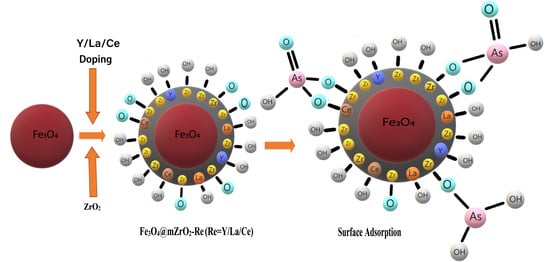
3.3.1. Effect of pH
The initial pH value of the solution has a significant impact on metal ion adsorption. This parameter is directly related to the capability of hydrogen ions and metal ions to adsorb on the active surface sites [
28]. Therefore, the effect of initial pH value on the adsorption performance of Fe
3O
4@mZrO
2 (Re = Y/La/Ce) was investigated. 10 mg of adsorbent was accurately measured and put into different conical flasks, and added 100 mL of 5 mg/L solutions of As
3+ and As
5+, and adjusted the pH to 3.0, 4.0, 5.0, 6.0, 7.0, 8.0 and 9.0 by using HCL and NaOH. The remaining process was done by following the method in the
Section 2.5.
From the
Figure 10, it can be clearly noticed that the initial pH value of the solution has a substantial effect on the adsorption. Under the strong basic conditions, the adsorption capacity of the adsorbent for As
3+ and As
5+ removal was extremely low, while under weak acidic conditions, the adsorption capacities of both metals were high. The highest adsorption capacities for both As
3+ and As
5+ were recorded at pH 6 and pH 5, respectively. The adsorption capacity of As
3+ from pH 5~pH 7 was almost near i.e., 30 mg/g, 32 mg/g and 30 mg/g, respectively. Based on initial pH studies the ideal adsorption pH conditions for both As
3+ and As
5+ were selected as 6 and 5, respectively.
The relevant studies suggest that that mesoporous zirconia-based nanostructures demonstrated good results for As(V) removal under lower acidic and neutral pH conditions [
29]. For as (V), it mainly exists in the form of H
2AsO
4− and in the range of pH = 3~7. At this time, the adsorbent surface has a positive charge, and the negatively charged H
2AsO
4− will be adsorbed on the adsorbent surface directly through electrostatic attraction. With the increase of pH, the positive charge on the surface of the adsorbent decreases gradually, so the adsorption capacity of the adsorbent for As
5+ decreases gradually; When the pH is greater than 7, the surface of the adsorbent begins to be occupied by negative charges [
30]. Some other studies have shown that under acidic conditions, the surface of metal oxides prone to “protonation”, so the adsorption is negatively charged H
2AsO
4− and HAsO
42−, and the protonation decreases with the increase of pH value. The protonation of the sorbent surface is supported by a lower pH. Improved protonation is supposed to escalate the positively charged spots, increase the attraction force between the sorbent surface and As anions, and hence boost adsorption in the lower pH range. The negatively charged sites dominate at higher pH levels, the repulsion effect increases, and the number of adsorption decreases. When the solution is alkaline, OH
− in the system will compete with arsenate anion for adsorption, resulting in the decrease of arsenic removal efficiency [
31]. May be the same inclination was observed with the As
3+, although the decline in As
3+ removal was not that clear as that of As
5+ with the increasing pH.
3.3.2. Adsorption Isothermal Analysis
For non-linear fitting adsorption isothermal analysis, the three widely used isothermal adsorption models are the Langmuir, Freundlich, and Redlich–Peterson models [
32]. The specific equation and application scope of each model are as follows:
Langmuir Isothermal non-linear adsorption equation is;
where:
qe (mg/g) adsorption capacity at equilibrium,
Ce (mg/L) is equilibrium concentration,
b (L/mg) is the Langmuir constant, and
qm (mg/g) is the Saturated adsorption capacity.
RL is a dimensionless constant separation factor. Its definition formula is;
For the adsorption process on the heterogeneous surfaces, Freundlich isotherms are more suitable. The isotherm provides expressions for surface heterogeneity as well as the exponential distribution of active centers and their associated energies. Non-monolayer adsorption is the most common in this method [
33]. The equation is as follows:
where:
qe (mg/g) is adsorption capacity,
KF is the Freundlich coefficient (mg/g (L/mg)
1/n),
Ce (mg/L) is Equilibrium concentration, and “
n” is the Freundlich constant.
The Redlich–Peterson model combines the Langmuir and Freundlich models formula to summarize the Redlich–Peterson isothermal model [
34], and the equation is as follows;
where:
K, α are Redlich–Peterson constant and “
β” is coefficient.
The accurately weighted 10 mg adsorbent was put into different 250 mL conical flasks, and 100 mL solutions of As
3+, and As
5+ with different concentrations like 5 mg/L, 10 mg/L, 20 mg/L, 40 mg/L, 60 mg/L, and 80 mg/L were added, respectively. The solution was adjusted by HCL and NaOH to the required pH value. The conical flasks were then put in the constant temperature oscillator at 298 K, 308 K, and 318 K temperature conditions oscillated at 200 r/min for 60 min. After one hour of oscillation, magnetic separation was used, and the supernatant was then filtered through membrane (0.45 μm), and heavy metals concentration was detected by ICP-OES. According to change in the Ion concentration before and after adsorption the adsorption isotherm was drawn.
Figure 11 and
Table 7 shows the different adsorption isothermal curves and parameters of the models.
From the Isothermal fitting curves (
Figure 11), and equations parameters of the Langmuir model, Freundlich model, and Redlich–Peterson Model, the best adsorption isothermal models for As
3+ and As
5+ removal was evaluated. The Langmuir model effectively described the adsorption isotherm data with all correlation coefficient (R
2) ranged between 0.995~0.997 for As
3+, and 0.988~0.990 for As
5+. The R
L values calculated by the Langmuir model at different temperatures for As
3+ and As
5+ were ranged from 0.389~0.954, and 0.473~0.531, respectively, all were found less than 1, which suggested that the adsorption is in the favorable direction [
35]. The results were found consistent, and the Qm fitted by the Langmuir model is slightly larger than the actual experimental results. The highest adsorption capacity of As
3+ ions at 298 K, 308 K, and 318 K, were 62.00 mg/g, 64.31 mg/g, and 68.33 mg/g, respectively, and for As
5+ at different temperatures were 59 mg/g, 64 g/g, and 84.23 mg/g, respectively. Based on R
2 analysis, the Langmuir model and Redlich–Peterson model shows better results than the Freundlich model, indicating they are best fitted for As
3+ and As
5+ adsorption isotherm. Based on the analyzed isothermal results of the adsorption isotherms of As
3+, and As
5+ ions are mainly monolayer layer adsorption and absorption sites on the adsorbent are distributed homogenously [
36].
The comparison of the prepared adsorbent Fe
3O
4@mZrO
2-Re (Re = Y/La/Ce) with some relevant studies is shown in
Table 8, where the comparison results revealed that the adsorbent is very effective in removing As
3+ and As
5+ from the aqueous solution.
3.3.3. Adsorption Kinetics of Fe3O4@mZrO2-Re (Re = Y/La/Ce)
The two widely used kinetics models are; (1) the Pseudo-First order kinetic model, a basic kinetic rate equation of stable adsorption reaction; (2) the second one is Pseudo-second order reaction kinetics model, where adsorption rate of adsorbent is directly proportional to the number of available active sites on the surface [
45,
46,
47]. In this study, pseudo-first-order and pseudo-second-order linear models were used to establish the rate constant and As(III) and As(V) controlling mechanism of adsorption onto the prepared adsorbent.
- (1)
Pseudo-first-order kinetics model
Converting the above equation into a linear equation:
where:
qe (mg/g) is adsorption capacity at equilibrium,
qt (mg/g) is the adsorption capacity of materials at time “
t”,
t (min) is adsorption reaction time, and
K1 (min
−1) is the first order adsorption rate constant.
- (2)
Pseudo-second-order kinetics model
The equation given above is converted into a linear equation:
where:
qt (mg/g) is the adsorption capacity at equilibrium,
qt (mg/g) adsorption capacity at time “
t”,
t (min) is the adsorption reaction time, and
K1 (min
−1) is the second kinetic order adsorption rate constant.
Adsorption kinetic tests were conducted with 0.01 g adsorbent at initial concentrations of (1.0 mg/L, 2.0 mg/L, and 5.0 mg/L), pH 6.0, temperature 298 K, and at time durations of 10, 20, 40, 60, 80, 100, and 120 min. First and second linear kinetic models using Origin 8.5 software were applied to establish the rate constant and As3+ and As5+ adsorption controlling mechanism. The R2 (correlation coefficient) was used to find the most suited model. The values of K1, K2, and qe were also calculated.
From the fitting curves (
Figure 12) and related parameters (
Table 9) fitted by the kinetic models, it can be noticed that the R
2 values obtained from the fittings of the two kinetic reaction models are significantly different. Among the two selected models, Pseudo-second-order kinetic model has a suitable effect, and R
2 values were found for both As
3+ and As
5+ between 0.991–0.997, and found relatively stable, because there is no obvious fluctuation noted. The range of R
2 values fitted by the Pseudo-first-order kinetics model are relatively wide ranged for both heavy metals, with values ranging from 0.921 to 0.987, with noticeable fluctuations. Therefore, the adsorption kinetics of As
3+ and As
5+ from solution by the adsorbent Fe
3O
4@mZrO
2-Re (Re = Y/La/Ce) is more suitably designated by the Pseudo-second-order reaction kinetic model, where rate of reaction depended directly on the square of the concentration of the reactants remaining in the solution [
48]. Many other researchers suggested that adsorption kinetics of Magnetic nanomaterials are supporting the Pseudo-second order Kinetics, and our results are also favoring the 2nd order kinetic model [
49,
50,
51].
3.3.4. Regeneration Efficiency of Fe3O4@mZrO2-Re (Re = Y/La/Ce)
Regeneration of materials is one the most important indicator to check the worth of any adsorbent, and the adsorbents with good regeneration abilities are very vital for the cost effective and environmental friendly adsorption process [
52]. The regeneration experiment was performed at room temperature where 0.1 g of adsorbent was put in a 250 mL conical flask, and added 100 mL of As
3+ and As
5+ solutions with a concentration of 100 mg/L, and adjusted the pH to 5. Then placed the conical flask in a constant temperature oscillator for 3 h oscillated at 200 rpm. After filtration, determined the ion concentration in the filtrate to calculate the adsorption capacity. After every cycle NaOH solution was used as an elution agent, then washed with deionized water and dried in oven, and repeated the experiment for seven cycles. The regeneration experiment results of the adsorbent Fe
3O
4@mZrO
2-Re (Re = Y/La/Ce) at different levels are shown in
Figure 13.
In the first regeneration cycle, the regeneration efficiencies of both As
3+ and As
5+ were 93.45% and 95.75%, respectively. It can be seen that the regeneration performance of Fe
3O
4@mZrO
2-Re (Re = Y/La/Ce) for As(III) and As(V) slightly decreases with the increase of regeneration cycles, but the adsorption efficiency of the adsorbent in all the later regeneration experiments tended to be stable. In the 7th cycle, the adsorption capacities of both As
3+ and As
5+ were 75.15% and 77.59%, respectively. The amount of adsorption slightly decreased after every cycle, and the possible reason for this loss is the structural uncertainty of the adsorbents, which during regeneration treatment might lose their adsorption sites and the structural order accessibility in the pores [
53]. By regenerating the nanomaterials during wastewater treatment, we can make the removal process more viable and economical.