Effects of Nano Aluminum Powder on the Mechanical Sensitivity of RDX-Based Explosives
Abstract
:1. Introduction
2. Experiment and Calculation
2.1. Reagents and Instruments
2.2. Explosive Preparation
2.3. Performance Test
2.3.1. Impact Sensitivity Test
2.3.2. Friction Sensitivity Test
2.4. Initial Structure Selection and Optimization Calculation of Explosive Components
2.4.1. RDX
2.4.2. Al2O3
2.4.3. Paraffin Molecule
2.4.4. Al2O3 + Paraffin Molecule
3. Results and Discussion
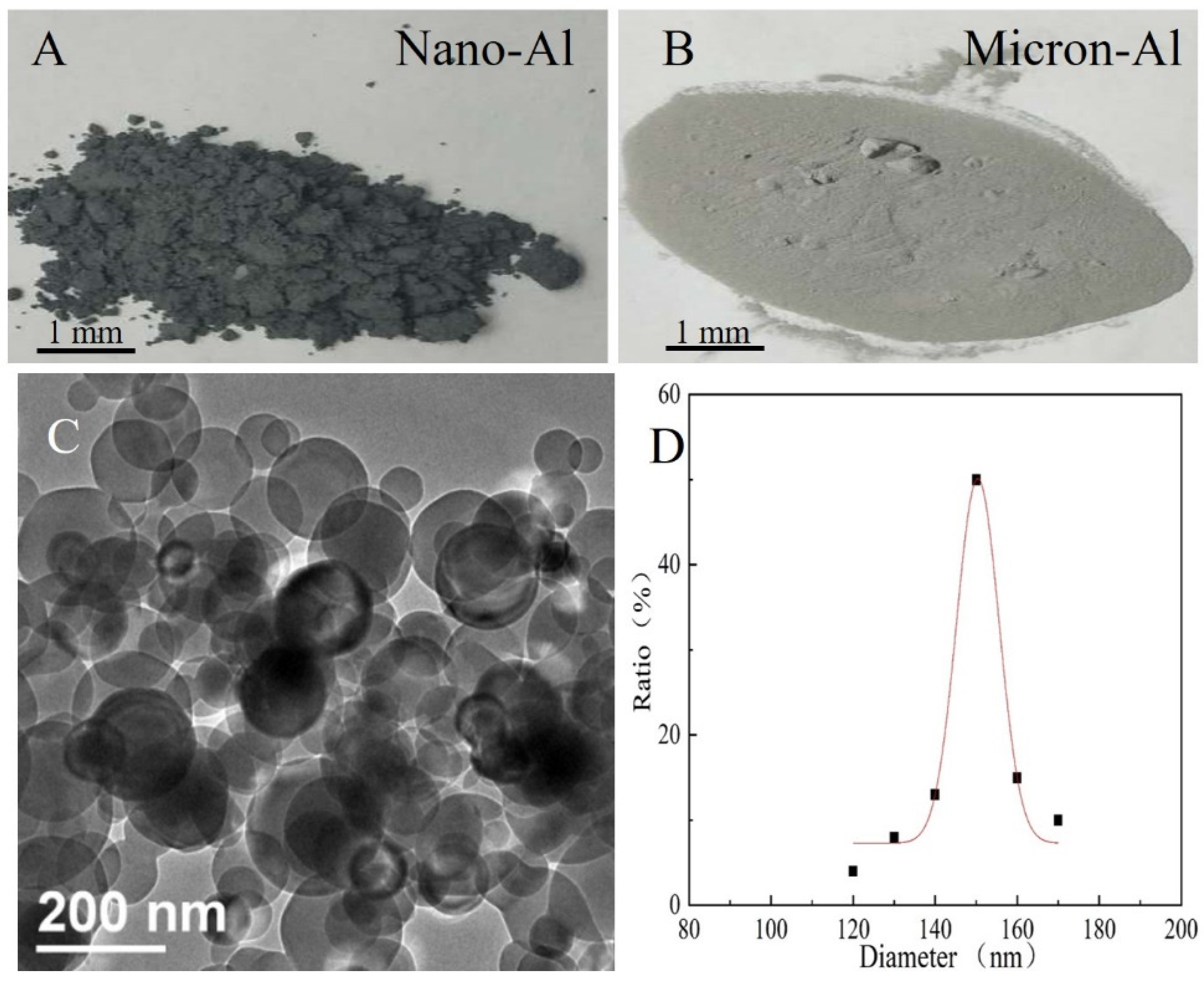
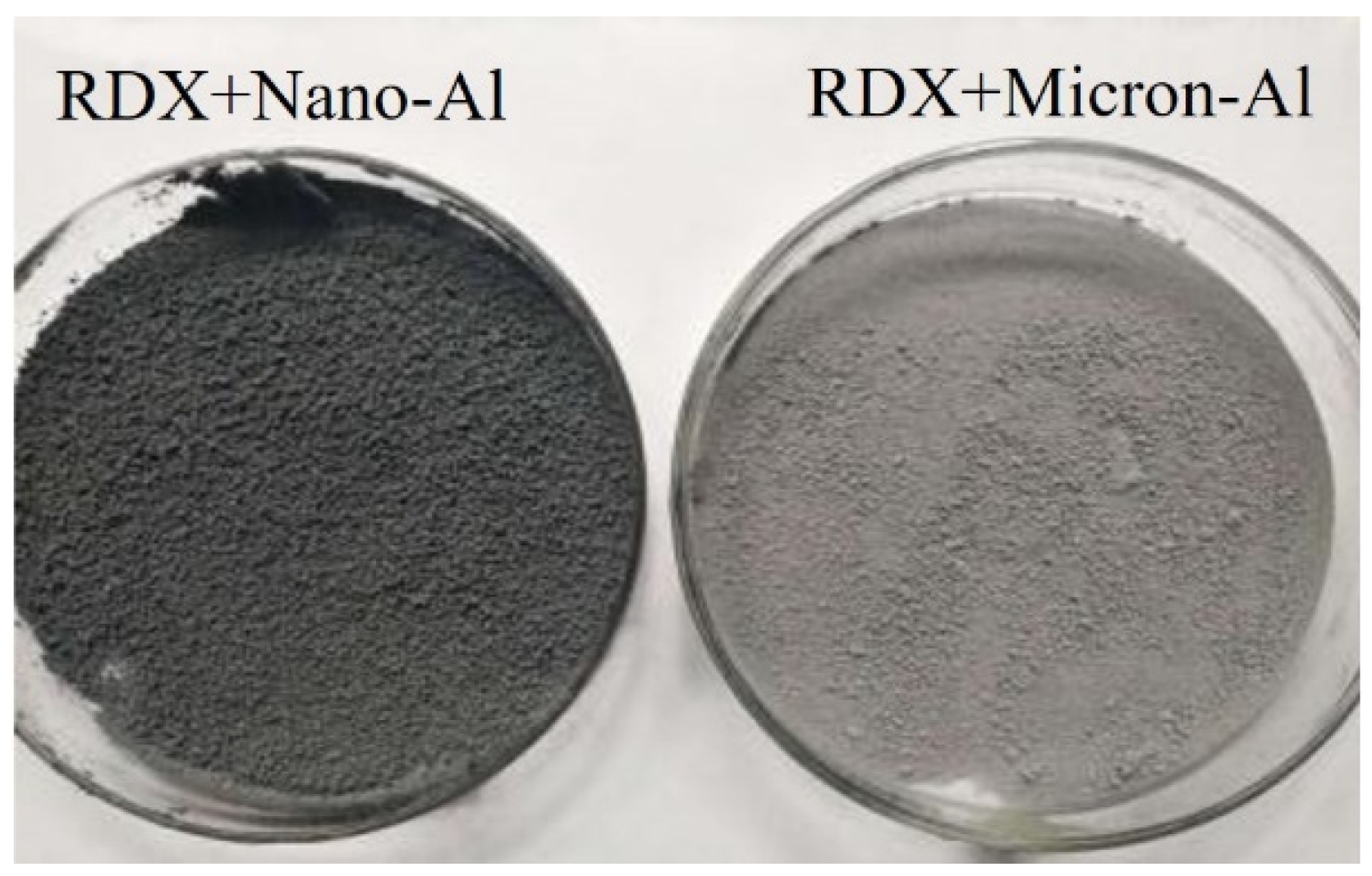
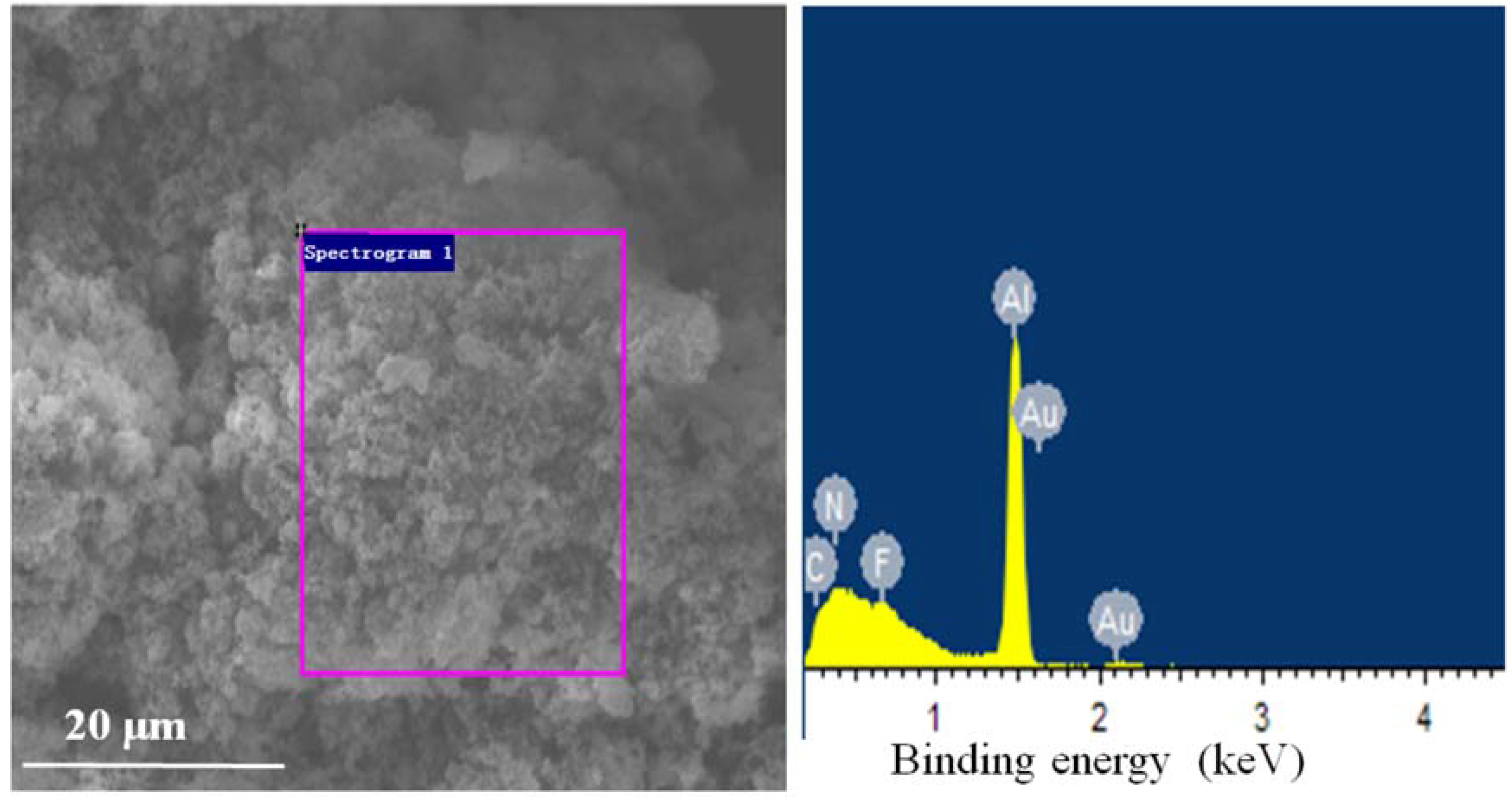
3.1. Microscopic Morphology and Surface Elemental Ratio of Explosives
3.2. Test and Analysis of Surface Energy and Adhesion Work of Materials
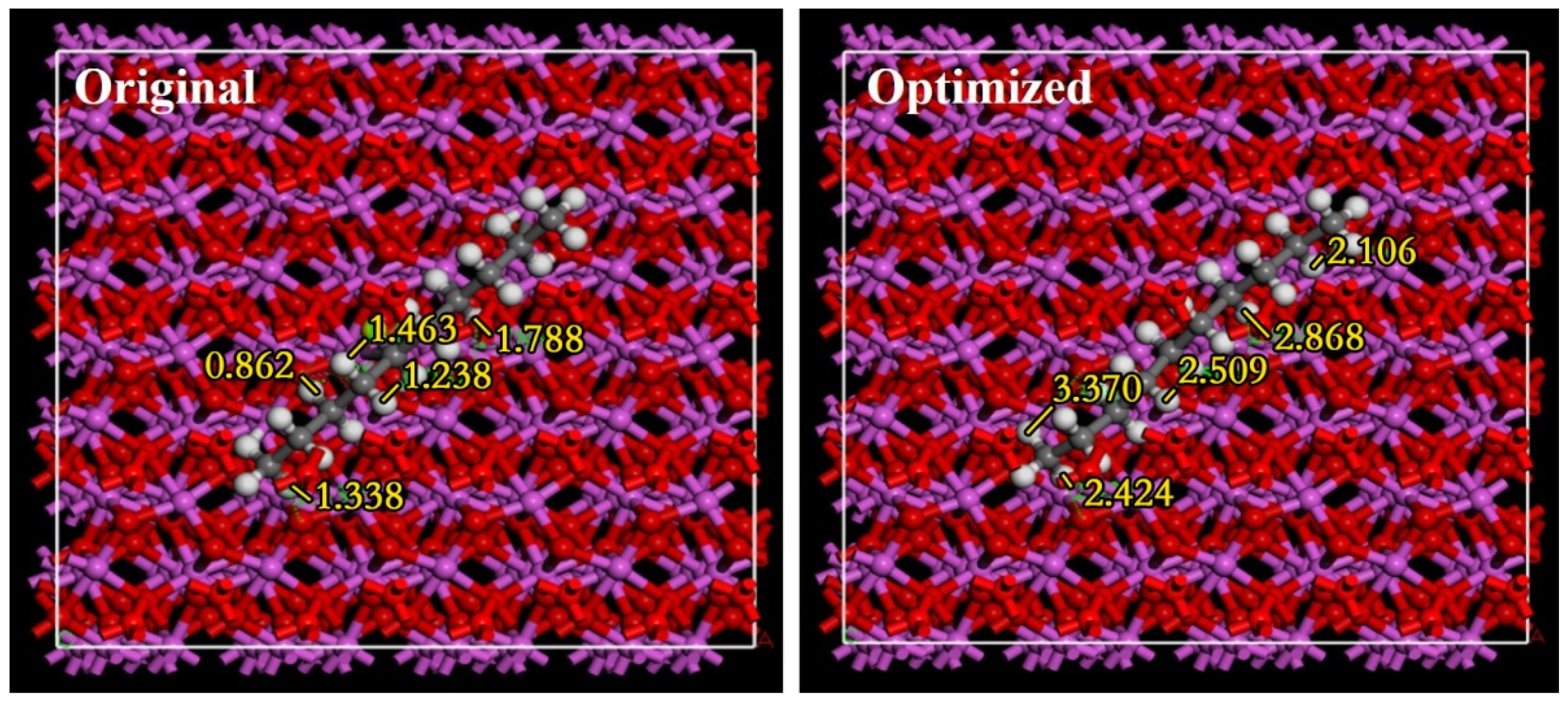
3.3. Analysis of Adsorption Energy between Explosive Components
3.4. Test and Analysis of Impact and Friction Sensitivity of Explosives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, H.; Li, X.; Zhao, F. Review on application of nano-metal powders in explosive and propellants. Chin. J. Energ. Mater. 2011, 19, 232–239. [Google Scholar]
- Wang, X.; Hao, Z. New development of explosive technology. Chin. J. Explos. Propellants 2002, 25, 35–38. [Google Scholar]
- Xu, L.; Guo, Z.; Jiang, H.; Xu, S.; Ma, J.; Hu, M.; Yu, J.; Zhao, F.; Huang, T. Dimethylglyoxime clathrate as ligand derived nitrogen-doped carbon-supported nano-metal particles as catalysts for oxygen reduction reaction. Nanomaterials 2021, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, V.; Goroshin, S.; Higgins, A.J. Aluminium particle combustion in high-speed detonation products. Combust. Sci. Technol. 2009, 181, 670–693. [Google Scholar] [CrossRef]
- Makhow, M.N. Effect of ageing on the heat of explosion of HMX-based compositions containing nano-sized aluminum. In Proceedings of the 40th International Annual Conference of ICT, Karlsruhe, Germany, 23–26 June 2009. [Google Scholar]
- Dong, J.; Wang, W.; Wang, S.; Qiu, S.; Du, M.; Tan, B.; Yang, Y.; Huang, T. Influences of multilayer graphene and boron decoration on the structure and combustion heat of Al3Mg2 alloy. Nanomaterials 2020, 10, 2013. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Z.; Zhou, H.; Chang, X.Y. Research on filling quality mechanism of ammunition. J. Proj. Rocket. Missiles Guid. 2005, 25, 59–60. [Google Scholar]
- Huang, H.; Huang, Y.; Li, S. Research on composite explosive with nano-aluminium. Chin. J. Explos. Propellants 2002, 25, 1–3. [Google Scholar]
- Komarov, V.F.; Sakovich, G.V.; Vorozhtsov, A.B. The role of nanometals in enhancement of the explosion performance of composite explosives. In Proceedings of the 40th International Annual Conference of ICT, Karlsruhe, Germany, 23–26 June 2009. [Google Scholar]
- Brousseau, P.; Dorsett, H.E.; Cliff, M.D. Detonation properties of explosives containing nanometric aluminum powder. In Proceedings of the 12th International Detonation Symposium, San Diego, CA, USA, 11–16 August 2002. [Google Scholar]
- Zhi, X. Computation on detonation velocity of single event FAE. Chin. J. Explos. Propellants 2005, 28, 76–78. [Google Scholar]
- Miao, Q.; Xu, G.; Wang, T. Mechanism analysis of the influence of Al shape and size on the detonation properties of aluminized explosives. Chin. J. Explos. Propellants 2002, 25, 4–8. [Google Scholar]
- Cook, M.A.; Filler, A.S.; Keyes, R.T. Aluminized explosives. J. Phys. Chem. 1957, 61, 189–196. [Google Scholar] [CrossRef]
- Miller, P.J. A reactive flow model with coupled reaction kinetics for detonation and combustion in non-ideal explosives. Mater. Res. Soc. Symp. Proc. 1996, 418, 413–420. [Google Scholar] [CrossRef]
- Howard, W.M.; Fried, L.E.; Souers, P.C. Kinetic modeling of non-ideal explosives with cheetah. In Proceedings of the 11th International Symposium on Detonation, Snowmass, CO, USA, 30 August–4 September 1998. [Google Scholar]
- Zhao, F.; Qin, G.; Cai, B. Research status and development trends of nanometer materials in the application of propellants and explosives. Chin. J. Explos. Propellants 2001, 24, 61–65. [Google Scholar]
- Yu, W.; Huang, H.; Nie, F. Research on nanocomposite energetic materials. Chin. J. Energ. Mater. 2005, 13, 340–343. [Google Scholar]
- Gai, G.; Yang, Y.; Jin, L. Micro-nano-sized metal particles composite and shape modification technology. J. Chin. Powder Technol. 2007, 4, 20–24. [Google Scholar]
- Yan, Z.; Deng, J.; Zhang, Y. Study on the ignition mechanism of aluminum nanoparticle by fast spectroscopy. Spectrosc. Spectr. Anal. 2010, 30, 2057–2061. [Google Scholar]
- Yao, L.; Wang, S.; Dai, Z.; Wang, H.Q.; Diao, X.Q.; Zhao, S.X. Influence of nano-aluminum on the explosion heat and thermal stability of DNTF pressed mixed explosives. Initiat. Pyrotech. 2014, 4, 47–49. [Google Scholar]
- Niu, G.; Feng, X.; Cao, S.; Yao, L. Underwater explosion test on aluminized explosive with nano-aluminum. Initiat. Pyrotech. 2013, 4, 49–52. [Google Scholar]
- Feng, X.; Huang, Y.; Xu, H. The influence of Al on the detonation parameters of aluminized explosives. Initiat. Pyrotech. 2012, 1, 38–44. [Google Scholar]
- Chen, L.; Zhang, S.; Zhao, Y. Study of the metal acceleration capacities of aluminized explosives with spherical aluminum particles of different diameter. Explos. Shock Waves 1999, 19, 250–255. [Google Scholar]
- Kaledin, L.; Tepper, F.R. Metallic nano-powders: Rocket propulsion. In Dekker Encyclopedia of Nano Science and Nano Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Wang, S.; Feng, X.; Yao, L.; Niu, G.; Cao, S. The influence of nanometer aluminum on the explosion heat of RDX-based explosive. Initiat. Pyrotech. 2014, 1, 21–24. [Google Scholar]
- Cao, S.; Niu, L.; Feng, X. The influence of nano-aluminum on the detonation velocity of RDX-based explosive. Initiat. Pyrotech. 2019, 1, 50–52. [Google Scholar]
- Huang, H.; Huang, H.; Huang, Y. The influence of aluminum particle size and oxidizer morphology in RDX- based aluminized explosives on their ability to accelerate metals. Explos. Shock Waves 2006, 26, 7–11. [Google Scholar]
- Feng, X.; Tian, X. Effect of nano-aluminum on the under-water detonation energy of explosive. Explos. Mater. 2016, 45, 1–4. [Google Scholar]
- Shen, F.; Wang, H.; Xu, S. Influence of micro-/nano-scaled particle gradation on the Dn(κ) relation of detonation wave front. Chin. J. Explos. Propellants 2018, 41, 61–65. [Google Scholar]
- Yao, L.; Feng, X.; Zhao, S.; Wang, S.; Wang, C. Influence of nano Al on mechanical sensitivity and flame sensitivity of RDX-based explosive. Chin. J. Explos. Propellants 2012, 35, 15–18. [Google Scholar]
- Chen, B.; Xia, Z.; Huang, L.; Ma, L. Characteristics of the combustion chamber of a boron-based solid propellant ducted rocket with a chin-type inlet. Aerosp. Sci. Technol. 2018, 82–83, 210–219. [Google Scholar] [CrossRef]
- Ankrevelen, D.W.V.; Nijenhuis, K.T. Proparties of Polymers: Their Correlation with Chemical Structure. Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Li, C.; Hu, C.; Deng, Z.; Hu, X.; Li, Y.; Wei, J. Dynamic ignition and combustion characteristics of agglomerated boron-magnesium particles in hot gas flow. Aerosp. Sci. Technol. 2012, 110, 106478. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, X.; Qu, X.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. High-energy metal–organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar]

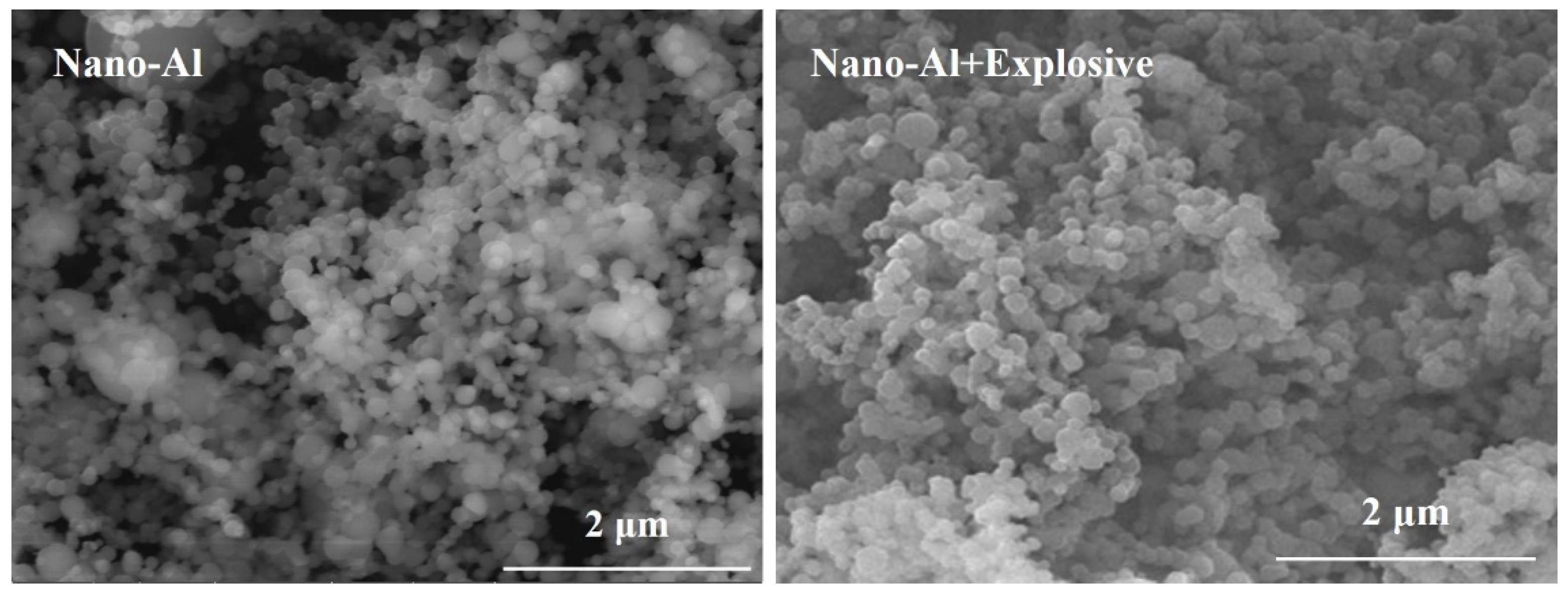
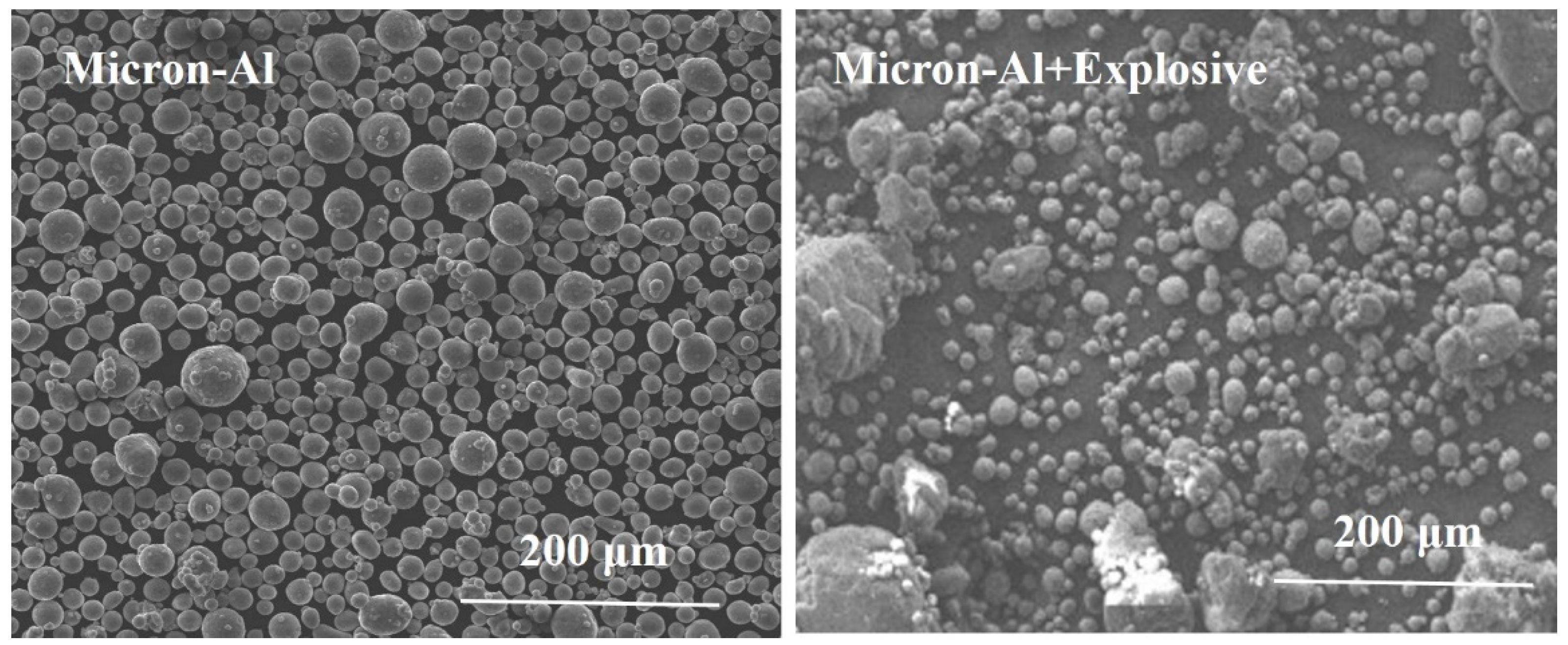
| Name | Composition and Weight Ratio (%) |
|---|---|
| Explosive 1 | nano-Al 30 |
| RDX 67 | |
| Paraffin wax 3 | |
| Explosive 2 | micron Al 30 |
| RDX 67 | |
| Paraffin wax 3 |
| Section No. | Crystal Plane | ||||||
|---|---|---|---|---|---|---|---|
| (110) | (101) | (011) | (111) | (100) | (010) | (001) | |
| 1 | −283.38324 eV | −293.38312 eV | −283.38323 eV | −293.0993 eV | −289.0031863 eV | −286.31209 eV | −286.3075026 eV |
| 2 | −278.86804 eV | −278.887853 eV | −278.886804 eV | −283.32663 eV | −289.0031755 eV | - | −286.3075033 eV |
| 3 | −286.58172 eV | −286.58182 eV | −286.58891 eV | −283.65849 eV | −289.0031783 eV | −286.0970793 eV | −286.0969497 eV |
| 4 | −286.65099 eV | −286.65109 eV | −286.65488 eV | −293.09043 eV | −289.0031823 eV | −289.0148331 eV | −289.0031925 eV |
| 5 | −278.31891 eV | −278.31902 eV | −278.31895 eV | −283.31319 eV | −289.0031799 eV | −289.4154393 eV | −289.0031915 eV |
| 6 | −293.38288 eV | −283.38337 eV | −293.39229 eV | −293.0993 eV | −289.0031804 eV | −286.3075868 eV | −286.3074286 eV |
| 7 | - | - | - | −293.0993 eV | −289.0031854 eV | −292.1069331 eV | −292.09674 eV |
| Element | Weight Percentage | Atomic Percentage |
|---|---|---|
| C | 38.10 | 54.53 |
| N | 7.38 | 9.06 |
| F | 6.24 | 5.64 |
| Al | 48.35 | 30.76 |
| Total | 100.00 | 100 |
| Element | Weight Percentage | Atomic Percentage |
|---|---|---|
| C | 28.55 | 44.89 |
| N | 7.38 | 9.96 |
| O | −0.41 | −0.48 |
| F | 1.68 | 1.67 |
| Al | 62.80 | 43.97 |
| Total | 100.00 | 100 |
| Reagent | Surface Energy (mJ) | Polar Component (mN/m) | Dispersion Component (mN/m) | Probe Liquid |
|---|---|---|---|---|
| RDX | 41.29 | 34.44 | 6.85 | water and formamide |
| Nano-Al | 40.01 | 12.52 | 27.49 | glycol and glycerol |
| Micron-Al | 18.55 | 5.48 | 13.07 | glycol and glycerol |
| Paraffin wax | 14.82 | 12.78 | 2.04 | water and glycol |
| Materials | Adhesion Work (mJ) |
|---|---|
| Paraffin with RDX | 49.40 |
| Paraffin with nano-Al | 40.31 |
| Paraffin with micron-Al | 27.06 |
| Structure | Van der Waals Force Correction Method | K-Point Grid | Calculation Accuracy | Adsorption Energy (eV) |
|---|---|---|---|---|
| Al2O3 | DFT-D3 | 9 × 9 × 1 | Default precision | −298.49886 |
| paraffin | DFT-D3 | 1 × 1 × 1 | Default precision | −173.074 |
| RDX | DFT-D3 | 3 × 3 × 1 | Default precision | −4173.2264 |
| Al2O3 + paraffin | DFT-D3 | 1 × 1 × 1 | Default precision | −4950.088 |
| RDX + paraffin | DFT-D3 | 2 × 1 × 1 | Default precision | −4328.7048 |
| Structure | Adsorption Energy (eV) |
|---|---|
| Paraffin-Micro-Al | −0.9301 |
| Paraffin-Nano-Al | −2.312 |
| Paraffin-RDX | 17.5956 |
| + | Impact Sensitivity (cm) | Friction Sensitivity (%) |
|---|---|---|
| Explosive 1 | 27.5 | 8 |
| Explosive 2 | 97.7 | 0 |
| RDX + paraffin wax | 94.5 | 0 |
| RDX | 95.7 | 84~76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Wang, W.; Wang, X.; Zhou, Q.; Miao, R.; Du, M.; Tan, B.; Wang, Y.; Zhang, T.; Li, Y.; et al. Effects of Nano Aluminum Powder on the Mechanical Sensitivity of RDX-Based Explosives. Nanomaterials 2021, 11, 2182. https://doi.org/10.3390/nano11092182
Dong J, Wang W, Wang X, Zhou Q, Miao R, Du M, Tan B, Wang Y, Zhang T, Li Y, et al. Effects of Nano Aluminum Powder on the Mechanical Sensitivity of RDX-Based Explosives. Nanomaterials. 2021; 11(9):2182. https://doi.org/10.3390/nano11092182
Chicago/Turabian StyleDong, Jun, Weili Wang, Xiaofeng Wang, Qiang Zhou, Run Miao, Maohua Du, Bo Tan, Yuanjing Wang, Tengyue Zhang, Yafei Li, and et al. 2021. "Effects of Nano Aluminum Powder on the Mechanical Sensitivity of RDX-Based Explosives" Nanomaterials 11, no. 9: 2182. https://doi.org/10.3390/nano11092182
APA StyleDong, J., Wang, W., Wang, X., Zhou, Q., Miao, R., Du, M., Tan, B., Wang, Y., Zhang, T., Li, Y., & Cao, F. (2021). Effects of Nano Aluminum Powder on the Mechanical Sensitivity of RDX-Based Explosives. Nanomaterials, 11(9), 2182. https://doi.org/10.3390/nano11092182






