Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review
Abstract
:1. Introduction
2. Body–Electrode Interface
2.1. Electrode–Electrolyte Interface
2.1.1. Current Transfer Mechanisms
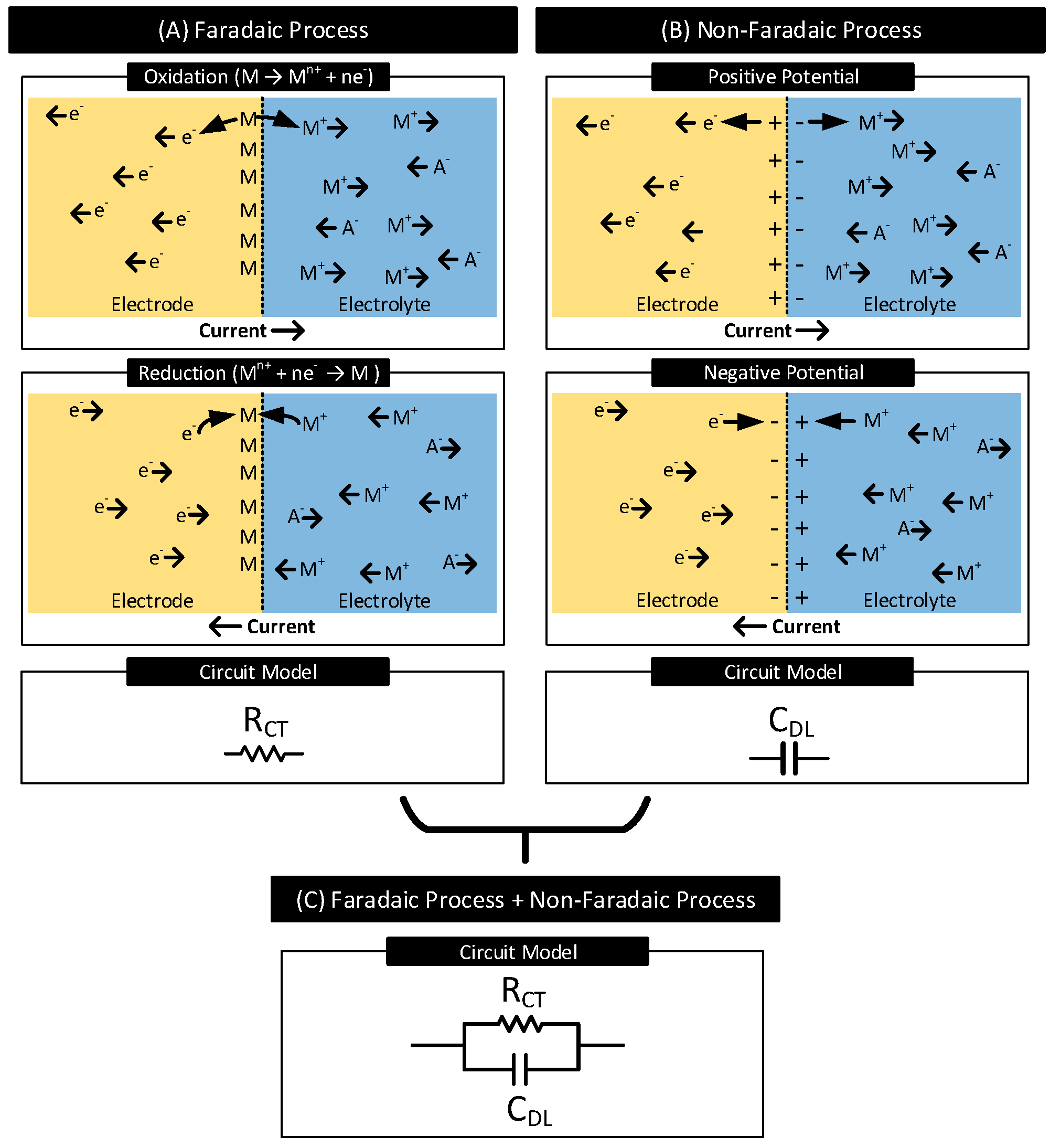
- Faradaic Process: In this process of current transfer, charges (electrons/ions) cross the electrode–electrolyte interface by electrochemical reactions: oxidation and reduction [1,2].Oxidation occurs when atoms in the electrode (M) leave electrons behind and goes into the electrolyte as a cation () or when anions () in the electrolyte transform to a neutral atom, leaving electrons to the electrode. The following reactions represent oxidation:where n is the valence of M and m is the valence of A. Reversal of oxidation reactions results in reduction reactions. The following reactions represent reduction:Figure 2A shows the oxidation and the reduction reactions involving M and and the direction of flow of charge carriers. In the presence of a driving current source, in the electrode moves in the opposite direction as the current. Charge carriers and in the electrolyte move in the opposite and same direction as the current, respectively. Oxidation reactions dominate when the current flows from electrode to electrolyte, and reduction reactions dominate when the current direction is the opposite.In a faradaic process, the charge transfer across the interface obeys Faraday’s law, i.e., the amount of chemical reactions resulting in charge transfer across the interface is proportional to the current that flows through the interface [30]. The electrode–electrolyte interface undergoing the faradaic process thus behaves like a resistor [29]. This resistor is known as the charge-transfer resistor, [31,32].
- Non-Faradaic Process: In this process of current transfer, charges never cross the interface. Rather, they accumulate across either side of the interface, polarizing the electrode. See Figure 2B. When current flows from electrode to electrolyte, near the interface, positive charges accumulate in the electrode ( moves away from the interface), and negative charges accumulate in the electrolyte ( moves away from the interface, moves closer to the interface) [33]. These charge redistributions result in the electrode acquiring a positive potential that increases with time. The charge and the voltage polarities reverse when the direction of the current is the opposite. The accumulation of charges across the interface results in the interface acting as a capacitor known as the interface double-layer capacitor, . The current transfer across the interface is due to the displacement current resulting from the charging and discharging of [29].
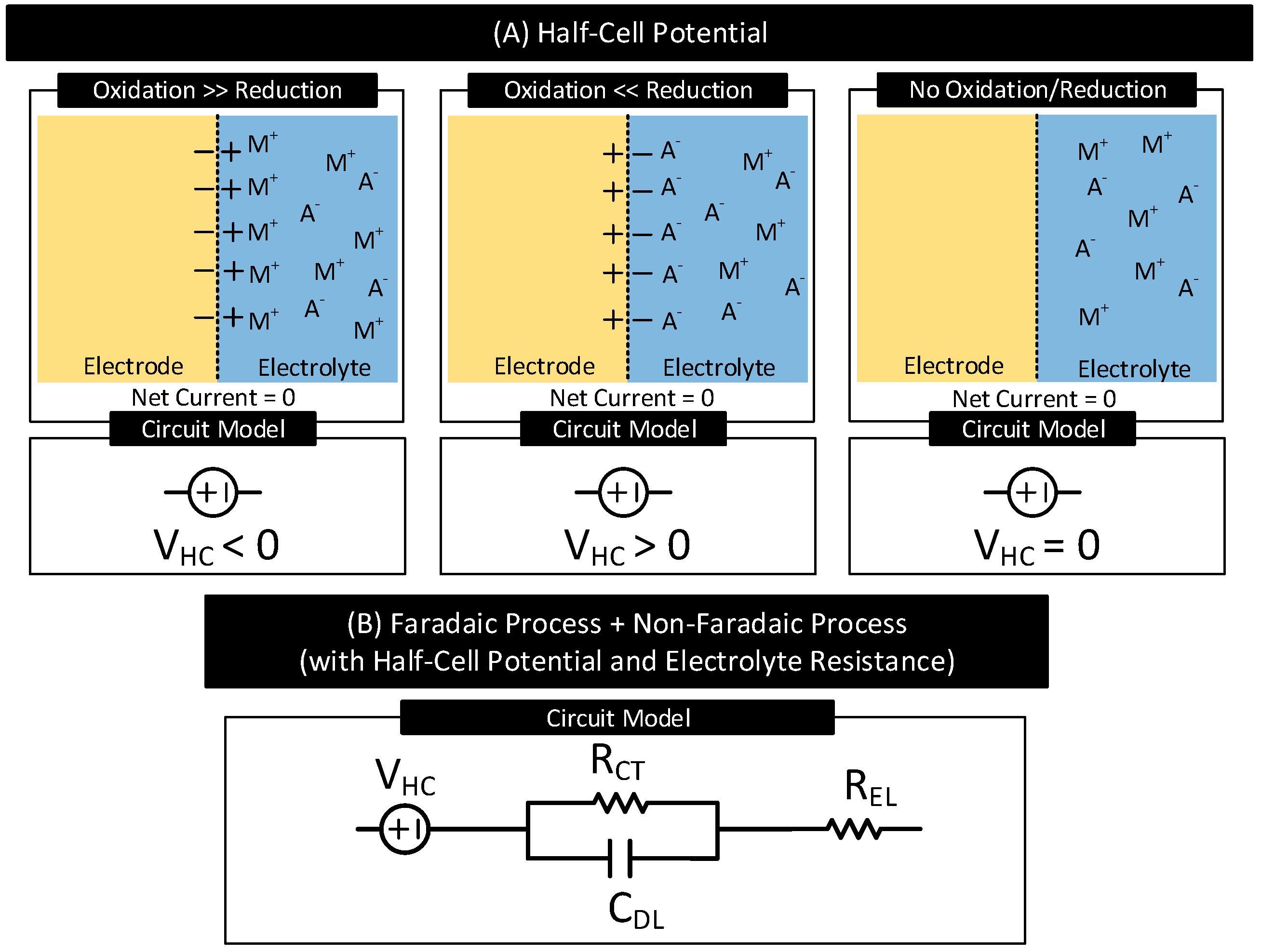
2.1.2. Half-Cell Potential
2.1.3. Electrode Polarization
- Current Transfer via Faradaic Process
- -
- Non-Polarizable (or Charge Transfer) Electrodes: The current transfer across the interface is solely due to the faradaic process. They have a non-zero charge-transfer resistance (i.e., ). The degree of polarization is proportional to .
- -
- Ideal Non-Polarizable Electrodes: They are non-polarizable electrodes with zero charge transfer resistance (i.e., ). Their interface behaves like an electrical short. They offer no resistance to direct current flow and thus do not polarize the electrode, i.e., the electrode potential does not change from its equilibrium value.
- Current Transfer via Non-Faradaic Process
- -
- Ideal Polarizable Electrodes: The current transfer across the interface is solely due to the non-faradaic process. Their interface behaves like a capacitor and thus blocks direct current. The direct current causes the magnitude of the electrode potential to increase over time without limits.
- Current Transfer via Faradaic and Non-Faradaic Processes
- -
- General Polarizable Electrodes: In these electrodes, both faradaic and non-faradaic processes contribute to the current transfer across the interface. The degree of polarization depends on the values of the charge transfer resistance and double-layer capacitance of the interface.
2.2. Electrode–Skin Interface
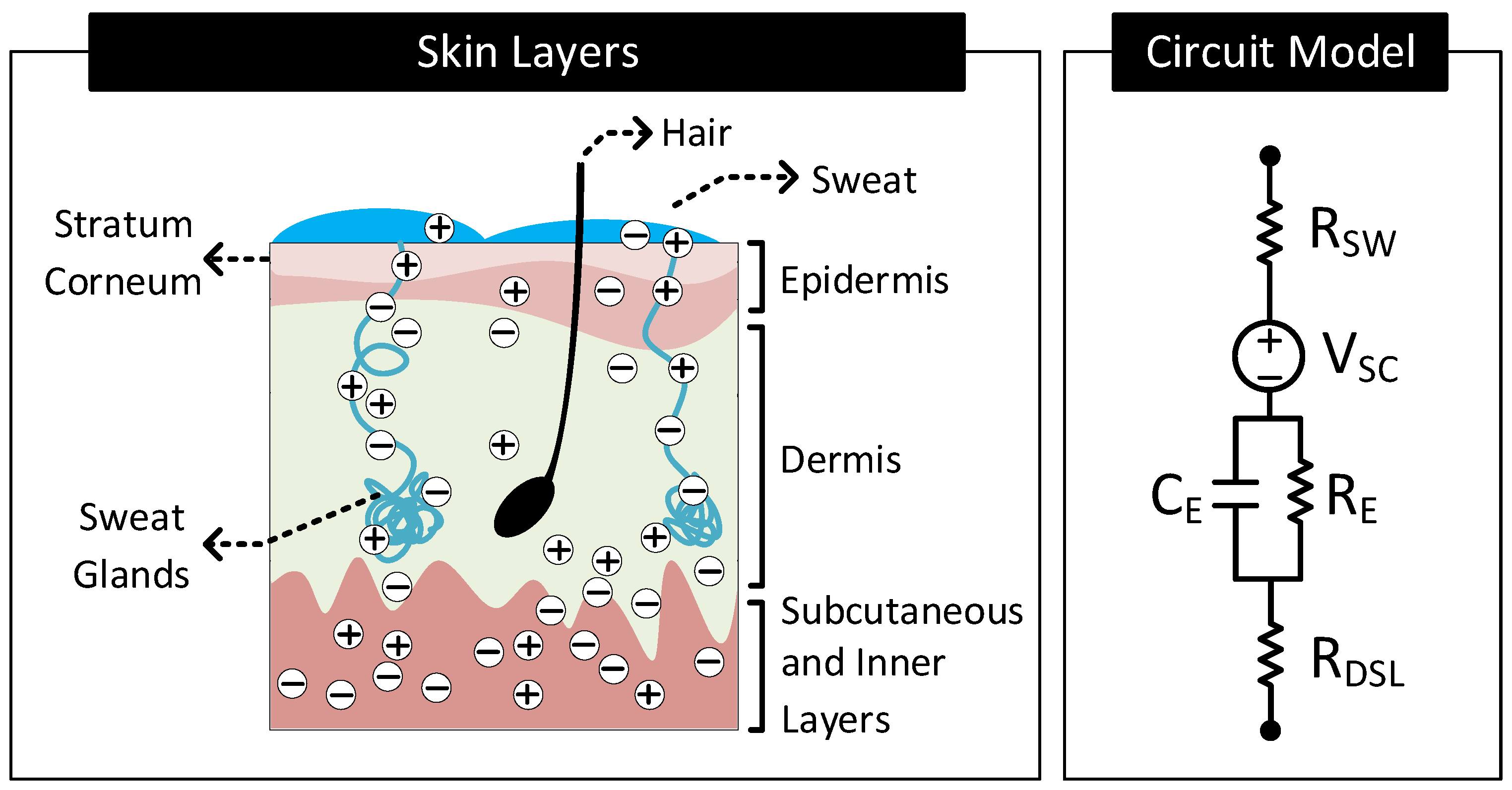
2.2.1. Electrical Model of the Skin
2.2.2. Types of Surface Electrodes
- Wet Electrode: These electrodes make contact with the skin through an explicitly added electrolyte layer on the skin surface. The electrolyte layer hydrates the skin layer and improves the electrical conductivity of the stratum corneum [34]. Figure 6B shows the layer stack and circuit model of the electrode–skin interface for a wet electrode. Model parameters , and are the half-cell potential, charge transfer resistance, and double-layer capacitance, respectively, of the electrode–electrolyte interface. is the resistance of the electrolyte layer. Model parameters , (≈10–100 K cm [34]), (≈10–50 nF cm [34]) and constitute the skin potential and impedance components.
- Dry-Contact Electrode: These electrodes make direct contact with the skin surface without relying on an externally applied electrolyte [34]. Here, the moisture or sweat on the skin surface plays the role of the electrolyte, hydrating the skin surface and lowerering the skin impedance [37,38]. However, since the moisture and sweat distribution is uneven, varies over time, and often has less ion concentration than off-the-shelf electrolytes, the electrode to skin impedance with dry electrodes is higher than the wet electrode’s case and changes over time. Additionally, the electrode–skin interface also traps air bubbles, blocking parts of the electrode surface from contacting the skin surface directly, adding a capacitive component to the interface impedance. The interface impedance also depends on the pressure applied to the electrode [39,40,41]. The dependency of the interface impedance on sweat, trapped air bubbles, and pressure mean that the electrode–skin characteristics of these electrodes vary with time, ambient conditions (e.g., temperature, humidity), subject’s movement, and electrode form factor (e.g., rigid or flexible electrodes) [42,43]. Figure 6C shows the layer stack and circuit model of the electrode–skin interface for a dry contact electrode. Model parameters , , and are the half-cell potential, charge transfer resistance, and double-layer capacitance, respectively, of the electrode–sweat interface. is the resistance of the sweat layer. models the capacitance of the electrode that does not make contact with the skin due to trapped air bubbles. Model parameters , (≈30–1000 K cm [34]), (≈10–50 nF cm [34]) and constitute the skin potential and impedance components.
- Dry-Capacitive Electrode: These electrodes do not make direct contact with the skin [34,44]. They are electrically isolated from the skin through an insulating layer (e.g., air or clothes). These electrodes eliminate the chance of skin irritation, protect the body from any electrical mishaps, and are easy to clean [45]. However, they are prone to motion artifacts, i.e., their contact impedances with the skin vary with the subject’s movements [44]. Figure 6D shows the layer stack and circuit model of the electrode–skin interface for these electrodes. The parameter (≈1 pF–10 nF cm [34]) models the insulation layer between the electrode and the skin surface. Since there does not exist any electrode–electrolyte interface, the circuit model of these electrodes does not include , , or typical of an electrode–electrolyte interface. Model parameters , (≈100–1000 K cm [34]), (≈10–50nF cm [34]) and constitute the skin potential and impedance components.
- Semi-Dry Electrode: These electrodes are placed directly on the skin surface like a dry electrode without direct application of electrolyte. However, unlike a dry electrode, a semi-dry electrode has inbuilt reservoirs which store electrolytes. While in contact with the body, the semi-dry electrode slowly releases the electrolyte in its reservoir to the skin surface (e.g., through porous columns in the reservoir) [46,47,48,49,50,51]. The released electrolyte hydrates the skin and improves the conductivity of the stratum corneum similarly to a wet electrode. Figure 6E shows the layer stack and circuit model of a semi-dry electrode that releases electrolyte in its reservoir through porous columns. The circuit model is similar to the wet electrode with the following difference. for the semi-dry electrode is generally higher due to higher electrolyte resistance at the porous columns [50].
- Electrolyte Amount: The electrolyte at the electrode–skin interface hydrates the stratum corneum layer of the skin and improves the contact impedance. Improvement in contact impedance with electrolytes is evident when comparing the contact impedance of wet/semi-dry electrodes with dry electrodes. The contact impedance of the dry electrode is approximately 50-fold higher than the wet/semi-dry electrodes that use electrolytes [59]. Using higher amounts of electrolytes is also desirable as it reduces the contact impedance by lowering both and . In particular, higher amounts of electrolytes can easily penetrate the skin layers and hydrate the layers lowering [50]. However, having higher amounts of electrolytes can cause discomforts to the user and cause possible inter-electrode shorts in cases where the electrodes are close to each other.
- Skin Preparation: Often, to improve the electrode–skin contact, skin preparations are performed at the electrode sites before placing the electrodes [60,61]. Skin preparations commonly involve cleaning, shaving, and abrasion. Cleaning and shaving help remove dirt and hairs from the skin, while abrasion removes the topmost stratum corneum layer. Skin preparation helps in reducing the interface impedance. For instance, performing skin abrasion can reduce interface impedance in dry-contact electrodes by about 80% [59]. Nevertheless, skin preparations can be time-consuming, costly, and painful to the subjects involved.
- Contact Pressure: Electrode–skin contact also improves with the application of pressure on the electrodes. The effect is significant, specifically in dry and semi-dry electrodes, where pressure significantly reduces the contact impedance [39,40,41,50]. Unlike wet electrodes, dry and semi-dry electrodes do not maintain stable contact with the skin due to the absence or relatively low amounts of electrolytes. For instance, authors in [50] find a decrease in contact impedance of 71% and 35% in dry-contact and semi-dry electrodes, respectively, with mild pressure. Application of pressure in these electrodes lowers both and . The decrease in is due to close contact of the electrode with the skin. In contrast, the drop in is due to compression of skin layers shortening the ionic-current channel and due to porous columns of the semi-dry electrode penetrating the top layers of the skin [50].
2.3. Signal Distortions: Types and Causes
- Offset Shift: For the signals passing through the interface, the electrode half-cell potential () together with skin potential () behaves like a series DC voltage source represented in Figure 7 as . The presence of shifts the DC offset of the passing signals. Since is in hundreds of millivolts, often the offset shift is many folds higher than the sensing and communication signal amplitudes which are approximately a few microvolts to millivolts in range. The amount of offset shift depends on the electrode and its position on the body. The dependency is because vary with the electrode (see Table 2) and vary with the location on the body [36].
- Baseline Wander: The offset added to the signals is often not a constant; it varies slowly with time (i.e., →) resulting in the baseline of the signal shifting over time. This phenomenon is called baseline wander. Following are the factors that can potentially cause baseline wander.
- (a)
- Motion Artifact: The distortion of the signal with the subject’s movement is known as motion artifact [63,64]. Motion artifact can cause the signal baseline to vary with time at frequencies of 1–15 Hz. Figure 8A depicts this behavior. Motion artifact has many causes.
- When the body is in motion, e.g., jogging or even during respiration, the electrode moves relative to the electrolyte. This movement can cause the electrode surface to see different electrolyte concentrations over time. Since depends on the electrolyte concentration near the electrode’s surface, the subject’s movement results in and thus to vary [1,2,41,65,66].
- (b)
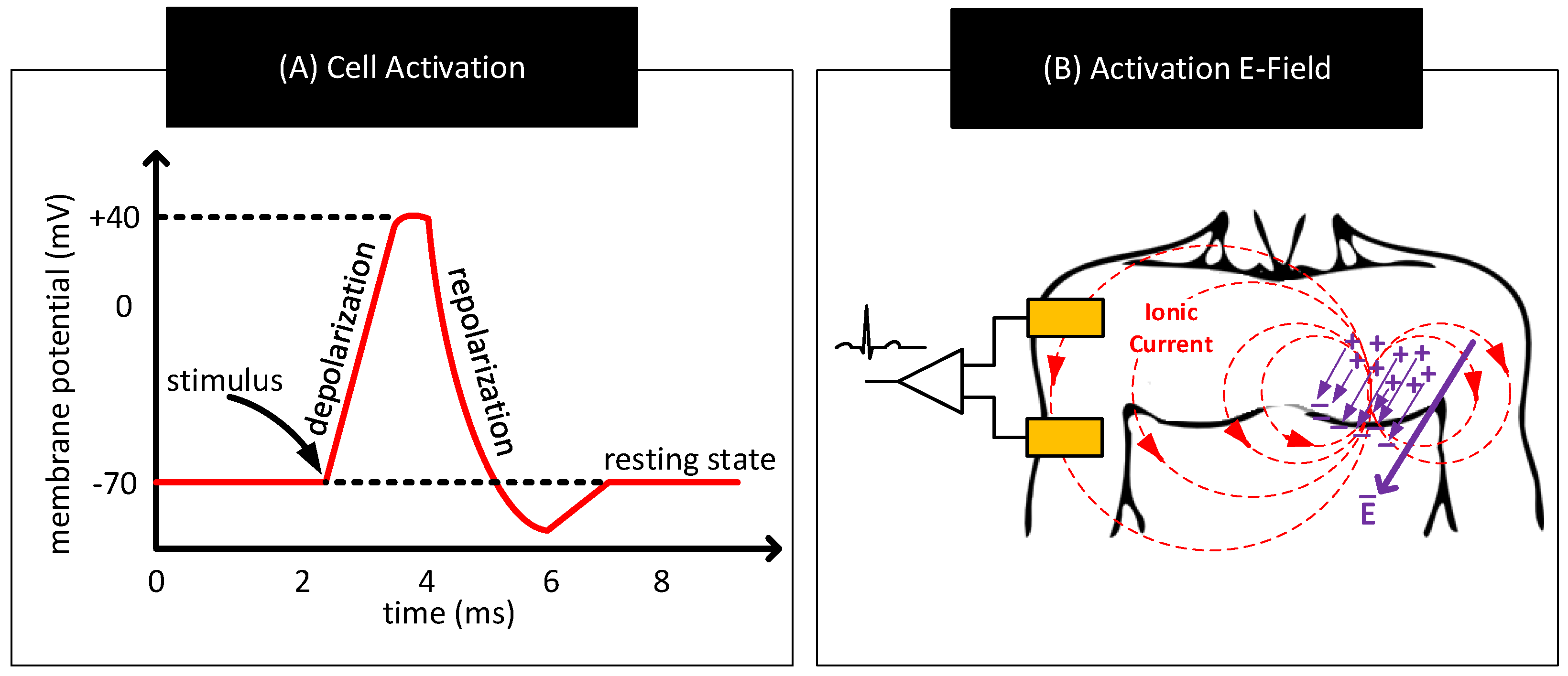
- Environmental Interference: Environmental noise sources such as AC mains near the body can potentially couple currents to the body [71]. When that happens, the currents can traverse through the electrode–body interface and create a time-varying voltage drop across , that adds to . The currents also polarize the electrode and change its , modifying further. Additionally, the currents flowing through the body can change the body’s potential. Since signals used in human-body sensing and communication use the body’s potential as the reference, any variation in the body’s potential will reflect in the signal measurements. Figure 8B shows the likely environmental noise sources that can interfere with the body and cause the signal baseline to wander over time.
- Signal Attenuation and Dispersion: When signals pass through the interface, due to non-zero impedance , a part of the signal drops across , attenuating the output. Signal dispersion occurs due to the capacitive component of , which results in different attenuation of low-frequency components than the high-frequency components of the signals. This frequency dependent transfer function through the body–electrode interface also depends on the termination impedance.
2.4. Practical Electrodes: Form Factors and Materials
2.4.1. Surface Electrodes
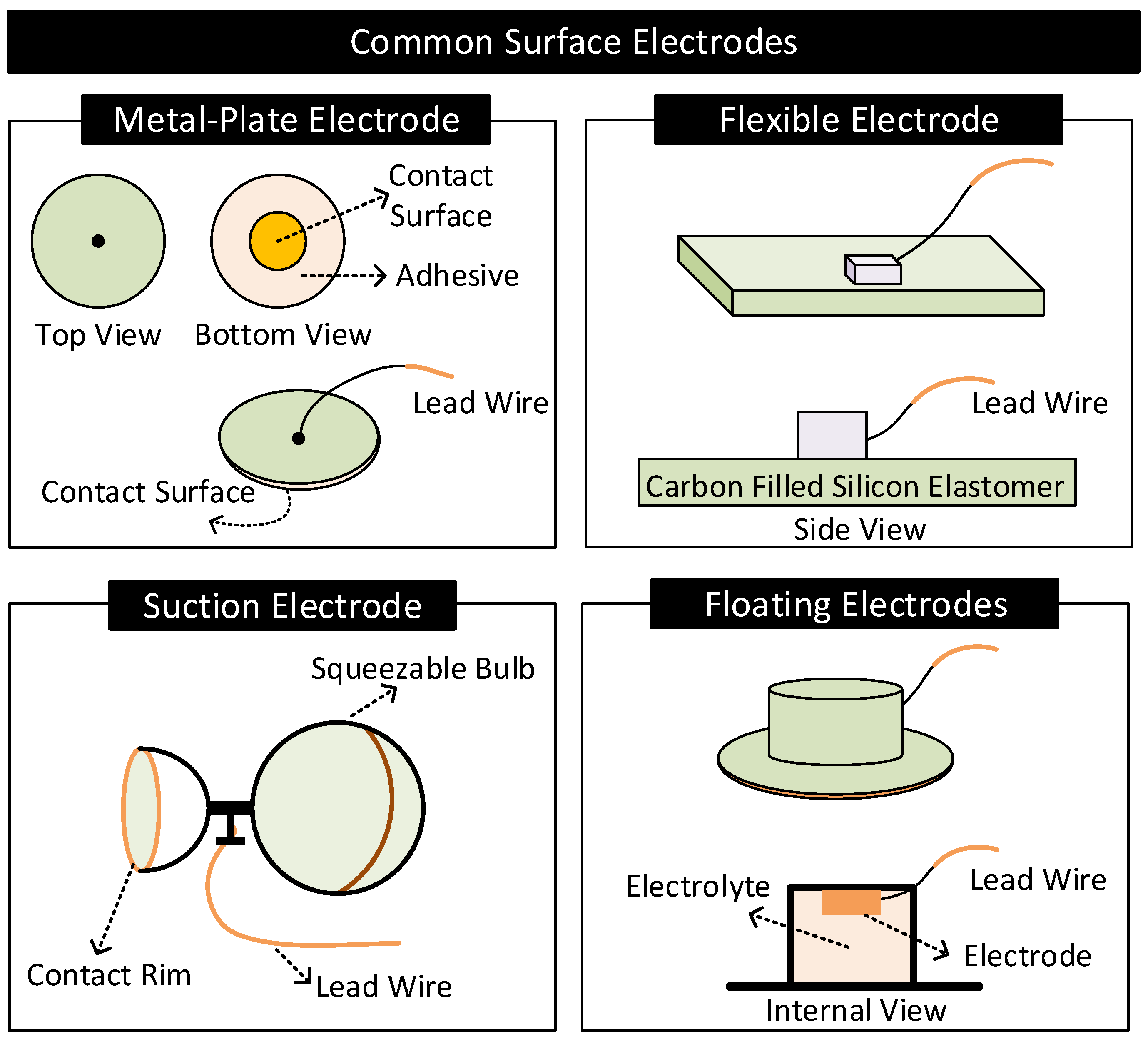
- Metal-Plate Electrodes: They consist of a metal plate with an attached lead wire [1,57]. They are fixed on the body with or without electrolytes using adhesives or surgical tapes. Metal-plate electrodes are highly prone to motion artifacts. The subject’s movements can cause electrolyte concentration near the electrode to vary, causing variations in the electrode’s half-cell potential.
- Flexible Electrodes: They consist of a thin metal foil of thickness less than a micrometer mounted on a flexible substrate such as polyester or polyimide [2]. Due to the flexibility, the electrode easily conforms to the shape of the subject’s body, improving contact impedance and enhancing the subject’s experience of wearing the electrode. Additionally, the thin metal foil allows X-rays to pass through, allowing X-ray diagnosis without removing electrodes [2].
- Suction Electrodes: They are primarily used for diagnostic purposes as they can be easily attached and transferred from one location to another on the body. They make contact with the skin surface via a suction cup. Suction electrodes are relatively large compared to metal-plate electrodes; however, the body contacts only the rim of the suction cup, resulting in high contact impedance [1,2].
- Floating Electrodes: They consist of an electrode recessed in a cup of electrolyte gel. The cup is then attached to the body using a medical-grade adhesive. Floating electrodes are less prone to motion artifacts. When the body moves, the concentration of the electrolyte near the skin may vary. However, the electrolyte near the electrode is less affected as the electrode resides at a distance away from the skin, ensuring relatively stable half-cell potential [1,2].
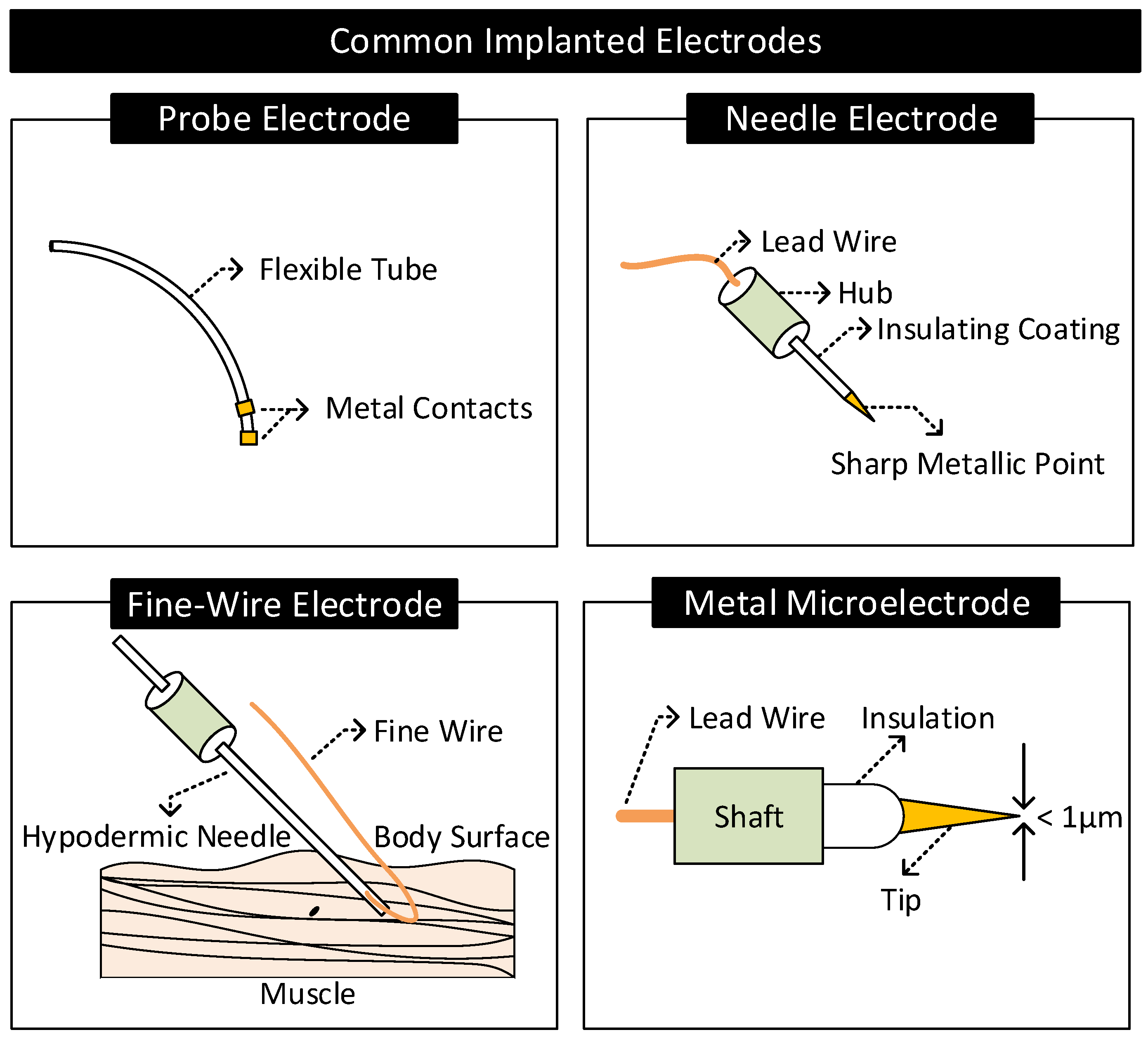
2.4.2. Implanted Electrodes
- Probe Electrodes: These electrodes are made of long flexible tubes with a metallic tip at the end. They are inserted in body cavities that occur naturally or through surgical procedures. The metal tip is connected to the external circuitry through a lead wire running internally through the tube [2].
- Needle Electrodes: They are rigid electrodes typically made of stainless steel with a sharp-pointed edge of diameter 100–500 m [1,2,73]. Their design allows it to penetrate the top layers of the skin and make contact with the internal body tissues. Needle electrodes are often coated with an insulating material throughout their surface except at the tip for localized signal transfers.
- Fine-Wire Electrodes: They are flexible single-strand wires with a diameter of 25–125 m [1,2]. Like needle electrodes, they are also coated with an insulating material throughout its surface except at the tip for localized signal transfers. Often, a hypodermic needle inserts these electrodes into the region of interest. The tip of these electrodes is bent in the form of a hook to prevent it from moving upon insertion.
- Microelectrodes: They are electrodes with a tip diameter of less than a micrometer used for transferring signals at the cellular level [74] (e.g., to sense the neural activity in the brain). They are of different types: glass micropipettes, metal microelectrodes, and solid-state microprobes [75]. Metal microelectrodes are similar in construction to a needle electrode but with their tips tapered to micrometer widths through an electrochemical etching process.
3. Biopotential Sensing
3.1. Background
3.2. Challenges
3.2.1. Contact Potential
- Restricted Amplification: The contact potential of several electrode–electrolyte interfaces used in biopotential sensing is in the order of hundreds of millivolts [2]. In contrast, biopotential signals are in the order of tens of microvolts to a couple of millivolts [87]. For the biopotential sensing circuit, the contact potential appears as a large DC offset in series to the feeble biopotential signal. Here, the large DC offset poses the following problem. It limits the amount of amplification the front-end amplifiers of the measurement circuit can apply to increase the SNR of the biopotential signal measurements. To give an example, if the contact potential is 200 mV and the biopotential signal is 1 mV, then for a 5 V amplifier that supports rail-to-rail operation, the maximum possible gain that can be set is lower than ≈5 V/200 mV = 25. Here, gain settings higher than 25 will result in the output of the front-end amplifier to stuck at rail voltage distorting or eliminating biopotential signals. Note that if the DC offset is zero in the previous case, the gain setting of the front-end amplifier could have been as high as ≈5000.
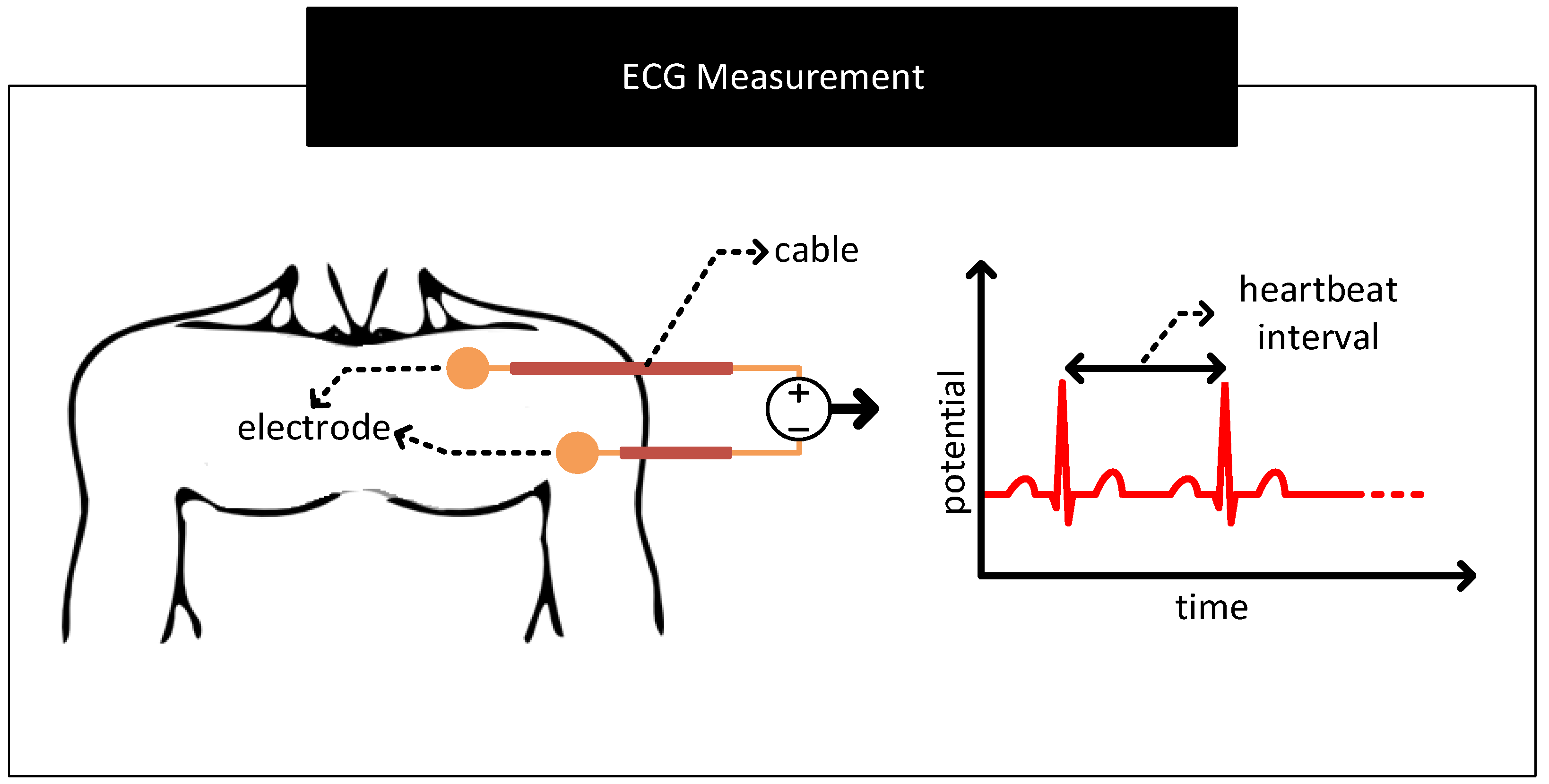
- Baseline Wander: In the scheme to measure ECG in Figure 13, if the contact potentials at each electrode to skin contact are the same, then the resulting output which is derived from the difference block will not contain any artefacts of contact potentials. However, in practice, there exist mismatches in the contact potentials. The mismatch is often due to electrode/electrolyte manufacturing variations or due to skin chemistry being different at different parts of the body. The mismatch will now be seen along with the biopotential signal at the output of the difference block. If the mismatch in contact potentials remains the same, then it can be removed from the output by subtracting the output with a constant. However, in practice, the electrode contact potentials and their mismatches varies slowly over time, making it challenging to separate it from biopotential signals having significant low-frequency components. The aforementioned mismatches result in biopotential signal measurements to ride over a baseline that slowly wanders over time, giving the problem its name baseline wander. Figure 14 shows representative ECG waveforms in the presence and absence of mismatches in contact potentials.
- Motion Artefact: The movement of the human body during respiration, while talking or while being stressed, can cause the skin near the electrodes to stretch/deform, resulting in time-varying mismatches in contact potentials [88,89]. These mismatches act as a source of noise and interference in biopotential signal measurements known as motion artefacts. Motion artefacts cause low-frequency fluctuations in the baseline of the ECG measurements which are often difficult to remove because their frequencies overlap with the frequency components of the biopotential signals.
3.2.2. Contact Impedance
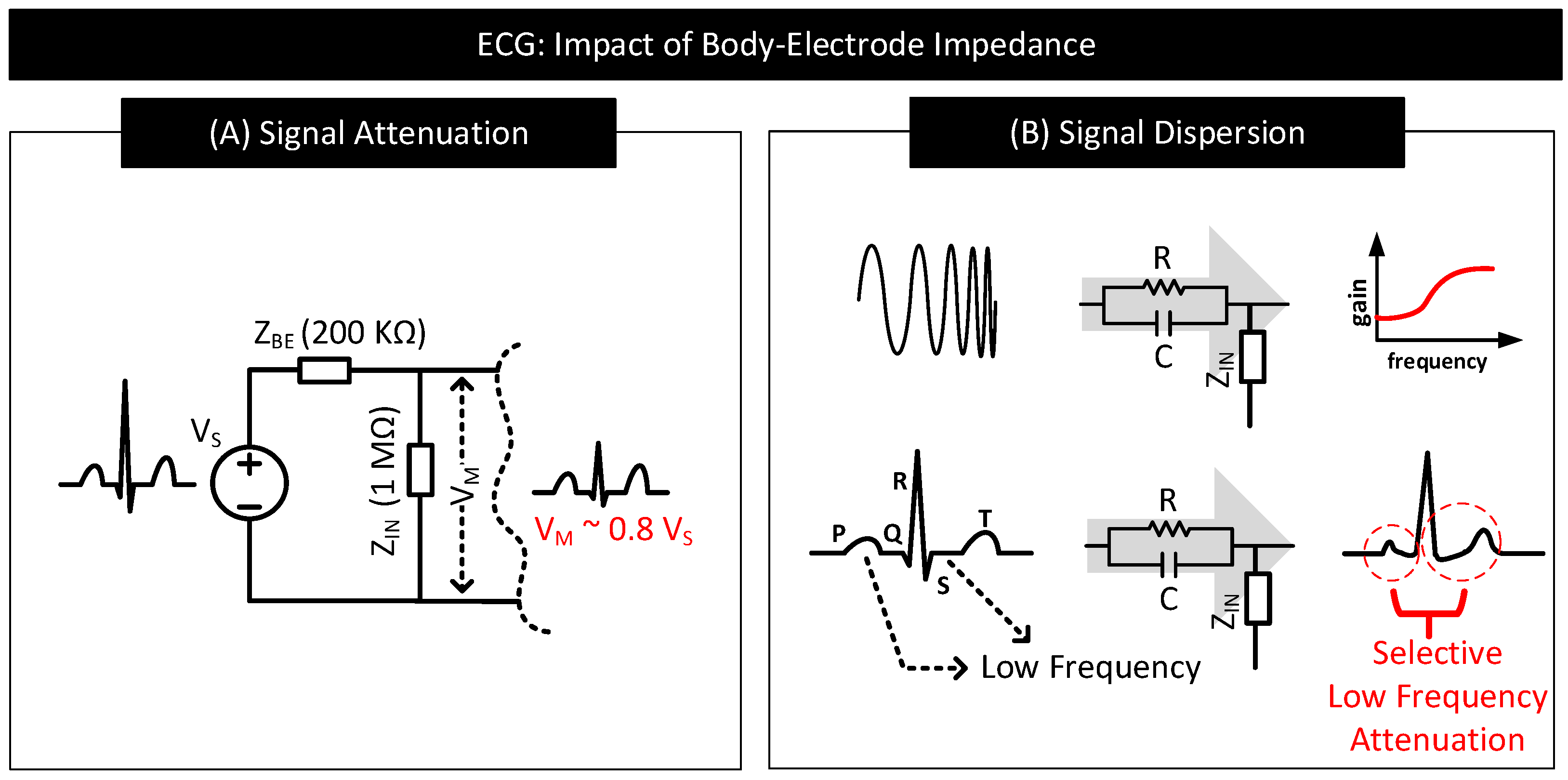
- Signal Attenuation: When measuring the biopotential signal, contact impedance acts as the source impedance of the biopotential source. If the contact impedance is significant in comparison to the input impedance of the measurement circuit, then a considerable part of the signal will be attenuated. Figure 15A depicts this behaviour.
- Signal Dispersion: Signal attenuation per se is not a big problem if all the frequency components constituting the biopotential signal experience attenuation by the same amount. Mere amplification of the signal can reverse the effect of attenuation. However, in practice, low-frequency components of biopotential signals suffer higher degrees of attenuation due to the capacitive component of the contact impedance offering higher resistance to low-frequency signal components. The result is dispersion in biopotential signal readings that are difficult to fix. Figure 15B shows a sample contact impedance consisting of a resistor in parallel to a capacitor, its bode plot, and how it affects the low-frequency, P, S, and T regions of ECG.
3.2.3. External Interferences
4. Human Body Communication
4.1. Background
4.2. Challenges
4.2.1. Contact Impedance
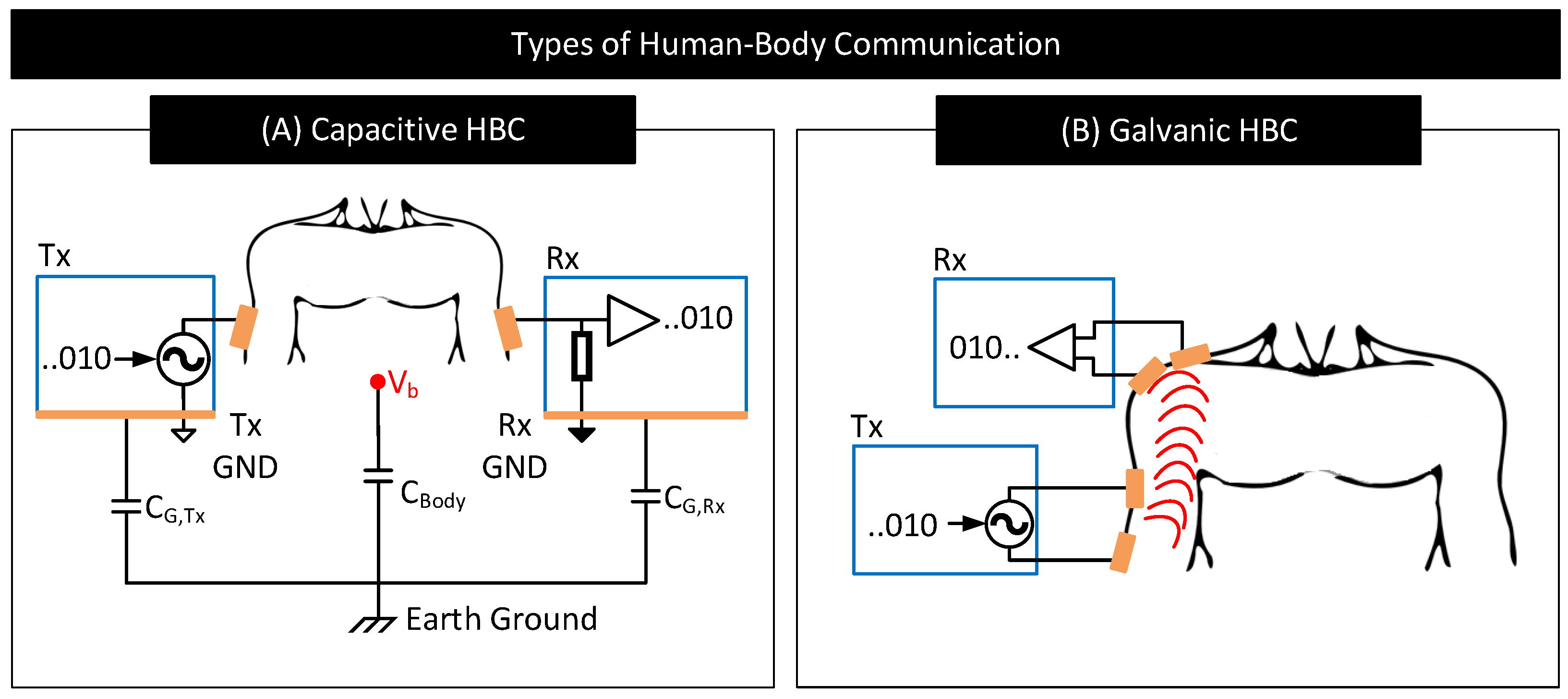
- Capacitive HBC: Figure 18 shows a simplified schematic and circuit model of capacitive HBC with body–electrode contact impedances [6]. At higher operating frequencies, when the contact impedances are higher in comparison to and , at the transmitter side, only a part of the potential applied to the transmit electrode, , drops across the bulk of the body () which results in a lower . Further, due to higher contact impedance at the receiver side, the measured body potential at the receiver will be lesser than the actuals (i.e., <). In short, at higher operating frequencies, the interface contact impedance deteriorates the received signal strength. At lower operating frequencies (e.g., <1 MHz), the contact impedances in Figure 18 are often negligible in comparison to parasitics , and [98] and thus their impact on the received signal is minimal provided that the signal termination impedance is high. However, if the termination impedance is lower or comparable to contact impedance, considerable signal attenuation can occur even at lower operating frequencies [106].
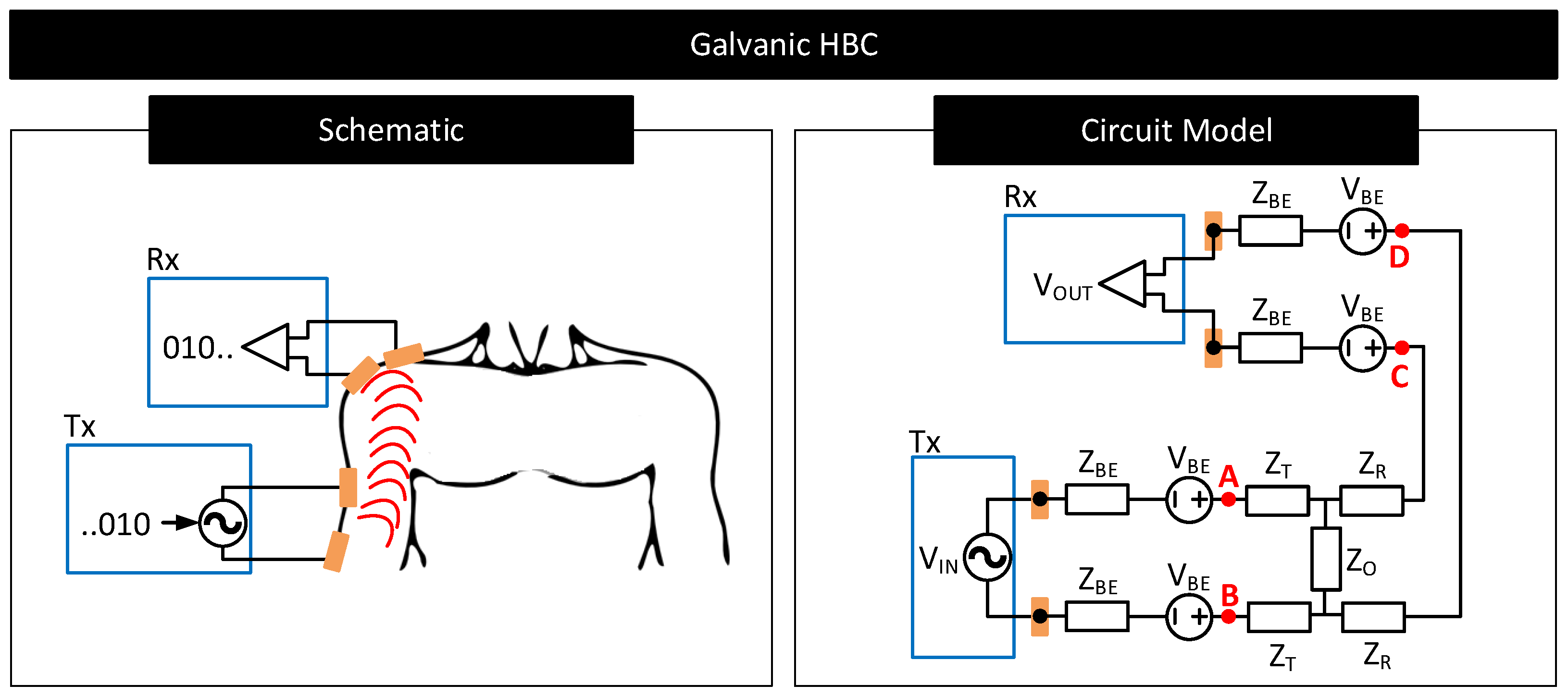
- Galvanic HBC: Figure 19 shows the circuit model of galvanic HBC with body–electrode impedances as in [102]. Here, the fields generated in the conductive core of the body are dependent on the potential difference across A and B, i.e., . In the presence of contact impedance, only a part of the differential voltage applied across the transmitter electrode pair drops across A and B, i.e., <, which lowers the strength of the field generated in the body. Consequently, the potential generated across C and D, i.e., , and thus , reduces. In short, the received signal strength deteriorates in the presence of contact impedances.
4.2.2. Electrode Configuration
- Capacitive HBC:
- (a)
- Ground Electrodes: The size, shape, and placement of the floating ground electrodes of the transmitter and receiver in capacitive HBC affect their parasitic capacitances to the earth’s ground ( and ) and the receiver-side load capacitance () in Figure 18 [108,109]. Note that the received signal strength in capacitive HBC depends on these capacitances. Higher values of and and lower values of are desired for reducing HBC channel loss [5,98]. When the size of the ground electrodes is large or when they are close to the earth’s ground, and increase. However, increasing the size of electrodes results in bulky transmitter and receiver designs, making it difficult for the user to move freely. Further, many applications fix the site to place the transmitter and receiver; meaning, the options to place the ground electrodes close to the earth’s ground are limited. Moreover, increasing the size of the receiver-side ground electrode increases the load capacitance, , which reduces the received signal strength.The distance between the transmitter and the receiver ground electrodes and their relative angle of orientations also matter [97,108]. At lower distances and when the ground electrodes of the transmitter and receiver are parallel, the inter-device coupling capacitor, , is higher (See Figure 18). Higher results in a part of the signal returning through instead of through the relatively high impedance path and improving the channel quality [107]. Again, applications generally dictate the locations and orientations for the transmitter and receiver ground electrodes; meaning, the options to increase is limited.
- (b)
- Signal Electrodes: In capacitive HBC, signal electrodes are placed in direct contact with the body. The size of these electrodes dictates their contact impedance. For instance, an electrode with a large surface area increases the capacitive component of the contact impedance [6]. A higher capacitive component is preferred when operating frequencies are high, as it lowers the impedance of the forward signal path. However, having large-sized signal electrodes can make the transmitter and receiver designs bulky and increase channel loss due to higher at the receiver side.
- Galvanic HBC:
- Ideal Galvanic Behaviour: Here, both the pair of electrodes at the transmitter and receiver sides are signal electrodes. They are placed in direct contact with the body (see Figure 17) and exited with signals often at low frequencies to ensure that the signal forward and return paths are well defined and confined within the body (see Section 4). For this reason, and since there exist no floating ground electrodes, the performance of galvanic HBC at low frequencies is independent of the position of the electrodes to the earth’s ground, the parasitics of the electrodes to the earth’s ground, or the shape of the electrode [3,102]. However, the distance between the transmitter and receiver electrodes and their orientation matters. The received signal strength drastically drops with increasing transmitter-receiver electrode distance [102,110]. Received signal strength also drops if the transmitter-side and receiver-side electrodes are facing away from each other.
- Non-Ideal Galvanic Behaviour: At significantly longer distances between the transmitter and the receiver, i.e., when the transmitter to the receiver distance is significant to the distance of separation of transmitter and receiver electrode pair, signal losses tend to stabilize. In this realm, the galvanic HBC channel behaves similar to capacitive HBC, with losses dependent on the electrodes’ parasitics, geometry, and configuration. Authors in [102] attribute this behavior to the presence of a non-zero common mode in the transmitted signal owing to the mismatches in the transmitter electrode parasitics and the subsequent common mode to differential-mode conversion at the receiver side.
4.2.3. External Interferences
- Capacitive HBC: Since the signal transfer in capacitive HBC is via modulating body potential, , any external source capable of modulating acts as a source of interference. AC mains or nearby high-frequency switch-mode supplies are likely sources of interference [71] as they can modulate (see Section 3.2.3). Further, since the signal return path in capacitive HBC is through the parasitic capacitances , , and , any source that can vary these capacitances can also act as a likely source for interference. Possible such sources include the presence of nearby wall wires, conductive objects, water, or humans [99]. The presence of grounded metallic objects in contact with the body is a strong source of interference. Such a condition drives to earth’s potential. When that happens, the transmitter electrodes cannot modulate and thus cannot transmit signals.
- Galvanic HBC: As mentioned in Section 4.1, at low frequencies, signal leakage to the environment is minimal in galvanic HBC. For this reason, galvanic HBC at low frequencies is less prone to external interferences that affect electrode parasitics. However, external interferences such as AC mains or nearby SMPS can vary the potential of the body and the local ground potential of the receiver circuit similarly to Figure 16. These potential variations may appear as common-mode noise to the receiver-side measurement circuit and lower the SNR of the measured signal.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Button, V.L.D.S.N. (Ed.) Electrodes for Biopotential Recording and Tissue Stimulation. In Principles of Measurement and Transduction of Biomedical Variables; Academic Press: Oxford, UK, 2015; pp. 25–76. [Google Scholar] [CrossRef]
- Neuman, M.R. Biopotential electrodes. Med. Instrum. Appl. Des. 1998, 4, 189–240. [Google Scholar]
- Zhao, J.F.; Chen, X.M.; Liang, B.D.; Chen, Q.X. A review on human body communication: Signal propagation model, communication performance, and experimental Issues. Wirel. Commun. Mob. Comput. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Naranjo-Hernández, D.; Callejón-Leblic, A.; Lučev Vasić, Ž.; Seyedi, M.; Gao, Y.M. Past results, present trends, and future challenges in intrabody communication. Wirel. Commun. Mob. Comput. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Maity, S.; Das, D.; Chatterjee, B.; Sen, S. Characterization and classification of human body channel as a function of excitation and termination modalities. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3754–3757. [Google Scholar]
- Maity, S.; He, M.; Nath, M.; Das, D.; Chatterjee, B.; Sen, S. Bio-physical modeling, characterization, and optimization of electro-quasistatic human body communication. IEEE Trans. Biomed. Eng. 2018, 66, 1791–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, S.; Chatterjee, B.; Chang, G.; Sen, S. BodyWire: A 6.3-pJ/b 30-Mb/s- 30-dB SIR-tolerant broadband interference-robust human body communication transceiver using time domain interference rejection. IEEE J. Solid State Circuits 2019, 54, 2892–2906. [Google Scholar] [CrossRef]
- Maity, S.; Das, D.; Sen, S. Wearable health monitoring using capacitive voltage-mode human body communication. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 1–4. [Google Scholar]
- Lucev, Ž.; Krois, I.; Cifrek, M. A capacitive intrabody communication channel from 100 kHz to 100 MHz. IEEE Trans. Instrum. Meas. 2012, 61, 3280–3289. [Google Scholar] [CrossRef]
- Park, J.; Garudadri, H.; Mercier, P.P. Channel modeling of miniaturized battery-powered capacitive human body communication systems. IEEE Trans. Biomed. Eng. 2016, 64, 452–462. [Google Scholar]
- Cho, N.; Yoo, J.; Song, S.J.; Lee, J.; Jeon, S.; Yoo, H.J. The human body characteristics as a signal transmission medium for intrabody communication. IEEE Trans. Microw. Theory Tech. 2007, 55, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Hao, Q.; Zhang, K. Review of the modeling, simulation and implement of intra-body communication. Def. Technol. 2013, 9, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Callejon, M.A.; Naranjo-Hernandez, D.; Reina-Tosina, J.; Roa, L.M. A comprehensive study into intrabody communication measurements. IEEE Trans. Instrum. Meas. 2013, 62, 2446–2455. [Google Scholar] [CrossRef]
- Das, D.; Maity, S.; Chatterjee, B.; Sen, S. Enabling covert body area network using electro-quasistatic human body communication. Sci. Rep. 2019, 9, 4160. [Google Scholar] [CrossRef]
- Fahier, N.; Fang, W.C. An HBC-based continuous bio-potential system monitoring using 30MHz OOK modulation. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Zio Monitoring. Available online: https://www.irhythmtech.com/providers/zio-service/zio-monitors (accessed on 14 July 2021).
- Home—VitalConnect. Available online: https://vitalconnect.com/ (accessed on 14 July 2021).
- Kardia Mobile EKG|Omron Healthcare. Available online: https://omronhealthcare.com/products/kardia-mobile-ekg-ac009uac/ (accessed on 14 July 2021).
- Portable EKG Monitor—Instant EKG Analysis with APP—Wellue Health. Available online: https://getwellue.com/products/duoek-hand-held-wearable-ekg-tracker (accessed on 14 July 2021).
- BardyDx. Available online: https://www.bardydx.com/ (accessed on 14 July 2021).
- MAX-ECGMONITOR Wearable ECG and Heart Monitor Evaluation and Development Platform—Maxim Integrated. Available online: https://www.maximintegrated.com/en/products/interface/sensor-interface/MAX-ECGMONITOR.html (accessed on 14 July 2021).
- Maity, S.; Modak, N.; Yang, D.; Avlani, S.; Nath, M.; Danial, J.; Das, D.; Mehrotra, P.; Sen, S. A 415 nw physically and mathematically secure electro-quasistatic hbc node in 65nm cmos for authentication and medical applications. In Proceedings of the 2020 IEEE Custom Integrated Circuits Conference (CICC), Boston, MA, USA, 22–25 March 2020; pp. 1–4. [Google Scholar]
- Chen, C.H.; Mak, P.I.; Zhang, T.T.; Vai, M.I.; Mak, P.U.; Pun, S.H.; Wan, F.; Martins, R. A 2.4 Hz-to-10 kHz-tunable biopotential filter using a novel capacitor multiplier. In Proceedings of the 2009 Asia Pacific Conference on Postgraduate Research in Microelectronics & Electronics (PrimeAsia), Shanghai, China, 19–21 January 2009; pp. 372–375. [Google Scholar]
- Harrison, R.R. A versatile integrated circuit for the acquisition of biopotentials. In Proceedings of the 2007 IEEE Custom Integrated Circuits Conference, San Jose, CA, USA, 16–19 September 2007; pp. 115–122. [Google Scholar]
- Anderson, J.; DiCarlo, S.E. “Virtual” experiment for understanding the electrocardiogram and the mean electrical axis. Adv. Physiol. Educ. 2000, 23, 1–17. [Google Scholar] [CrossRef]
- Milner-Brown, H.; Stein, R. The relation between the surface electromyogram and muscular force. J. Physiol. 1975, 246, 549–569. [Google Scholar] [CrossRef]
- Subha, D.P.; Joseph, P.K.; Acharya, R.; Lim, C.M. EEG signal analysis: A survey. J. Med. Syst. 2010, 34, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Maling, N.; McIntyre, C. Local Field Potential Analysis for Closed-Loop Neuromodulation. In Closed Loop Neuroscience; Academic Press: London, UK, 2016; pp. 67–80. [Google Scholar]
- Biesheuvel, P.; Porada, S.; Dykstra, J. The difference between Faradaic and non-Faradaic electrode processes. arXiv 2018, arXiv:1809.02930. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233. [Google Scholar] [CrossRef]
- Xiong, F.; Chen, D.; Chen, Z.; Jin, C.; Dai, S. Impedance characteristics of the skin-electrode interface of dry textile electrodes for wearable electrocardiogram. In Advances in Body Area Networks I; Springer: Berlin/Heidelberg, Germany, 2019; pp. 343–356. [Google Scholar]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Wang, C.; Zhao, R.; Du, L.; Fang, Z.; Guo, X.; Zhao, Z. Review of stratum corneum impedance measurement in non-invasive penetration application. Biosensors 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulds, I.; Barker, A. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, H.; Chen, K.; Zhang, Y.; Zhang, Y.; Liu, Y.; Zhu, C.; Ouyang, S.C.; Kong, G.W.; Yu, C.; et al. Epidermal impedance sensing sheets for precision hydration assessment and spatial mapping. IEEE Trans. Biomed. Eng. 2013, 60, 2848–2857. [Google Scholar] [CrossRef]
- Clar, E.; Her, C.; Sturelle, C. Skin impedance and moisturization. J. Soc. Cosmet. Chem. 1975, 26, 337–353. [Google Scholar]
- Yokus, M.A.; Jur, J.S. Fabric-based wearable dry electrodes for body surface biopotential recording. IEEE Trans. Biomed. Eng. 2015, 63, 423–430. [Google Scholar] [CrossRef]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Cömert, A.; Honkala, M.; Hyttinen, J. Effect of pressure and padding on motion artifact of textile electrodes. Biomed. Eng. Online 2013, 12, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Li, Z.; Chen, J. A flexible dry electrode based on APTES-anchored PDMS substrate for portable ECG acquisition system. Microsyst. Technol. 2016, 22, 2027–2034. [Google Scholar] [CrossRef]
- Baek, J.Y.; An, J.H.; Choi, J.M.; Park, K.S.; Lee, S.H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators A Phys. 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Nanomaterial-enabled dry electrodes for electrophysiological sensing: A review. JOM 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Cheng, H.; Yeo, W.; Huang, X.; Liu, Y.; Zhang, Y.; Huang, Y.; Rogers, J. Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv. Healthc. Mater. 2014, 3, 642–648. [Google Scholar] [CrossRef]
- Wang, F.; Li, G.; Chen, J.; Duan, Y.; Zhang, D. Novel semi-dry electrodes for brain–computer interface applications. J. Neural Eng. 2016, 13, 046021. [Google Scholar] [CrossRef]
- Li, G.L.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Li, M.; Duan, Y.Y. Towards real-life EEG applications: Novel superporous hydrogel-based semi-dry EEG electrodes enabling automatically ‘charge–discharge’ electrolyte. J. Neural Eng. 2021, 18, 046016. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators B Chem. 2016, 237, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 2018, 277, 250–260. [Google Scholar] [CrossRef]
- The Wet-EEG Cap: Semi-Dry, Saline & Gel EEG Caps|Bitbrain. Available online: https://www.bitbrain.com/blog/wet-eeg-cap (accessed on 8 May 2021).
- Ha, S.; Kim, C.; Chi, Y.M.; Akinin, A.; Maier, C.; Ueno, A.; Cauwenberghs, G. Integrated circuits and electrode interfaces for noninvasive physiological monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1522–1537. [Google Scholar]
- Rai, P.; Oh, S.; Shyamkumar, P.; Ramasamy, M.; Harbaugh, R.E.; Varadan, V.K. Nano-bio-textile sensors with mobile wireless platform for wearable health monitoring of neurological and cardiovascular disorders. J. Electrochem. Soc. 2013, 161, B3116. [Google Scholar] [CrossRef]
- Assambo, C.; Baba, A.; Dozio, R.; Burke, M. Determination of the parameters of the skin-electrode impedance model for ECG measurement. In Proceedings of the 6th WSEAS International Conference on Electronics, Hardware, Wireless and Optical Communications, Corfu Island, Greece, 16–19 February 2007; pp. 90–95. [Google Scholar]
- Kusche, R.; Kaufmann, S.; Ryschka, M. Dry electrodes for bioimpedance measurements—Design, characterization and comparison. Biomed. Phys. Eng. Express 2018, 5, 015001. [Google Scholar] [CrossRef] [Green Version]
- Liao, L.D.; Wang, I.J.; Chen, S.F.; Chang, J.Y.; Lin, C.T. Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Mohan, R.; Van Helleputte, N.; Mitra, S. Design and optimization of ICs for wearable EEG sensors. In CMOS Circuits for Biological Sensing and Processing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 163–185. [Google Scholar]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- McAdams, E.; Jossinet, J.; Lackermeier, A.; Risacher, F. Factors affecting electrode-gel-skin interface impedance in electrical impedance tomography. Med. Biol. Eng. Comput. 1996, 34, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Oster, C.D. Proper Skin Prep Helps Ensure ECG Trace Quality. Available online: https://multimedia.3m.com/mws/media/358372O/proper-skin-prep-ecg-trace-quality-white-paper.pdf (accessed on 6 August 2021).
- Chen, W.C.; Guido, K.; Kiourti, A. Passive Impedance Matching for Implanted Brain–Electrode Interfaces. IEEE J. Electromagn. Microw. Med. Biol. 2019, 3, 233–239. [Google Scholar] [CrossRef]
- Webster, J.G. Reducing motion artifacts and interference in biopotential recording. IEEE Trans. Biomed. Eng. 1984, 12, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Tankisi, H.; Burke, D.; Cui, L.; de Carvalho, M.; Kuwabara, S.; Nandedkar, S.D.; Rutkove, S.; Stålberg, E.; van Putten, M.J.; Fuglsang-Frederiksen, A. Standards of instrumentation of EMG. Clin. Neurophysiol. 2020, 131, 243–258. [Google Scholar] [CrossRef]
- Seok, D.; Lee, S.; Kim, M.; Cho, J.; Kim, C. Motion artifact removal techniques for wearable EEG and PPG sensor systems. Front. Electron. 2021, 2, 4. [Google Scholar] [CrossRef]
- Buxi, D.; Kim, S.; Van Helleputte, N.; Altini, M.; Wijsman, J.; Yazicioglu, R.F.; Penders, J.; Van Hoof, C. Correlation between electrode-tissue impedance and motion artifact in biopotential recordings. IEEE Sens. J. 2012, 12, 3373–3383. [Google Scholar] [CrossRef]
- de Talhouet, H.; Webster, J.G. The origin of skin-stretch-caused motion artifacts under electrodes. Physiol. Meas. 1996, 17, 81. [Google Scholar] [CrossRef]
- Burbank, D.P.; Webster, J.G. Reducing skin potential motion artefact by skin abrasion. Med. Biol. Eng. Comput. 1978, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Simakov, A.; Webster, J. Motion artifact from electrodes and cables. IJECE 2010, 9, 139–143. [Google Scholar]
- Triboelectric Noise in Medical Cables and Wires|Experience Molex. Available online: https://experience.molex.com/triboelectric-noise-in-medical-cables-and-wires/ (accessed on 18 July 2021).
- Yang, D.; Mehrotra, P.; Weigand, S.; Sen, S. In-The-Wild Interference Characterization and Modelling for Electro-Quasistatic-HBC with Miniaturized Wearables. IEEE Trans. Biomed. Eng. 2021. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Xia, Y.; Wu, Y.; Tian, Y.; Liu, J.; Chen, D.; He, Q. Towards emerging EEG applications: A novel printable flexible Ag/AgCl dry electrode array for robust recording of EEG signals at forehead sites. J. Neural Eng. 2020, 17, 026001. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, T.; Andary, M.; Dumitru, D. Electrodiagnostic medicine. In Braddom’s Physical Medicine and Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 115–152. [Google Scholar]
- Zeuthen, T. Microelectrodes. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 25–32. [Google Scholar] [CrossRef]
- Mendelson, Y. Chapter 10—Biomedical Sensors. In Introduction to Biomedical Engineering, 3rd ed.; Enderle, J.D., Bronzino, J.D., Eds.; Biomedical Engineering; Academic Press: Boston, FL, USA, 2012; pp. 609–666. [Google Scholar] [CrossRef]
- Bain, L. Materials for Implantable Electrodes. MRS Bull. 1986, 11, 23–25. [Google Scholar] [CrossRef] [Green Version]
- McCreery, D.; Agnew, W.; Yuen, T.; Bullara, L. Comparison of neural damage induced by electrical stimulation with faradaic and capacitor electrodes. Ann. Biomed. Eng. 1988, 16, 463–481. [Google Scholar] [CrossRef]
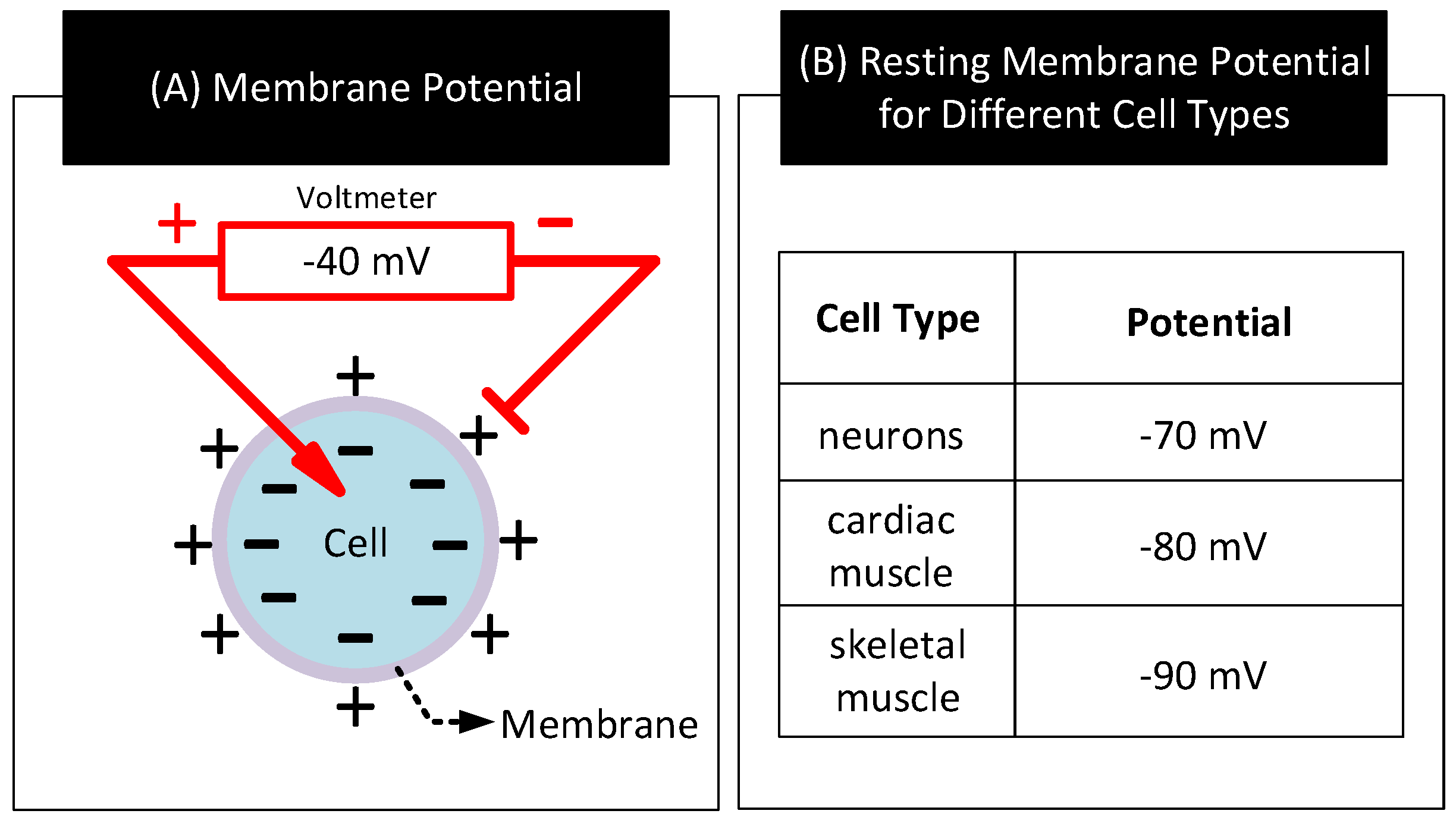
- Wright, S.H. Generation of resting membrane potential. Adv. Physiol. Educ. 2004, 28, 139–142. [Google Scholar] [CrossRef]
- Hopkins, P.M. Skeletal muscle physiology. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- PASCO CI-6539A EKG SENSOR User Manual. Available online: https://manualmachine.com/pasco/ci6539aekgsensor/1700487-user-manual/ (accessed on 16 August 2021).
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef]
- Bansal, A.; Joshi, R. Portable out-of-hospital electrocardiography: A review of current technologies. J. Arrhythm. 2018, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.A.; Kochhäuser, S.; Duning, T.; Reinke, F.; Pott, C.; Dechering, D.G.; Eckardt, L.; Ringelstein, E.B. Occult atrial fibrillation in cryptogenic stroke: Detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013, 44, 1449–1452. [Google Scholar] [CrossRef] [Green Version]
- Wyngaarden, J.B.; Smith, L.H., Jr. Cecil Textbook of Medicine; W B Saunders: Philadelphia, PA, USA, 1982. [Google Scholar]
- Gupta, M.; Kurmi, M.P.; Sharma, B.R.; Chen, L.; Shahi, R.; Jian, S. Clinical significance of ST segment depression in lead aVR to predict culprit artery in an acute inferior wall myocardial infarction. Nepal. Heart J. 2015, 12, 5–9. [Google Scholar] [CrossRef]
- Coppola, G.; Carità, P.; Corrado, E.; Borrelli, A.; Rotolo, A.; Guglielmo, M.; Nugara, C.; Ajello, L.; Santomauro, M.; Novo, S.; et al. ST segment elevations: Always a marker of acute myocardial infarction? Indian Heart J. 2013, 65, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Samuel, O.W.; Liu, X.; Wang, X.; Idowu, P.O.; Li, P.; Chen, F.; Zhu, M.; Geng, Y.; Wu, F.; et al. Effective biopotential signal acquisition: Comparison of different shielded drive technologies. Appl. Sci. 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Physio Control Inc. Minimizing ECG Artifact. In Physio-Control; Physio Control Inc.: Kalamazoo, MI, USA, 2015. [Google Scholar]
- An, X.; Stylios, G.K. Comparison of motion artefact reduction methods and the implementation of adaptive motion artefact reduction in wearable electrocardiogram monitoring. Sensors 2020, 20, 1468. [Google Scholar] [CrossRef] [Green Version]
- Babusiak, B.; Borik, S.; Smondrk, M. Two-Electrode ECG for Ambulatory Monitoring with Minimal Hardware Complexity. Sensors 2020, 20, 2386. [Google Scholar] [CrossRef] [PubMed]
- Acharya, V. Improving Common-Mode Rejection Using the Right-Leg Drive Amplifier; Texas Instruments: Dallas, TX, USA, 2011. [Google Scholar]
- Yamamoto, Y. Impedance balancing analysis for power-line interference elimination in ECG signal. In Proceedings of the IMTC/98 Conference Proceedings, IEEE Instrumentation and Measurement Technology Conference, Where Instrumentation is Going (Cat. No. 98CH36222), St. Paul, MN, USA, 18–21 May 1998; Volume 1, pp. 235–238. [Google Scholar]
- Becchetti, C.; Neri, A. Medical Instrument Design and Development: From Requirements to Market Placements; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Nash, E.; Devices, A. Common Mode and Instrumentation Amplifiers; Analog Devices: Cambridge, MA, USA, 1998. [Google Scholar]
- Dobrev, D.; Daskalov, I. Two-electrode biopotential amplifier with current-driven inputs. Med. Biol. Eng. Comput. 2002, 40, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Koo, N.; Cho, S. A 24.8-μW Biopotential Amplifier Tolerant to 15-VPP Common-Mode Interference for Two-Electrode ECG Recording in 180-nm CMOS. IEEE J. Solid-State Circuits 2020, 56, 591–600. [Google Scholar] [CrossRef]
- Pereira, M.D.; Alvarez-Botero, G.A.; de Sousa, F.R. Characterization and modeling of the capacitive HBC channel. IEEE Trans. Instrum. Meas. 2015, 64, 2626–2635. [Google Scholar] [CrossRef]
- Nath, M.; Maity, S.; Sen, S. Toward understanding the return path capacitance in capacitive human body communication. IEEE Trans. Circuits Syst. II Express Briefs 2019, 67, 1879–1883. [Google Scholar] [CrossRef]
- Nath, M.; Maity, S.; Avlani, S.; Weigand, S.; Sen, S. Inter-body coupling in electro-quasistatic human body communication: Theory and analysis of security and interference properties. Sci. Rep. 2021, 11, 4378. [Google Scholar] [CrossRef]
- Wegmüller, M.S. Intra-Body Communication for Biomedical Sensor Networks. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2007. [Google Scholar]
- Avlani, S.; Nath, M.; Maity, S.; Sen, S. A 100KHz-1GHz termination-dependent human body communication channel measurement using miniaturized wearable devices. In Proceedings of the 2020 Design, Automation & Test in Europe Conference & Exhibition (DATE), Grenoble, France, 9–13 March 2020; pp. 650–653. [Google Scholar]
- Modak, N.; Nath, M.; Chatterjee, B.; Maity, S.; Sen, S. Bio-Physical Modeling of Galvanic Human Body Communication in Electro-Quasistatic Regime. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wegmueller, M.S.; Oberle, M.; Felber, N.; Kuster, N.; Fichtner, W. Signal transmission by galvanic coupling through the human body. IEEE Trans. Instrum. Meas. 2009, 59, 963–969. [Google Scholar] [CrossRef]
- Seyed Mazloum, N. Body-Coupled Communications-Experimental Characterization, Channel Modeling and Physical Layer Design. Master’s Thesis, Chalmers University of Technology, Göteborg, Sweden, 2008. [Google Scholar]
- Bae, J.; Cho, H.; Song, K.; Lee, H.; Yoo, H.J. The signal transmission mechanism on the surface of human body for body channel communication. IEEE Trans. Microw. Theory Tech. 2012, 60, 582–593. [Google Scholar] [CrossRef]
- Sen, S. Wearable Health Monitoring System and Method Using Human Body Communication. U.S. Patent 10,998,925, 4 May 2021. [Google Scholar]
- Datta, A.; Nath, M.; Yang, D.; Sen, S. Advanced biophysical model to capture channel variability for eqs capacitive hbc. IEEE Trans. Biomed. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, H.; Zhao, B. An investigation on ground electrodes of capacitive coupling human body communication. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Hnyk, P.; Kvarda, L.; Vojtech, L.; Neruda, M.; Zitta, T. Electrode Shapes and Frequency Band Analysis for Human Body Communication. In Proceedings of the 2018 18th International Conference on Mechatronics-Mechatronika (ME), Brno, Czech Republic, 5–7 December 2018; pp. 1–6. [Google Scholar]
- Gao, Y.M.; Wu, Z.M.; Pun, S.H.; Mak, P.U.; Vai, M.I.; Du, M. A novel field-circuit FEM modeling and channel gain estimation for galvanic coupling real IBC measurements. Sensors 2016, 16, 471. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.H.; Kang, T.W.; Kwon, J.H.; Park, S.O. Effect of electromagnetic interference on human body communication. IEEE Trans. Electromagn. Compat. 2016, 59, 48–57. [Google Scholar] [CrossRef]








| Biopotential Signal | Biopotential Source | Application |
|---|---|---|
| Electrocardiogram (ECG) [25] | Heart activity. Measured from the body’s surface. | Diagnosis of heart related diseases. |
| Electromyogram (EMG) [26] | Muscular activity. Measured from the body’s surface. | Analyzis of biomechanics of body movements. |
| Electroencephalogram (EEG) [27] | Brain activity. Measured from the surface of the scalp. | Diagnosis of abnormal brain activities resulting from epilepsy, strokes, sleep disorders etc. |
| Local Field Potentials (LFP) [28] | Brain activity. Measured from brain tissues. | Responsive stimulation of brain. |
| Neural Spikes [24] | Neural action potential. Measured from a single neuron. | Analyzis of brain activity. |
| Electrode | (V) | Electrode | (V) |
|---|---|---|---|
| Aluminium | −1.71 | Silver Chloride | +0.23 |
| Iron | −0.41 | Copper | +0.34 |
| Nickel | −0.23 | Silver | +0.80 |
| Lead | −0.13 | Gold | +1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polachan, K.; Chatterjee, B.; Weigand, S.; Sen, S. Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review. Nanomaterials 2021, 11, 2152. https://doi.org/10.3390/nano11082152
Polachan K, Chatterjee B, Weigand S, Sen S. Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review. Nanomaterials. 2021; 11(8):2152. https://doi.org/10.3390/nano11082152
Chicago/Turabian StylePolachan, Kurian, Baibhab Chatterjee, Scott Weigand, and Shreyas Sen. 2021. "Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review" Nanomaterials 11, no. 8: 2152. https://doi.org/10.3390/nano11082152
APA StylePolachan, K., Chatterjee, B., Weigand, S., & Sen, S. (2021). Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review. Nanomaterials, 11(8), 2152. https://doi.org/10.3390/nano11082152






