Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Immobilized Coenzyme
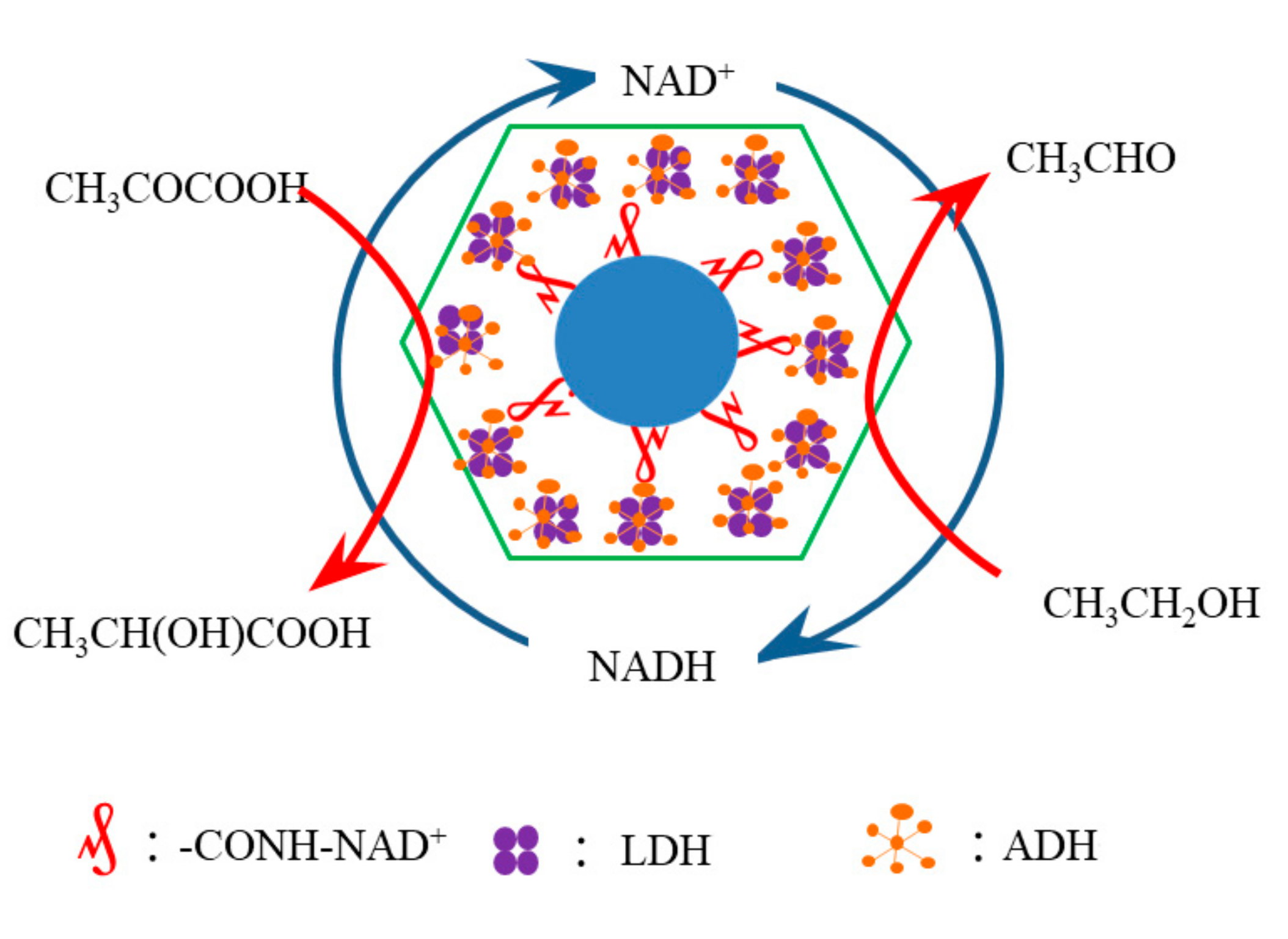
2.3. Preparation of ZIF@ADH/NAD-Polymer/LDH
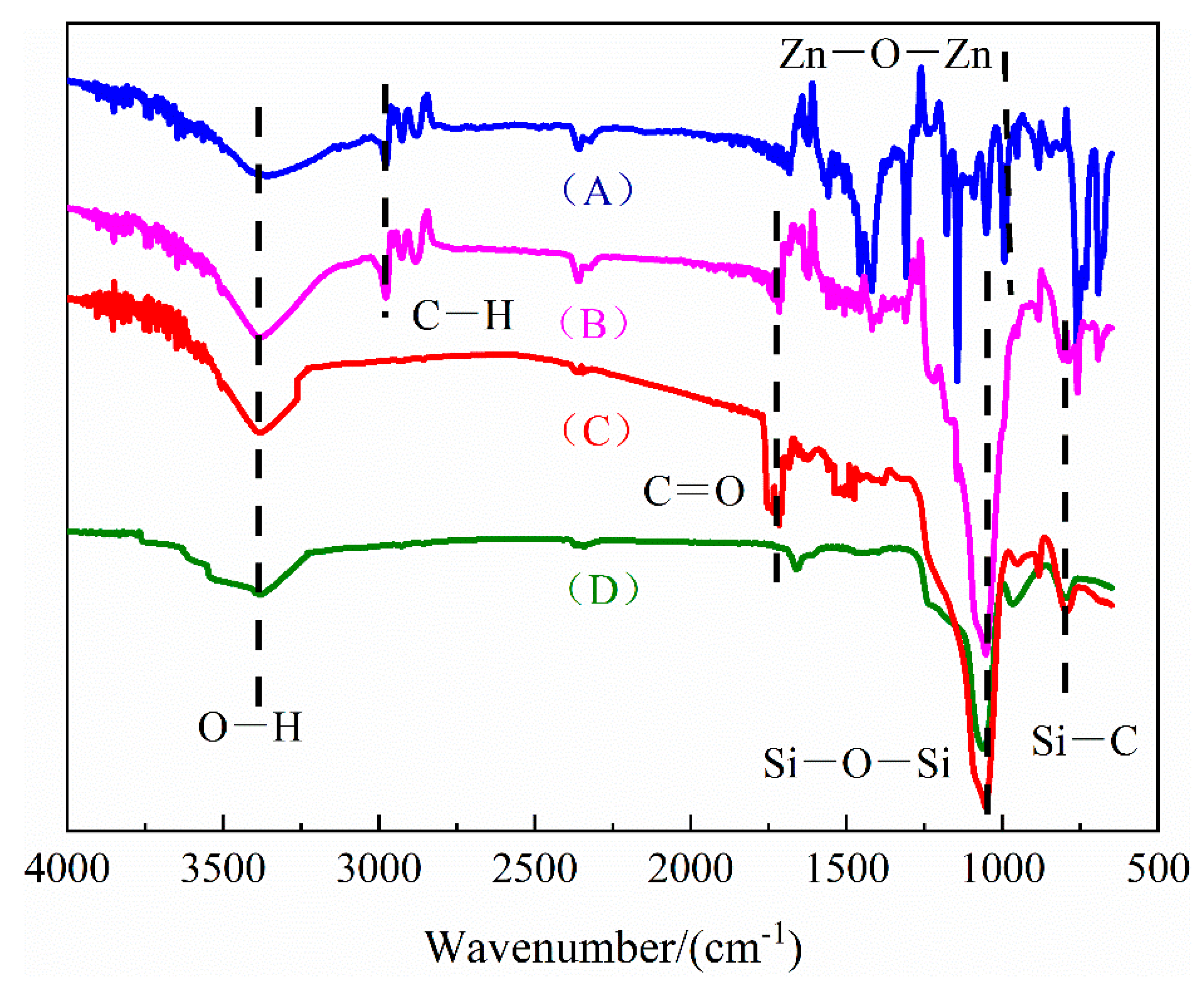
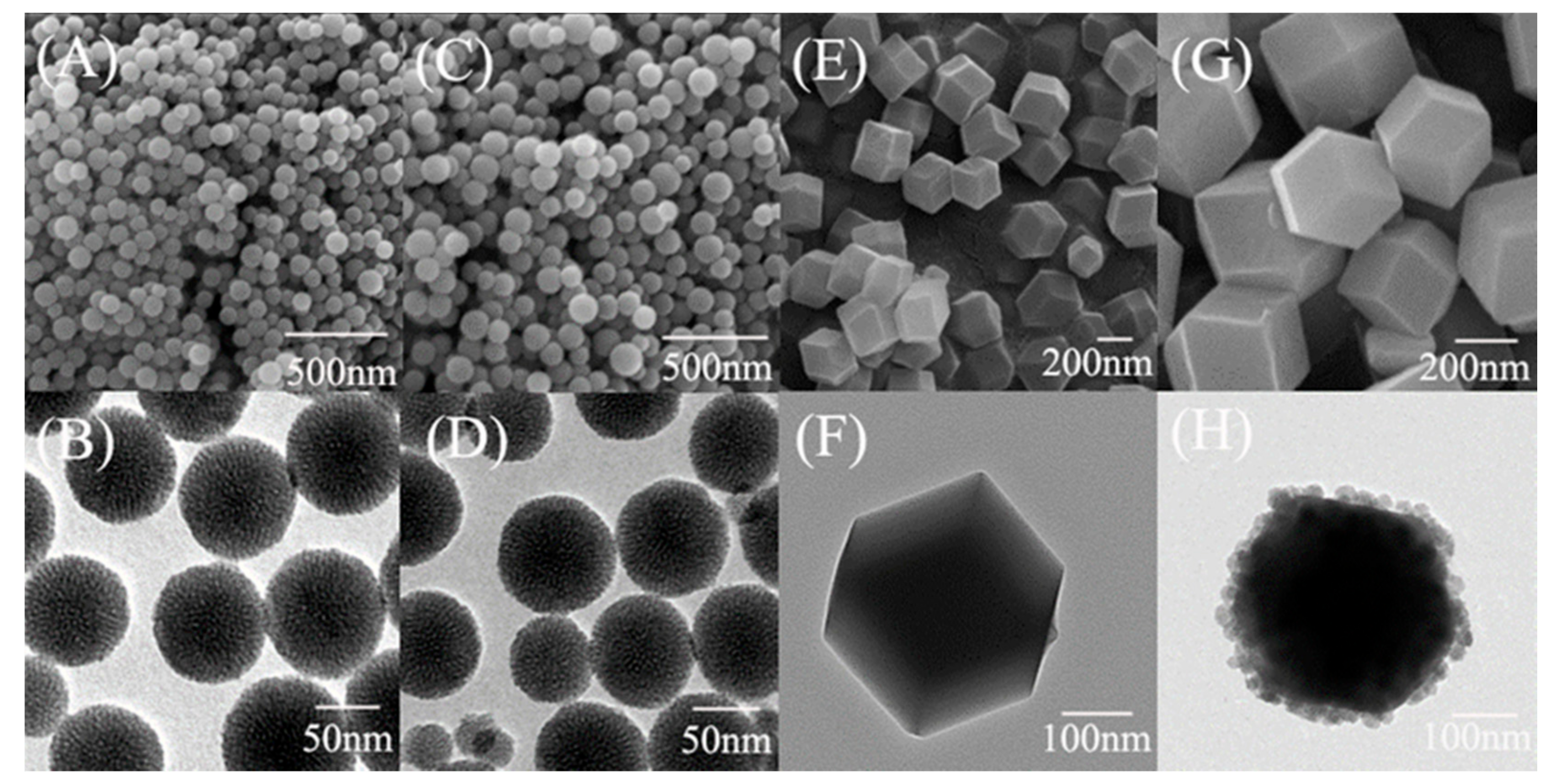
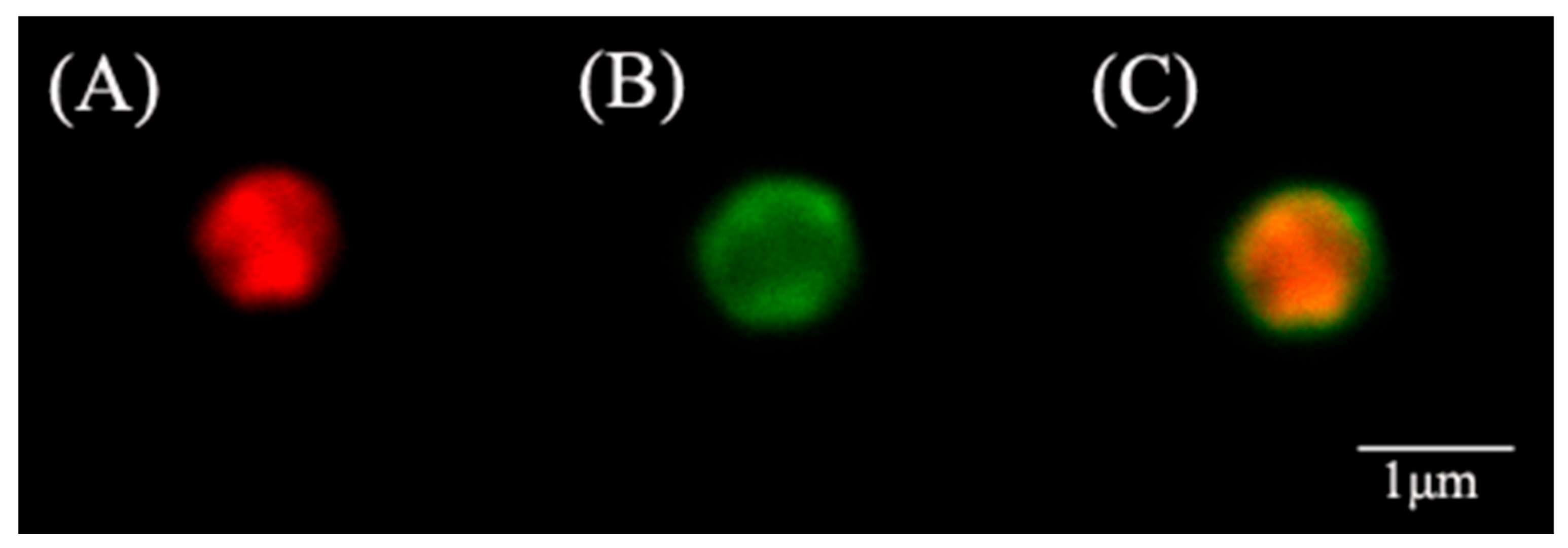
2.4. Materials Characterization
2.5. NAD+-Mediated Enzyme-Linked Reaction
2.6. Determination of Protein Concentration and Enzyme Activity
2.7. Stability of ZIF@ADH/NAD-MSN/LDH
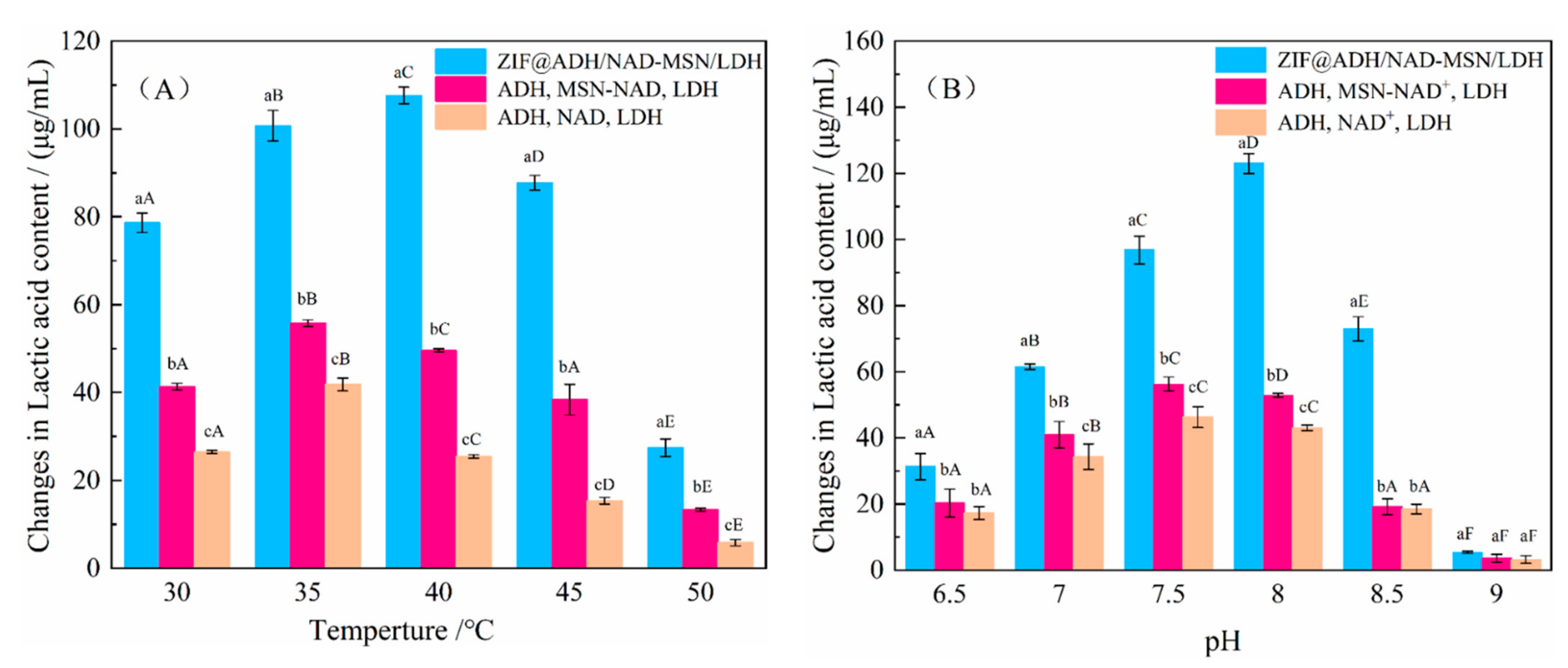
2.7.1. Stability Experiment of Temperature and pH for ZIF@ADH/NAD-MSN/LDH
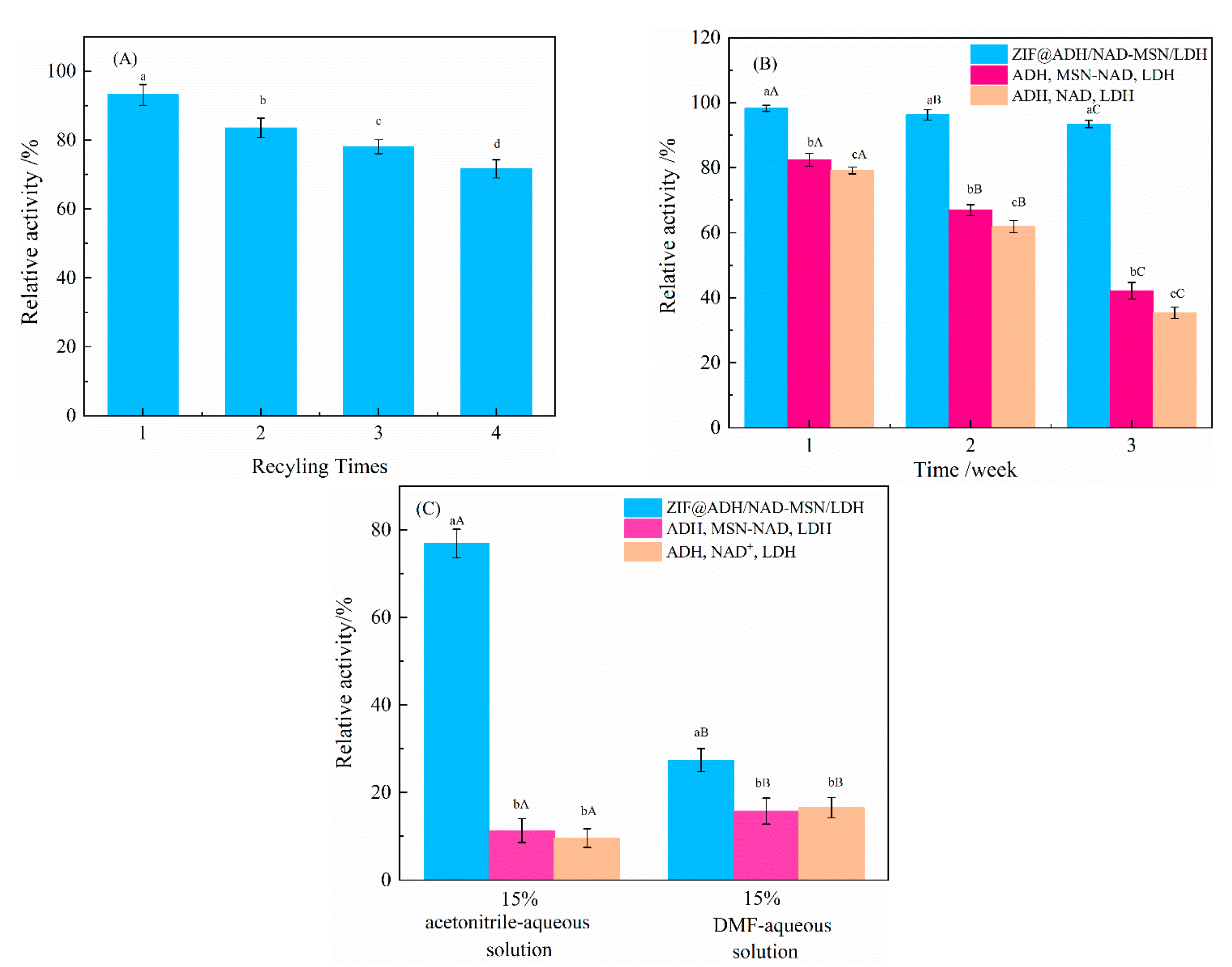
2.7.2. Storage and Experimental Stability of ZIF@ADH/NAD-MSN/LDH
2.7.3. Stability of Organic Reagents
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Textural Properties of ZIF@ADH/NAD-MSN/LDH
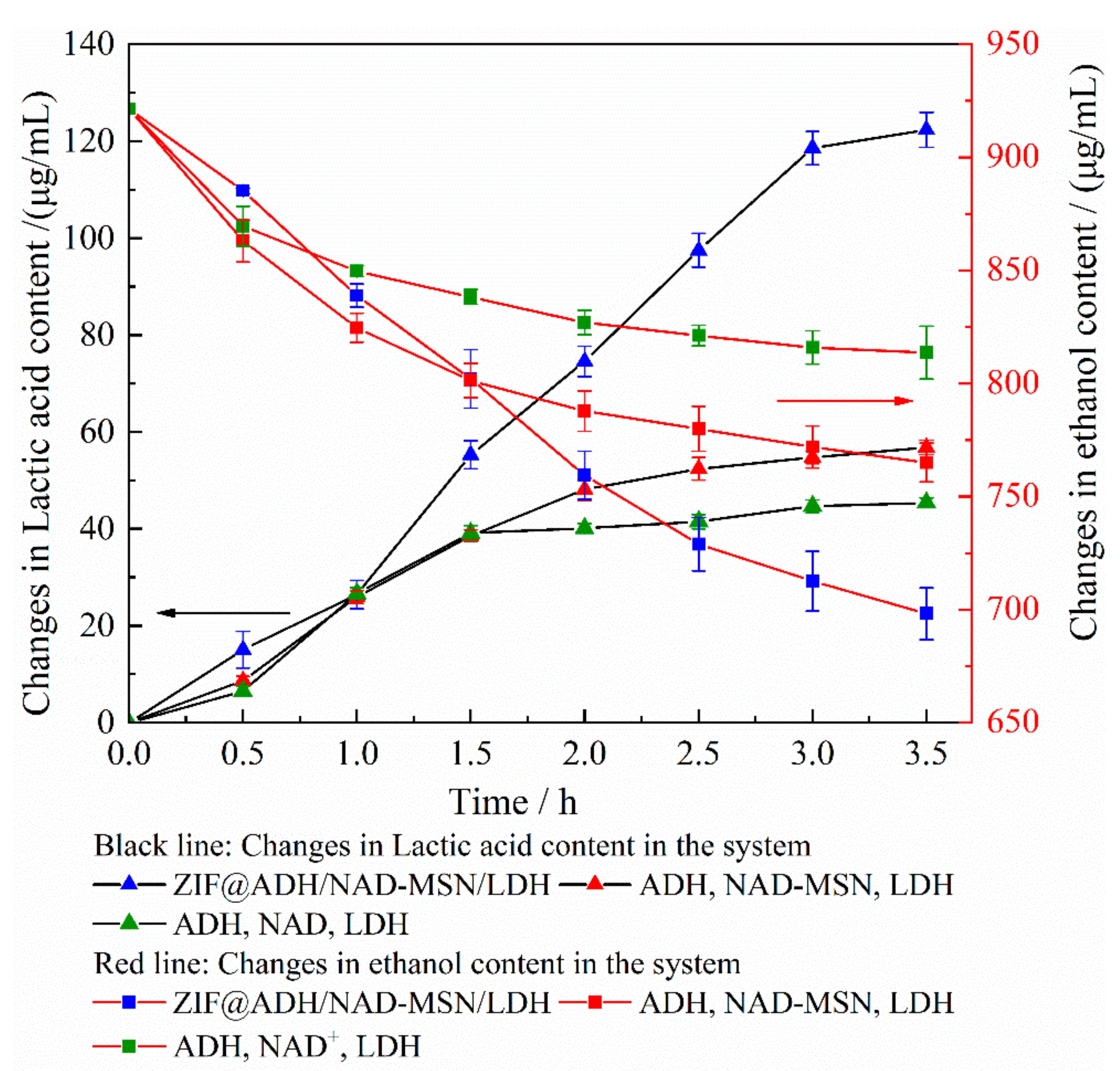
3.2. Evaluation of the Efficiency of ZIF@ADH/NAD-MSN/LDH Cascade Reaction in Nanoreactor
3.3. Study on the Reusability and Stability of ZIF@ADH/NAD-MSN/LDH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wichmann, R.; Wandrey, C.; Bückmann, A.F.; Kula, M.R. Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD(H) regeneration. Biotechnol. Bioeng. 1981, 23, 2789–2802. [Google Scholar] [CrossRef]
- Gasparrini, M.; Sorci, L.; Raffaelli, N. Enzymology of extracellular NAD metabolism. Cell. Mol. Life Sci. 2021, 78, 3317–3331. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Kim, T.W.; Yadav, R.K.; Kumar, K.; Yadav, B.C. Anthracene-based g-C3N4 photocatalyst for regeneration of NAD(P)H and sulfide oxidation based on Z-scheme nature. Int. J. Energy Res. 2021, 45, 13117–13129. [Google Scholar] [CrossRef]
- Anne, A.; Bourdillon, C.; Daninos, S.; Moiroux, J. Can the combination of electrochemical regeneration of NAD+, selectivity of L-α-amino-acid dehydrogenase, and reductive amination of α- keto-acid be applied to the inversion of configuration of a L-α-amino-acid? Biotechnol. Bioeng. 1999, 64, 101–107. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, S.; Myung, N.; Chen, W. DNA-guided assembly of a five-component enzyme cascade for enhanced conversion of cellulose to gluconic acid and H2O2. J. Biotechnol. 2017, 263, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, L.C.; Su, B.M.; Xu, X.Q.; Lin, J. Multi-enzyme cascade for improving β-hydroxy-α-amino acids production by engineering L-threonine transaldolase and combining acetaldehyde elimination system. Bioresour. Technol. 2020, 310, 123439. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Li, J.; Su, Y.; Gao, M.; Jin, T.; Chen, G. Co-immobilization of multi-enzyme on reversibly soluble polymers in cascade catalysis for the one-pot conversion of gluconic acid from corn straw. Bioresour. Technol. 2021, 321, 124509. [Google Scholar] [CrossRef]
- Findrik, Z.; Vasić-Rački, D. Overview on reactions with multi-enzyme systems. Chem. Biochem. Eng. Q. 2009, 23, 545–553. [Google Scholar]
- Faber, K. Multi-Step Enzyme Catalysis: Biotransformations and Chemoenzymatic Synthesis. Edited by Eduardo García-Junceda. ChemBioChem 2009, 10, 2266. [Google Scholar] [CrossRef]
- Wilner, O.I.; Weizmann, Y.; Gill, R.; Lioubashevski, O.; Freeman, R.; Willner, I. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotechnol. 2009, 4, 249–254. [Google Scholar] [CrossRef]
- Vong, T.; Schoffelen, S.; van Dongen, S.F.M.; van Beek, T.A.; Zuilhof, H.; van Hest, J.C.M. A DNA-based strategy for dynamic positional enzyme immobilization inside fused silica microchannels. Chem. Sci. 2011, 2, 1278–1285. [Google Scholar] [CrossRef]
- Xin, L.; Zhou, C.; Yang, Z.; Liu, D. Regulation of an enzyme cascade reaction by a DNA machine. Small 2013, 9, 3088–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, S.; Wu, S.; Li, Y.; Mao, C.; Liu, Y.; Yan, H. Complex wireframe DNA origami nanostructures with multi-arm junction vertices. Nat. Nanotechnol. 2015, 10, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-cleas): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.P.; Schwarz, B.; Waters, R.S.; Gedeon, T.; Douglas, T. Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem. Biol. 2014, 9, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pescador, P.; Katakis, I.; Toca-Herrera, J.L.; Donath, E. Efficiency of a bienzyme sequential reaction system immobilized on polyelectrolyte multilayer-coated colloids. Langmuir 2008, 24, 14108–14114. [Google Scholar] [CrossRef] [PubMed]
- Fontes, C.M.G.A.; Gilbert, H.J. Cellulosomes: Highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 2010, 79, 655–681. [Google Scholar] [CrossRef] [PubMed]
- Oroz-Guinea, I.; García-Junceda, E. Enzyme catalysed tandem reactions. Curr. Opin. Chem. Biol. 2013, 17, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Delaittre, G.; Reynhout, I.C.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Cascade reactions in an all-enzyme nanoreactor. Chem. Eur. J. 2009, 15, 12600–12603. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.M.; Nallani, M.; Vriezema, D.M.; Cornelissen, J.J.L.M.; Van Hest, J.C.M.; Nolte, R.J.M.; Rowan, A.E. Enzymes containing porous polymersomes as nano reaction vessels for cascade reactions. Org. Biomol. Chem. 2008, 6, 4315–4318. [Google Scholar] [CrossRef]
- Torabi, S.F.; Khajeh, K.; Ghasempur, S.; Ghaemi, N.; Siadat, S.O.R. Covalent attachment of cholesterol oxidase and horseradish peroxidase on perlite through silanization: Activity, stability and co-immobilization. J. Biotechnol. 2007, 131, 111–120. [Google Scholar] [CrossRef]
- Gan, J.S.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2021, 167, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.L.B.; de Sousa, E.Y.A.; de França Serpa, J.; Oliveira, R.C.; Dos Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Yu, X.; Liao, W.C.; Sohn, Y.S.; Cecconello, A.; Kozell, A.; Nechushtai, R.; Willner, I. ATP-Responsive Aptamer-Based Metal–Organic Framework Nanoparticles (NMOFs) for the Controlled Release of Loads and Drugs. Adv. Funct. Mater. 2017, 27, 1702102. [Google Scholar] [CrossRef]
- Chen, W.H.; Yu, X.; Cecconello, A.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem. Sci. 2017, 8, 5769–5780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.H.; Liao, W.C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal–Organic Framework Nanoparticles for Controlled Drug Release. Adv. Funct. Mater. 2018, 28, 1705137. [Google Scholar] [CrossRef]
- Chouyyok, W.; Panpranot, J.; Thanachayanant, C.; Prichanont, S. Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. J. Mol. Catal. B Enzym. 2009, 56, 246–252. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Homouz, D.; Stagg, L.; Wittung-Stafshede, P.; Cheung, M.S. Macromolecular crowding modulates folding mechanism of α/β protein apoflavodoxin. Biophys. J. 2009, 96, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Yang, C.; Ge, J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Bioprocess. 2017, 4, 24. [Google Scholar] [CrossRef]
- Cao, X.; Ni, Y.; Zhang, A.; Xu, S.; Chen, K.; Ouyang, P. Encapsulation of enzymes in metal ion-surfactant nanocomposites for catalysis in highly polar solvents. Chem. Commun. 2017, 53, 3134–3137. [Google Scholar] [CrossRef]
- Wang, K.; Ren, H.; Li, N.; Tan, X.; Dang, F. Ratiometric fluorescence sensor based on cholesterol oxidase-functionalized mesoporous silica nanoparticle@ZIF-8 core-shell nanocomposites for detection of cholesterol. Talanta 2018, 188, 708–713. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, P. Nanoparticle-supported multi-enzyme biocatalysis with in situ cofactor regeneration. J. Biotechnol. 2009, 139, 102–107. [Google Scholar] [CrossRef]
- Yang, S.; Song, S.; Han, K.; Wu, X.; Chen, L.; Hu, Y.; Wang, J.; Liu, B. Characterization, in vitro evaluation and comparative study on the cellular internalization of mesoporous silica nanoparticle-supported lipid bilayers. Microporous Mesoporous Mater. 2019, 284, 212–224. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Zhang, X.; Zhou, X.; Teng, X.; Yan, M.; Bi, H. Horseradish peroxidase-immobilized magnetic mesoporous silica nanoparticles as a potential candidate to eliminate intracellular reactive oxygen species. Nanoscale 2015, 7, 2941–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, L.; Sha, Y.; Wu, D.; Wei, Q.; Chen, D.; Yang, S.; Jia, F.; Yuan, Q.; Han, X.; Wang, J. Surfactant induces ROS-mediated cell membrane permeabilization for the enhancement of mannatide production. Process Biochem. 2020, 91, 172–180. [Google Scholar] [CrossRef]
- Niu, B.; Wu, D.; Wang, J.; Wang, L.; Zhang, W. Salt-sealing-pyrolysis derived Ag/ZnO@C hollow structures towards efficient photo-oxidation of organic dye and water-born bacteria. Appl. Surf. Sci. 2020, 528, 146965. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Quan, Z.; Li, C.; Kang, X.; Lian, H.; Lin, J. Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier. Biomaterials 2008, 29, 4341–4347. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.L.; Wang, L.; Ma, J.; Yang, Y.W.; Gao, H. Nanoassembles constructed from mesoporous silica nanoparticles and surface-coated multilayer polyelectrolytes for controlled drug delivery. Microporous Mesoporous Mater. 2014, 185, 245–253. [Google Scholar] [CrossRef]
- Qin, Y.; Han, X.; Li, Y.; Han, A.; Liu, W.; Xu, H.; Liu, J. Hollow Mesoporous Metal-Organic Frameworks with Enhanced Diffusion for Highly Efficient Catalysis. ACS Catal. 2020, 10, 5973–5978. [Google Scholar] [CrossRef]
- Cui, J.; Wang, L.; Han, Y.; Liu, W.; Li, Z.; Guo, Z.; Hu, Y.; Chang, Z.; Yuan, Q.; Wang, J. ZnO nano-cages derived from ZIF-8 with enhanced anti mycobacterium-tuberculosis activities. J. Alloys Compd. 2018, 766, 619–625. [Google Scholar] [CrossRef]
- Sarrouh, B. Up-To-Date Insight on Industrial Enzymes Applications and Global Market. J. Bioprocess. Biotech. 2012, s4, 2. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Song, Y.; Hu, D.; Liu, F.; Chen, S.; Wang, L. Fabrication of fluorescent SiO2@zeolitic imidazolate framework-8 nanosensor for Cu2+ detection. Analyst 2015, 140, 623–629. [Google Scholar] [CrossRef]
- Son, J.; Lee, H.J.; Oh, M. Systematic formation of multilayered core-shell microspheres through the multistep growth of coordination polymers. Chem. Eur. J. 2013, 19, 6546–6550. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, T.; Sun, Z.; Hu, X. Preparation of copper based metal organic framework materials and its effective adsorptive removal of ceftazidime from aqueous solutions. Appl. Surf. Sci. 2020, 532, 147411. [Google Scholar] [CrossRef]
- Chen, W.H.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat. Catal. 2018, 1, 689–695. [Google Scholar] [CrossRef]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Construction of multiple enzyme metal–organic frameworks biocatalyst via DNA scaffold: A promising strategy for enzyme encapsulation. Chem. Eng. J. 2019, 363, 174–182. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Ma, X.; Xia, M.; Zhang, Y. Adsorption of cholesterol oxidase and entrapment of horseradish peroxidase in metal-organic frameworks for the colorimetric biosensing of cholesterol. Talanta 2019, 200, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Hofstetter, K.; Schmid, A. Non-enzymatic regeneration of nicotinamide and flavin cofactors for monooxygenase catalysis. Trends Biotechnol. 2006, 24, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhou, X.; You, C. Efficient Multi-Enzymes Immobilized on Porous Microspheres for Producing Inositol From Starch. Front. Bioeng. Biotechnol. 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Eggert, M.W.; Byrne, M.E.; Chambers, R.P. Impact of high pyruvate concentration on kinetics of rabbit muscle lactate dehydrogenase. Appl. Biochem. Biotechnol. 2011, 165, 676–686. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L.; Wu, C.; Wang, X.; Zhang, X. Synthesis of 3D-Ordered Macro/Microporous Yolk-Shelled Nanoreactor with Spatially Separated Functionalities for Cascade Reaction. ACS Appl. Mater. Interfaces 2019, 11, 33978–33986. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Batencor, L.; Hidalgo, A.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. One-pot conversion of cephalosporin C to 7-aminocephalosporanic acid in the absence of hydrogen peroxide. Adv. Synth. Catal. 2005, 347, 1804–1810. [Google Scholar] [CrossRef]
- Garcia, J.; Zhang, Y.; Taylor, H.; Cespedes, O.; Webb, M.E.; Zhou, D. Multilayer enzyme-coupled magnetic nanoparticles as efficient, reusable biocatalysts and biosensors. Nanoscale 2011, 3, 3721–3730. [Google Scholar] [CrossRef]
- Chen, S.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic metal-organic frameworks as scaffolds for spatial co-location and positional assembly of multi-enzyme systems enabling enhanced cascade biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Martín, J.; de Las Rivas, B.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational co-immobilization of bi-enzyme cascades on porous supports and their applications in bio-redox reactions with insitu recycling of soluble cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W.; Wang, P. Nanobiocatalysis and its potential applications. Trends Biotechnol. 2008, 26, 639–646. [Google Scholar] [CrossRef]
- Knedel, T.O.; Ricklefs, E.; Schlüsener, C.; Urlacher, V.B.; Janiak, C. Laccase Encapsulation in ZIF-8 Metal-Organic Framework Shows Stability Enhancement and Substrate Selectivity. ChemistryOpen 2019, 8, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Pan, T.; Xie, Z.; Xu, X.; Jin, Z. Co-immobilization of β-fructofuranosidase and glucose oxidase improves the stability of Bi-enzymes and the production of lactosucrose. LWT Food Sci. Technol. 2020, 128, 109460. [Google Scholar] [CrossRef]
- He, H.; Han, H.; Shi, H.; Tian, Y.; Sun, F.; Song, Y.; Li, Q.; Zhu, G. Construction of Thermophilic Lipase-Embedded Metal-Organic Frameworks via Biomimetic Mineralization: A Biocatalyst for Ester Hydrolysis and Kinetic Resolution. ACS Appl. Mater. Interfaces 2016, 8, 24517–24524. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Facile synthesis of multiple enzyme-containing metal-organic frameworks in a biomolecule-friendly environment. Chem. Commun. 2015, 51, 13408–13411. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent immobilization of β-glucosidase into mesoporous silica nanoparticles from anhydrous acetone enhances its catalytic performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ma, C.; Ru, X.; Guo, Z.; Wu, D.; Zhang, S.; Yu, G.; Hu, Y.; Wang, J. Facile synthesis of ZnO hollow microspheres and their high performance in photocatalytic degradation and dye sensitized solar cells. J. Alloys Compd. 2015, 647, 57–62. [Google Scholar] [CrossRef]
| Reaction Conditions | The Types of Products | |
|---|---|---|
| ZIF-8 Immobilized Components 1 | Reactant 2 | |
| ADH 3 | C2H5OH CH3COCOOH | No reaction |
| LDH 4 | C2H5OH CH3COCOOH | No reaction |
| LDH MSN-NAD 5 | C2H5OH CH3COCOOH | No reaction |
| ADH MSN-NAD+ | C2H5OH CH3COCOOH | CH3CHO |
| ADH LDH | C2H5OH CH3COCOOH | No reaction |
| ADH LDH MSN-NAD+ | C2H5OH | CH3CHO |
| ADH LDH MSN-NAD+ | CH3COCOOH | No reaction |
| ADH LDH MSN-NAD+ | C2H5OH CH3COCOOH | CH3CHO CH3CH(OH)COOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Sun, P.; Yang, Y.; Qiao, H.; Tian, H.; Wu, D.; Yang, S.; Yuan, Q.; Wang, J. Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade. Nanomaterials 2021, 11, 2171. https://doi.org/10.3390/nano11092171
Wang L, Sun P, Yang Y, Qiao H, Tian H, Wu D, Yang S, Yuan Q, Wang J. Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade. Nanomaterials. 2021; 11(9):2171. https://doi.org/10.3390/nano11092171
Chicago/Turabian StyleWang, Le, Pengxue Sun, Yiyu Yang, Hanzhen Qiao, Hailong Tian, Dapeng Wu, Shuoye Yang, Qipeng Yuan, and Jinshui Wang. 2021. "Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade" Nanomaterials 11, no. 9: 2171. https://doi.org/10.3390/nano11092171
APA StyleWang, L., Sun, P., Yang, Y., Qiao, H., Tian, H., Wu, D., Yang, S., Yuan, Q., & Wang, J. (2021). Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade. Nanomaterials, 11(9), 2171. https://doi.org/10.3390/nano11092171





