties of Fe3O4@Au CSNPs produced via Rapid sonochemical synthesis for MRI and CT imaging. Mater. Today Commun. 2020, 25, 101368. [Google Scholar] [CrossRef]
- Keshtkar, M.; Shahbazi-Gahrouei, D.; Mehrgardi, M.; Aghaei, M.; Khoshfetrat, S. Synthesis and Cytotoxicity Assessment of Gold-coated Magnetic Iron Oxide Nanoparticles. J. Biomed. Phys. Eng. 2018, 8, 357–364. [Google Scholar]
- Nosrati, H.; Salehiabar, M.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. Theranostic nanoparticles based on magnetic nanoparticles: Design, preparation, characterization, and evaluation as novel anticancer drug carrier and MRI contrast agent. Drug Dev. Ind. Pharm. 2018, 44, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile One-Pot Synthesis of Fe3O4@Au Composite Nanoparticles for Dual-Mode MR/CT Imaging Applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tung, G.A.; Sun, S. Size and Concentration Effect of Gold Nanoparticles on X-ray Attenuation As Measured on Computed Tomography. Chem. Mater. 2008, 20, 4167–4169. [Google Scholar] [CrossRef] [Green Version]
- Dheyab, M.; Aziz, A.; Jameel, M.; Khaniabadi, P.; Mehrdel, B.; Khaniabadi, B. Gold-coated iron oxide nanoparticles as a potential photothermal therapy agent to enhance eradication of breast cancer cells. J. Phys. Conf. Ser. 2020, 1497, 012003. [Google Scholar] [CrossRef]
- Premaratne, G.; Dharmaratne, A.C.; Al Mubarak, Z.H.; Mohammadparast, F.; Andiappan, M.; Krishnan, S. Multiplexed surface plasmon imaging of serum biomolecules: Fe3O4@Au Core/shell nanoparticles with plasmonic simulation insights. Sens. Actuators B Chem. 2019, 299, 126956. [Google Scholar] [CrossRef]
- Zhou, H.; Oh, S.; Kim, J.E.; Zou, F.; Hwang, D.Y.; Lee, J. In Vivo Study of Spiky Fe3O4@Au Nanoparticles with Different Branch Lengths: Biodistribution, Clearance, and Biocompatibility in Mice. ACS Appl. Bio Mater. 2019, 2, 163–170. [Google Scholar] [CrossRef]
- Kang, N.; Xu, D.; Han, Y.; Lv, X.; Chen, Z.; Zhou, T.; Ren, L.; Zhou, X. Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater. Sci. Eng. C 2019, 98, 545–549. [Google Scholar] [CrossRef]
- Jency, D.A.; Sathyavathi, K.; Umadevi, M.; Parimaladevi, R. Enhanced bioactivity of Fe3O4-Au nanocomposites—A comparative antibacterial study. Mater. Lett. 2020, 258, 126795. [Google Scholar] [CrossRef]






| No. | Nanoparticles Structure | Synthesis Method | Size/Shape | Applications | Ref |
|---|---|---|---|---|---|
| 1 | Core@shell | Growth method | 5 nm/spherical | Food application | [21] |
| 2 | Core@shell | Sonochemical | ~40 nm/flower | Food application | [20] |
| 3 | Core@shell | Green method | 31/spherical | Antimicrobial activity | [28] |
| 4 | Core@shell | Green method | 3.49–4.11 nm/semispherical | Drug delivery | [29] |
| 5 | Core@shell | Reduction | 10 nm/amorphous | Enzyme immobilization | [30] |
| 6 | Core@shell | Sonochemical | 20–50 nm/spherical | Cancer biomarkers | [31] |
| 7 | Core@shell | Seeds growth | 9.49, 10.04 and 8.95 nm/flower | Catalytic reduction of RhB | [32] |
| 8 | Core@shell | Seeding technique | 15–57 nm/ | [33] | |
| 9 | Core@shell | Nano-emulsion technique | 11 nm/semispherical | [34] | |
| 10 | Core@shell | Laser ablation | 20 nm/spherical | [35] | |
| 11 | Core@shell | Reduction | 20–50 nm/semispherical | Cytotoxicity assay in MDCK cell line | [36] |
| 12 | Core@shell | Reduction | ~100 nm/flower | [37] | |
| 13 | HNPs | Reduction | 10 nm/spherical | Hyperthermia | [38] |
| 14 | Au/PDA hybrid | In situ redox-oxidize polymerization | 25 nm/spherical | Catalysis and adsorption | [39] |
| 15 | Fe3O4@Au@CeO2 hybrid | Redox reaction | 17 nm/nanofibers | Catalysis | [40] |
| 16 | HNPs | Thermal decomposition | 25 nm/octahedral | Theranostics | [41] |
| 17 | HNPs | Seeds growth | 90 nm/spiky | Multimodal in vivo imaging | [42] |
| 18 | HNPs | Chemical reduction | 31 nm/spherical | [43] | |
| 19 | Core@satellite | Seed-mediated growth | 65 nm/cubic | Catalysis | [44] |
| 20 | Core@satellite | Hydrothermal treatment, and freeze-drying technologies | 300–400 nm/spherical | Microbial fuel cells | [45] |
| 21 | Dumbbell NPs | Reduction | 22 nm/spherical | Radiation therapy | [46] |
| 22 | Dumbbell NPs | Thermal decomposition | 7 nm/spherical | [47] |
| No. | Nanoparticles Type | Application | Results | Ref |
|---|---|---|---|---|
| 1 | Core–shell Fe3O4/Au | Anticancer | The Fe3O4@Au NPs display 235 μg/mL of inhibitory concentration (IC)50 against colorectal cancer cells (HT-29). | [61] |
| 2 | Fe3O4@Au HNPs | CT-MR dual-modality contrast agents | In vitro phantom studies revealed that these NPs provided superior contrast enhancement for CT and MR imaging. | [62] |
| 3 | Fe3O4@Au NPs | MRI and CT imaging | The in vitro findings (r2 = 222.28 mM−1 s−1, HU = 418) substantiate the effectiveness of Fe3O4@Au NPs in MRI and CT imaging. | [63] |
| 4 | Fe3O4@Au NPs | Photothermal therapy | The findings demonstrated that Fe3O4@Au NPs have the ability to be used as a phototherapeutic agent to enhance the eradication of breast cancer cells. | [68] |
| 5 | Fe3O4@Au core/shell | Biosensors | Fe3O4@Au NPs as new multiplex biosensors of real laboratory testing in complex matrices. | [69] |
| 6 | Spiky Fe3O4@Au NPs | Theranosticagents | The serum biochemistry results showed that the spiky Fe3O4@Au NPs had no discernible toxicity in vivo and could not accurately depict liver and kidney failure. | [70] |
| 7 | Fe3O4@Au NPs | Dual-modal imaging | The Fe3O4@Au NPs proved to be a successful candidate to image tumors for Vivo PA/MR through intravenous injection. | [71] |
| 8 | Fe3O4@Au NPs | Antibacterial study | Fe3O4@Au NPs revealed good antibacterial activity against Gram-positive and Gram-negative pathogens which are found in water. | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Dheyab, M.; Abdul Aziz, A.; Jameel, M.S.; Moradi Khaniabadi, P. Recent Advances in Synthesis, Medical Applications and Challenges for Gold-Coated Iron Oxide: Comprehensive Study. Nanomaterials 2021, 11, 2147. https://doi.org/10.3390/nano11082147
Ali Dheyab M, Abdul Aziz A, Jameel MS, Moradi Khaniabadi P. Recent Advances in Synthesis, Medical Applications and Challenges for Gold-Coated Iron Oxide: Comprehensive Study. Nanomaterials. 2021; 11(8):2147. https://doi.org/10.3390/nano11082147
Chicago/Turabian StyleAli Dheyab, Mohammed, Azlan Abdul Aziz, Mahmood S. Jameel, and Pegah Moradi Khaniabadi. 2021. "Recent Advances in Synthesis, Medical Applications and Challenges for Gold-Coated Iron Oxide: Comprehensive Study" Nanomaterials 11, no. 8: 2147. https://doi.org/10.3390/nano11082147