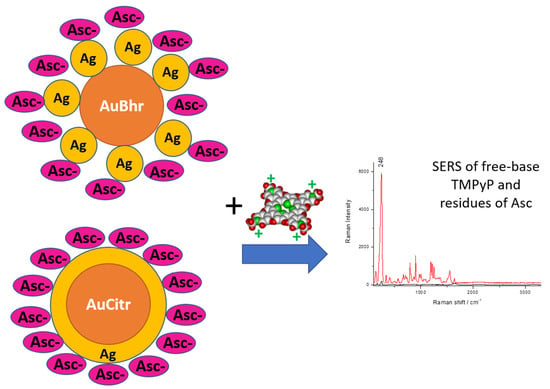
Distinctly Different Morphologies of Bimetallic Au-Ag Nanostructures and Their Application in Submicromolar SERS-Detection of Free Base Porphyrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Nomenclature
2.3. Syntheses of the AuNP Serving as Seeds
- (a)
- synthesis of AuBH-fr
- (b)
- synthesis of AuBOH-fr
- (c)
- synthesis of AuBhr (borohydride-reduced AuNPs aged for 7 months)
- (d)
- synthesis of AuCitr-fr and AuCitr (citrate-reduced AuNPs freshly prepared and aged for 7 months, respectively)
2.4. Syntheses of the Final Colloidal Au-AgNSs
- (i)
- AuBH-fr_Ag+_Asc: 0.5 mL of AuBH-fr was added to 12.5 mL of 0.2 mM AgNO3 aqueous solution, followed by 250 µL of 10 mM ascorbic acid aqueous solution. The final colloidal Au-AgNSs were stirred at the rate of 1350 rpm for 10 s, then at the rate of 850 rpm for additional 25 min.
- (ii)
- AuBOH-fr_Ag+_Asc: the same synthetic procedure as (i), however, 0.5 mL of AuBOH-fr was employed. The stirring and timing were the same as in (i).
- (iii)
- AuBhr_Ag+_Asc: the same synthetic procedure as (i), however, 0.5 mL of AuBhr colloid was added. The stirring and timing were the same as in (i).
- (iv)
- AuCitr-fr_Ag+_Asc: the synthetic procedure was the same as in (i), however, 0.5 mL of AuCitr-fr was introduced. The stirring and timing were the same as in (i).
- (v)
- AuCitr_Ag+_Asc: the synthetic procedure was the same as in (i), however, 0.5 mL of AuCitr (i.e., aged AuCitr seeds) was employed. The stirring and timing were the same as in (i).
- (vi)
- AuBhr_Asc_Ag+: the procedure of this Au-AgNSs preparation was very similar to that described in (iii) with the only exception: a reversed order of reactants was used during the second step. Briefly, 0.5 mL of the seed solution AuBhr (aged for 7 months) was added to 250 µL of 10 mM ascorbic acid aqueous solution, followed by the addition of 12.5 mL of 0.2 mM AgNO3 aqueous solution. The stirring and timing were the same as in (i).
- (vii)
- AuCitr_Asc_Ag+: the synthetic procedure was the same as in (vi), however, 0.5 mL of AuCitr (instead of AuBhr) was employed.
2.5. Methods
3. Results and Discussion
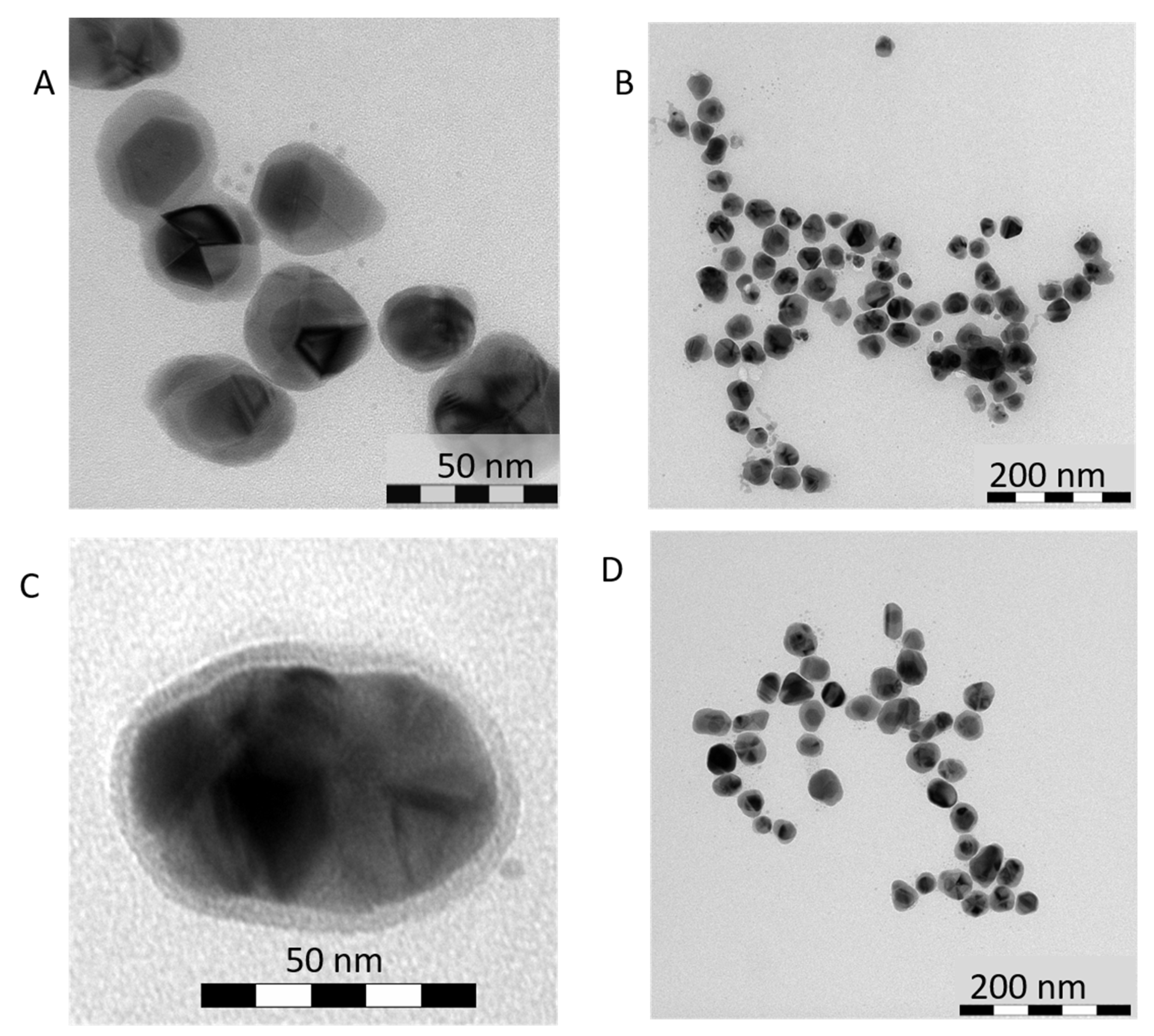
3.1. Direct Impact of Au Seed Type on Morphologies of Final Au-AgNSs
3.1.1. Au-AgNSs Stemming from Au Seeds Prepared by Reduction Induced by Borohydride
3.1.2. Au-AgNSs Stemming from Au Seeds Prepared by Reduction Induced by Citrate
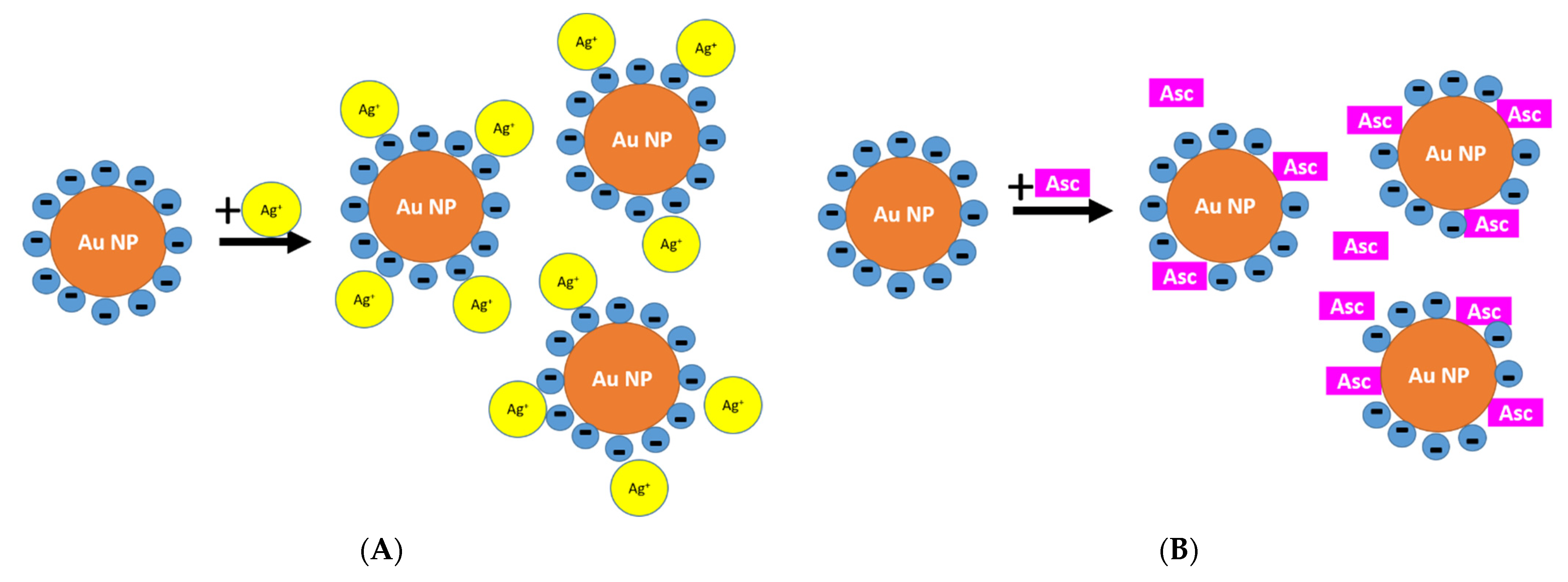
3.1.3. Reason for Different Morphologies of Au-AgNSs
3.2. Other Characteristics of Au-AgNSs Prepared by Using AuBhr or AuCitr Seeds
3.2.1. Sizes of Au-AgNSs Determined by DLS and Values Discussed in Comparison to TEM
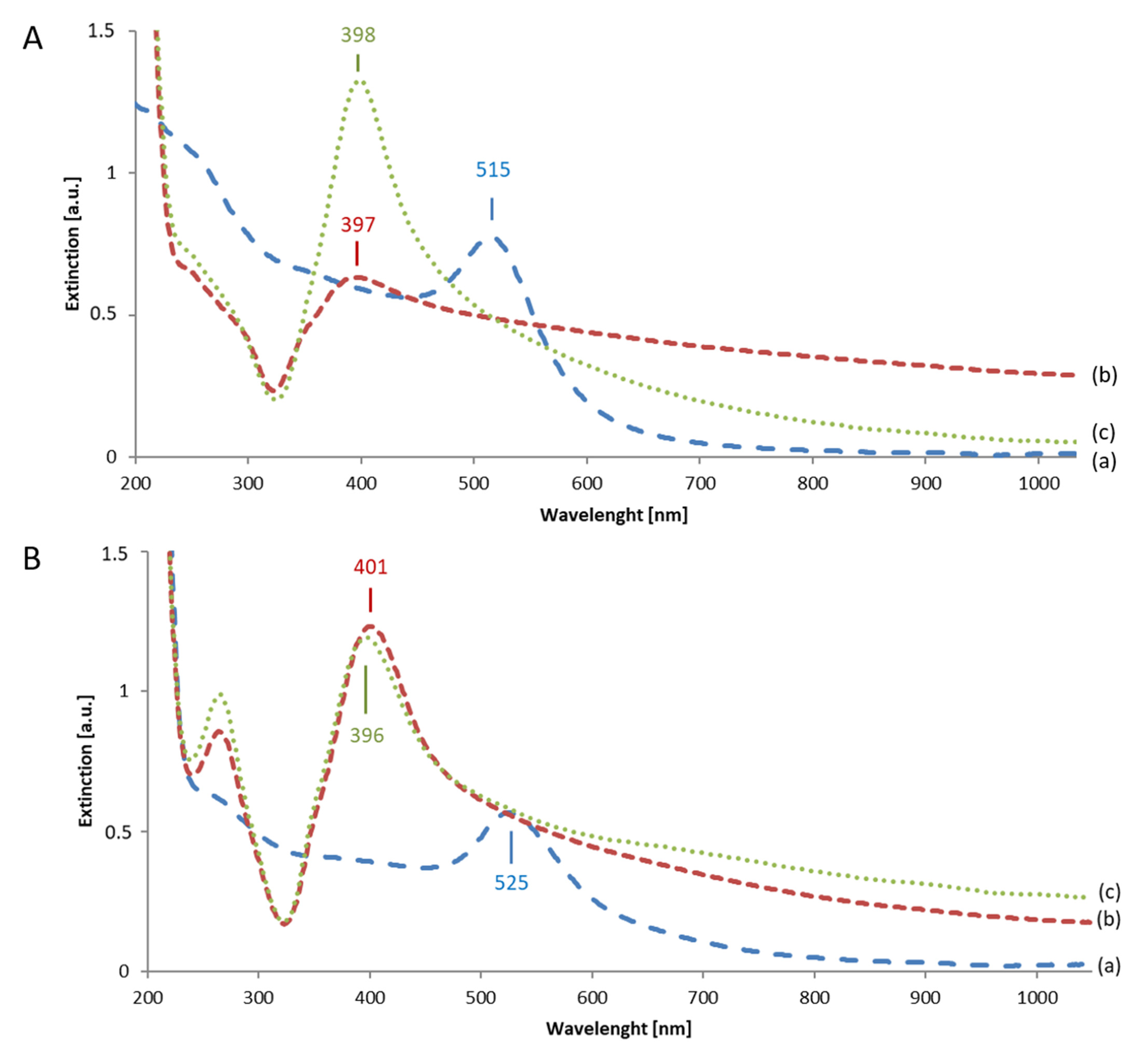
3.2.2. Extinction Spectra of Au Seeds and of Final Au-AgNSs
3.3. Classical vs. Reverse Order of Reactants in Seed-Mediated Growth Procedure of Au-AgNSs
3.4. Kinetics of Au-AgNSs Generation
3.5. SERS Application of Au-AgNSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, T.; Lu, P.; Zhang, K.; Zhou, P.; Liu, Y.; Guo, Z.; Lu, X. Gold/silver bimetallic nanocrystals: Controllable synthesis and biomedical applications. J. Biomed. Nanotechnol. 2017, 13, 1178–1209. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Odom, T.W.; Nehl, C.L. How gold nanoparticles have stayed in the light: The 3M’s principle. ACS Nano 2008, 2, 612–616. [Google Scholar] [CrossRef]
- Schwartzberg, A.M.; Zhang, J.Z. Novel optical properties and emerging applications of metal nanostructures. J. Phys. Chem. C 2008, 112, 10323–10337. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir. 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Vinod, M.; Gopchandran, K.G. Bimetallic Au-Ag nanochains as SERS substrates. Curr. Appl. Phys. 2015, 15, 857–863. [Google Scholar] [CrossRef]
- Kumari, M.M.; Jacob, J.; Philip, D. Green synthesis and applications of Au-Ag bimetallic nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsuo, R.; Jiang, P.; Miyamae, N.; Ueyama, D.; Nishio, M.; Hikino, S.; Kumagae, H.; Kamarudin, K.S.N.; Tang, X.L. Shape-dependent evolution of Au@Ag core-shell nanocrystals by PVP-assisted N,N-dimethylformamide reduction. Cryst. Growth Des. 2008, 8, 2528–2536. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Liu, Z.; Ye, J.; Khlebtsov, N.G. Au@Ag core/shell cuboids and dumbbells: Optical properties and SERS response. J. Quant. Spectrosc. Radiat. Transf. 2015, 167, 64–75. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, J.; Li, J.J.; Zhao, J.W. Multi-mode optical detection of iodide based on the etching of silver-coated gold nanobipyramids. Sensors Actuators B Chem. 2017, 253, 612–620. [Google Scholar] [CrossRef]
- Song, L.; Mao, K.; Zhou, X.; Hu, J. A novel biosensor based on Au@Ag core-shell nanoparticles for SERS detection of arsenic (III). Talanta 2016, 146, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, F.; Gao, Y.; Feng, Y.; Chen, S.; Wu, A. A rapid colorimetric detection method of trace Cr (VI) based on the redox etching of Agcore-Aushell nanoparticles at room temperature. Talanta 2012, 101, 122–127. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Kawamura, G.; Nogami, M. Preparation of Au-Ag, Ag-Au core-shell bimetallic nanoparticles for surface-enhanced Raman scattering. Scr. Mater. 2008, 58, 862–865. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Arsalani, S.; Neishaboorynejad, T. Synthesis and biosensor application of Ag@Au bimetallic nanoparticles based on localized surface plasmon resonance. Appl. Surf. Sci. 2014, 301, 230–234. [Google Scholar] [CrossRef]
- Tang, X.; Tsuji, M. Synthesis of Au core Au/Ag alloy shell nanoparticles using branched Au nanoparticles as seeds. Cryst. Eng. Comm. 2011, 13, 72–76. [Google Scholar] [CrossRef]
- Mallin, M.P.; Murphy, C.J. Solution-Phase Synthesis of Sub-10 nm Au—Ag Alloy Nanoparticles. Nano Lett. 2002, 11, 1235–1237. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, Y.; Kim, K. Dodecanethiol-derivatized Au/Ag bimetallic nanoparticles: TEM, UV/VIS, XPS, and FTIR analysis. J. Colloid Interface Sci. 1998, 278, 272–278. [Google Scholar] [CrossRef]
- Imura, Y.; Akiyama, R.; Furukawa, S.; Kan, R.; Morita-Imura, C.; Komatsu, T.; Kawai, T. Au–Ag Nanoflower Catalysts with Clean Surfaces for Alcohol Oxidation. Chem.-An Asian J. 2019, 14, 547–552. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Zhao, M.; Yang, T.H.; Gilroy, K.D.; da Silva, A.G.M.; Camargo, P.H.C.; Xia, Y. Synthesis of Colloidal Metal Nanocrystals: A Comprehensive Review on the Reductants. Chem. Eur. J. 2018, 24, 16944–16963. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Scaiano, J.C. Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles. J. Am. Chem. Soc. 2010, 132, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Mao, X.; Fu, W.; Liu, C. Seed-mediated growth of bimetallic nanoparticles as an effective strategy for sensitive detection of Vitamin C. Sensors Actuators B Chem. 2016, 231, 95–101. [Google Scholar] [CrossRef]
- Ding, S.J.; Zhu, J. Tuning the surface enhanced Raman scattering activity of gold nanocubes by silver coating. Appl. Surf. Sci. 2015, 357, 487–492. [Google Scholar] [CrossRef]
- Pei, L.; Ou, Y.; Yu, W.; Fan, Y.; Huang, Y.; Lai, K. Au-Ag Core-Shell Nanospheres for Surface-Enhanced Raman Scattering Detection of Sudan i and Sudan II in Chili Powder. J. Nanomater. 2015, 16, 215. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Caceres, R.; Dawson, C.; Formanek, P.; Fischer, D.; Simon, F.; Janke, A.; Uhlmann, P.; Stamm, M. Polymers as templates for au and Au@Ag bimetallic nanorods: Uv-vis and surface enhanced raman spectroscopy. Chem. Mater. 2013, 25, 158–169. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Possmayer, F.; Petersen, N.O. Role of different phospholipids in the synthesis of pearl-necklace-type gold-silver bimetallic nanoparticles as bioconjugate materials. J. Phys. Chem. C 2007, 111, 14113–14124. [Google Scholar] [CrossRef]
- Dai, L.; Song, L.; Huang, Y.; Zhang, L.; Lu, X.; Zhang, J.; Chen, T. Bimetallic Au/Ag Core-Shell Superstructures with Tunable Surface Plasmon Resonance in the Near-Infrared Region and High Performance Surface-Enhanced Raman Scattering. Langmuir 2017, 33, 5378–5384. [Google Scholar] [CrossRef]
- Zheng, P.; Hu, J.; Shen, G.; Jiang, J.; Yu, R.; Liu, G. Synthesis, structure and growth mechanism of size and shape tunable Au/Ag bimetallic nanoparticles. Chinese J. Chem. 2009, 27, 2137–2144. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, H.T. Anisotropic syntheses of boat-shaped core-shell Au-Ag nanocrystals and nanowires. Nanotechnology 2006, 17, 2304–2310. [Google Scholar] [CrossRef]
- Lu, L.; Burkey, G.; Halaciuga, I.; Goia, D.V. Core-shell gold/silver nanoparticles: Synthesis and optical properties. J. Colloid Interface Sci. 2013, 392, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Mori, T.; Morita-Imura, C.; Kataoka, H.; Akiyama, R.; Kurata, H.; Kawai, T. Preparation and length control of water-dispersible ultrathin gold and silver bimetallic nanowires. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 543, 9–14. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Yao, Y.; Shen, M.; Wang, X. Green synthesis of AuAtAg nanostructures through a seed-mediated method and their application in SERS. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 492, 263–272. [Google Scholar] [CrossRef]
- Cotton, T.M.; Schultz, S.G.; van Duyne, R.P. Surface-Enhanced Resonance Raman Scattering from Cytochrome c and Myoglobin Adsorbed on a Silver Electrode. J. Am. Chem. Soc. 1980, 102, 7960–7962. [Google Scholar] [CrossRef]
- Šmejkal, P.; Vlčková, B.; Procházka, M.; Mojzeš, P.; Pfleger, J. SERRS spectra of cationic free-base porphyrin species adsorbed on laser ablated Ag colloids modified by mercaptoacetate spacers. J. Mol. Struct. 1999, 482–483, 225–229. [Google Scholar] [CrossRef]
- Šišková, K.; Bečička, O.; Mašek, V.; Šafářová, K.; Zbořil, R. Spacer-free SERRS spectra of unperturbed porphyrin detected at 100 fM concentration in Ag hydrosols prepared by modified Tollens method. J. Raman Spectrosc. 2012, 43, 689–691. [Google Scholar] [CrossRef]
- Siskova, K.; Vlckova, B.; Turpin, P.Y.; Thorel, A.; Grosjean, A. Porphyrins as SERRS spectral probes of chemically functionalized Ag nanoparticles. Vib. Spectrosc. 2008, 48, 44–52. [Google Scholar] [CrossRef]
- Vlčková, B.; Šmejkal, P.; Michl, M.; Procházka, M.; Mojzeš, P.; Lednický, F.; Pfleger, J. Surface-enhanced resonance Raman spectroscopy of porphyrin and metalloporphyrin species in systems with Ag nanoparticles and their assemblies. J. Inorg. Biochem. 2000, 79, 295–300. [Google Scholar] [CrossRef]
- Procházka, M.; Turpin, P.Y.; Štěpánek, J.; Vlčková, B. SERRS of free base porphyrin in laser-ablated colloids: Evidence for three different spectral porphyrin forms. J. Raman Spectrosc. 2002, 33, 758–760. [Google Scholar] [CrossRef]
- Šišková, K.; Vlćková, B.; Turpin, P.Y.; Thorel, A.; Procházka, M. Laser ablation of silver in aqueous solutions of organic species: Probing Ag nanoparticle-Adsorbate systems evolution by surface-enhanced raman and surface plasmon extinction spectra. J. Phys. Chem. C 2011, 115, 5404–5412. [Google Scholar] [CrossRef]
- Šmejkal, P.; Šišková, K.; Vlčková, B.; Pfleger, J.; Šloufová, I.; Šlouf, M.; Mojzeš, P. Characterization and surface-enhanced Raman spectral probing of silver hydrosols prepared by two-wavelength laser ablation and fragmentation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2321–2329. [Google Scholar] [CrossRef]
- Jia, G.; Feng, Z.; Wei, C.; Li, C. Multifunctional human serum albumin in the surface-enhanced Ramanspectroscopy of porphyrin: Demetalation promoter, molecular spacer andstabilizer. J. Raman Spectrosc. 2010, 41, 1615–1620. [Google Scholar] [CrossRef]
- Vlčková, B.; Matějka, P.; Šimonová, J.; Čermáková, K.; Pančoška, P.; Baumruk, V. Surface-enhanced resonance Raman spectra of free base 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin and its silver complex in systems with silver colloid: Direct adsorption in comparison to adsorption via molecular spacer. J. Phys. Chem. 1993, 97, 9719–9729. [Google Scholar] [CrossRef]
- Song, C.; Abell, J.L.O.; He, Y.; Murph, S.H.; Cui, Y.; Zhao, Y. Gold-modified silver nanorod arrays: Growth dynamics and improved SERS properties. J. Mater. Chem. 2012, 22, 1150–1159. [Google Scholar] [CrossRef]
- van Hyning, D.L.; Zukoski, C.F. Formation mechanisms and aggregation behavior of borohydride reduced silver particles. Langmuir 1998, 14, 7034–7046. [Google Scholar] [CrossRef]
- Polte, J.; Tuaev, X.; Wuithschick, M.; Fischer, A.; Thuenemann, A.F.; Rademann, K.; Kraehnert, R.; Emmerling, F. Formation mechanism of colloidal silver nanoparticles: Analogies and differences to the growth of gold nanoparticles. ACS Nano 2012, 6, 5791–5802. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Erler, R.; Thünemann, A.F.; Sokolov, S.; Ahner, T.T.; Rademann, K.; Emmerling, F.; Kraehnert, R. Nucleation and growth of gold nanoparticles studied via in situ small angle X-ray scattering at millisecond time resolution. ACS Nano. 2010, 4, 1076–1082. [Google Scholar] [CrossRef]
- Gardiner, J.A.; Collat, J.W. Kinetics of the Stepwise Hydrolysis of Tetrahydroborate Ion. J. Am. Chem. Soc. 1965, 87, 1692–1700. [Google Scholar] [CrossRef]
- Gardiner, J.A.; Collat, J.W. The Hydrolysis of Sodium Tetrahydroborate. Identification of an Intermediate. J. Am. Chem. Soc. 1964, 86, 3165–3166. [Google Scholar] [CrossRef]
- Andrieux, J.; Demirci, U.B.; Hannauer, J.; Gervais, C.; Goutaudier, C.; Miele, P. Spontaneous hydrolysis of sodium borohydride in harsh conditions. Int. J. Hydrogen Energy. 2011, 36, 224–233. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape control of silver nanoparticles by stepwise citrate reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles - a new perspective. Cryst. Eng. Comm. 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Wuithschick, M.; Witte, S.; Kettemann, F.; Rademann, K.; Polte, J. Illustrating the formation of metal nanoparticles with a growth concept based on colloidal stability. Phys. Chem. Chem. Phys. 2015, 17, 19895–19900. [Google Scholar] [CrossRef] [Green Version]
- Šišková, K.M.; Machala, L.; Tuček, J.; Kašlík, J.; Mojzeš, P.; Zbořil, R. Mixtures of L-amino acids as reaction medium for formation of iron nanoparticles: The order of addition into a ferrous salt solution matters. Int. J. Mol. Sci. 2013, 14, 19452–19473. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Ko, F.H.; Tai, M.R.; Liu, F.K.; Chang, Y.C. Au-Ag core-shell nanoparticles with controllable shell thicknesses for the detection of adenosine by surface enhanced Raman scattering. Sensors Actuators B Chem. 2015, 211, 283–289. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Fasciani, C.; Bueno-Alejo, C.J.; Schwartz-Narbonne, R.; Scaiano, J.C. Photochemical strategies for the seed-mediated growth of gold and gold-silver nanoparticles. Langmuir 2012, 28, 16148–16155. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reguera, J.; Langer, J.; de Aberasturi, D.J.; Liz-Marzán, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef] [PubMed]
- González-Rubio, G.; Scarabelli, L.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Surfactant-Assisted Symmetry Breaking in Colloidal Gold Nanocrystal Growth. Chem. Nano. Mat. 2020, 6, 698–707. [Google Scholar] [CrossRef]
- Lohse, S.E.; Burrows, N.D.; Scarabelli, L.; Liz-Marzán, L.M.; Murphy, C.J. Anisotropic noble metal nanocrystal growth: The role of halides. Chem. Mater. 2014, 26, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef] [PubMed]
- Nermin, Y. Vitamin C—An Update on Current Uses and Functions; Chapter in Book; LeBlanc, J.G., Ed.; InTech Open: London, UK, 2018; ISBN 978-1-78923-896-9. [Google Scholar] [CrossRef] [Green Version]
- Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering. Langmuir 1995, 11, 3715–3720. [Google Scholar] [CrossRef]


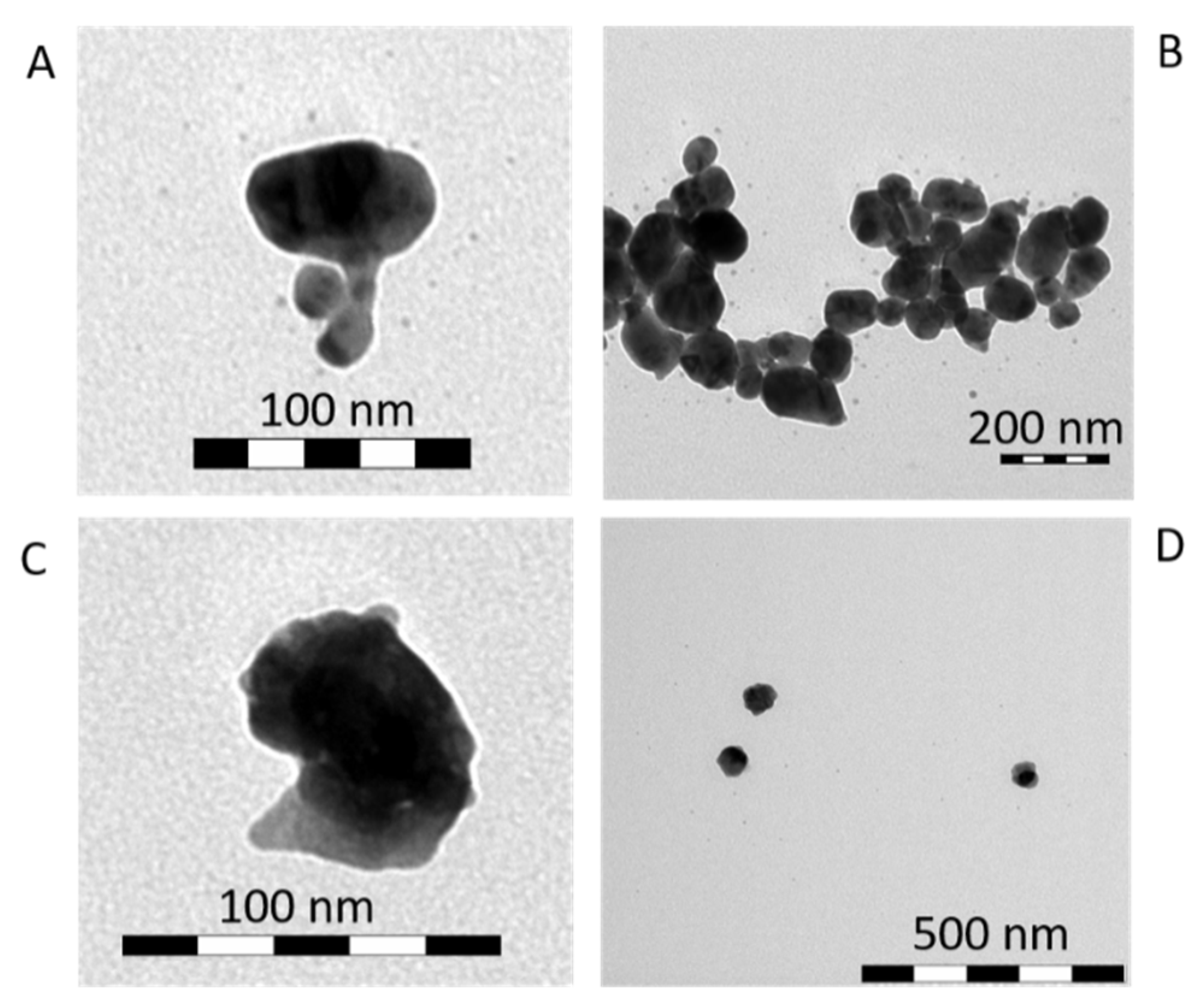
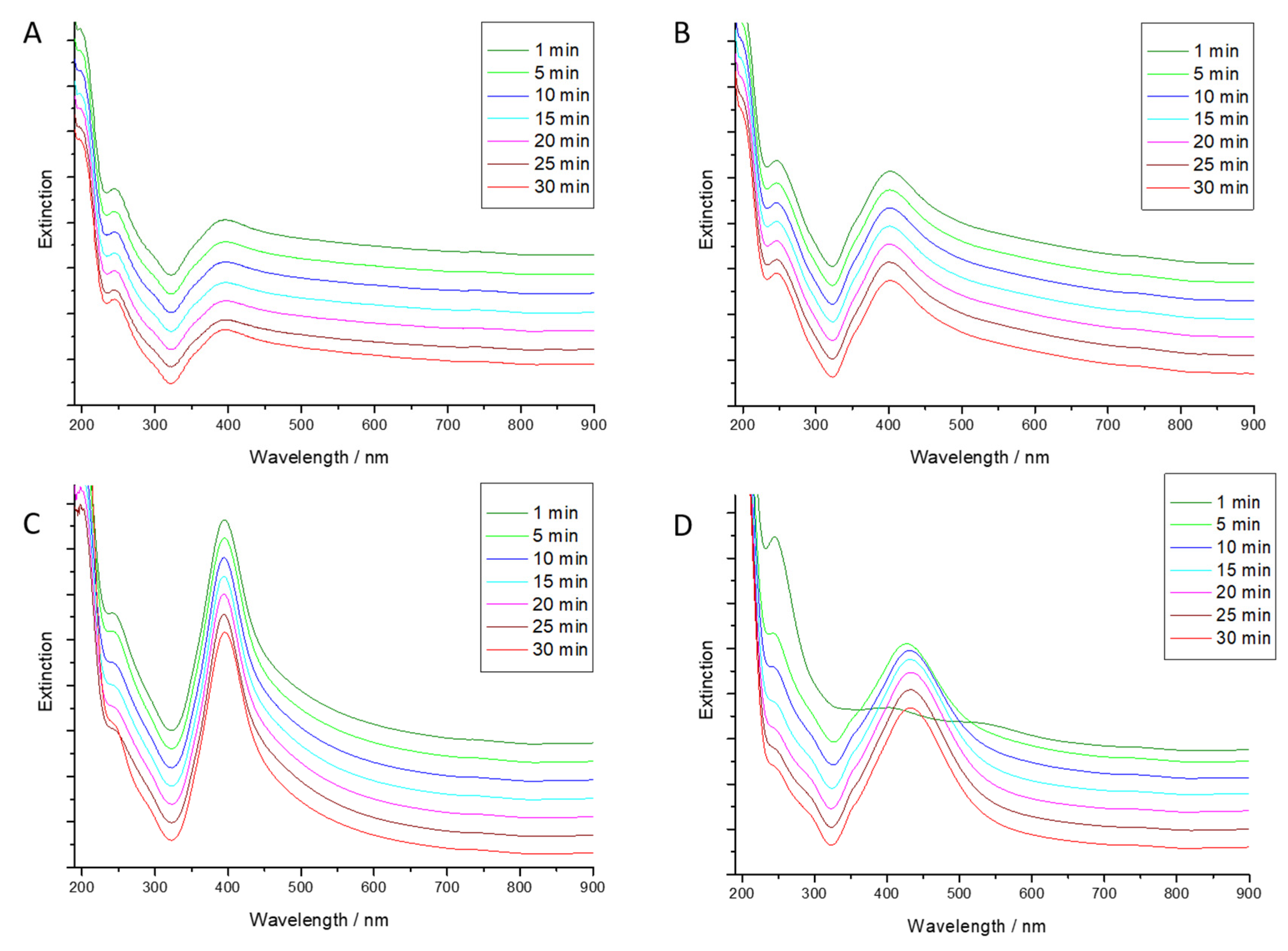
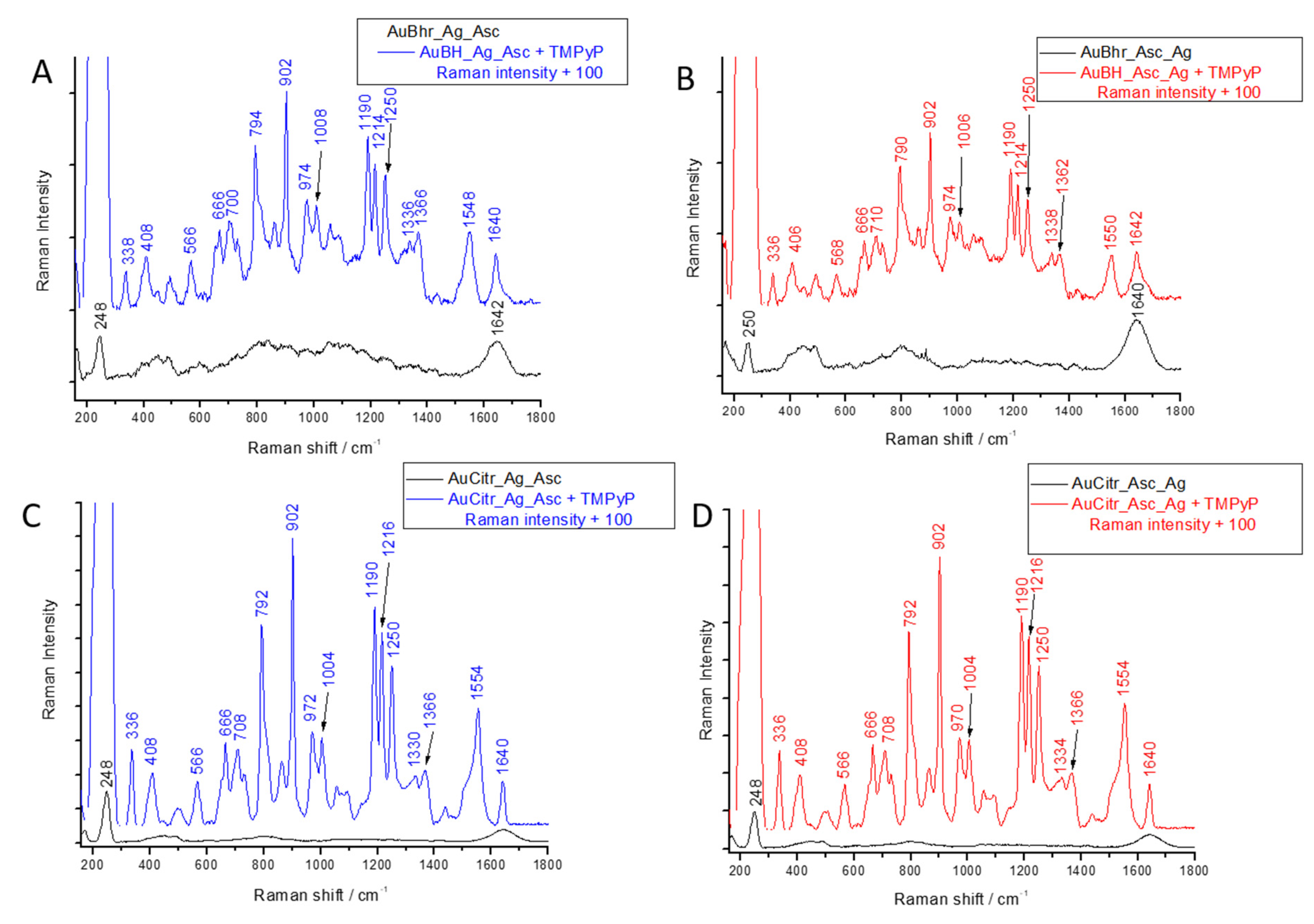
| Seed Solution Code | Final Colloidal Au-AgNSs Code |
|---|---|
| AuBH-fr | AuBH-fr_Ag+_Asc |
| AuBOH-fr | AuBOH-fr_Ag+_Asc |
| AuCitr-fr | AuCitr-fr_Ag+_Asc |
| AuBhr | AuBhr_Ag+_Asc |
| AuBhr | AuBhr_Asc_Ag+ |
| AuCitr | AuCitr_Ag+_Asc |
| AuCitr | AuCitr_Asc_Ag+ |
| Sample Code | Mean Particle Sizes and Their Percentual Contents Based on Light Intensity Changes | pH Values | |||
|---|---|---|---|---|---|
| [nm] | [%] | [nm] | [%] | ±0.05 | |
| AuBhr seeds | 44 ± 12 | 46 | 9 ± 1 | 54 | 7.80 |
| AuBhr_Ag+_Asc | 67 ± 38 | 76 | 11 ± 4 | 24 | 3.44 |
| AuBhr_Asc_Ag+ | 55 ± 27 | 70 | 7 ± 3 | 30 | 3.49 |
| AuCitr seeds | 41 ± 13 | 59 | 14 ±4 | 35 | 6.02 |
| AuCitr_Ag+_Asc | 65 ± 34 | 80 | 10 ± 4 | 20 | 3.69 |
| AuCitr_Asc_Ag+ | 74 ± 39 | 76 | 13 ± 5 | 24 | 3.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilímová, I.; Šišková, K. Distinctly Different Morphologies of Bimetallic Au-Ag Nanostructures and Their Application in Submicromolar SERS-Detection of Free Base Porphyrin. Nanomaterials 2021, 11, 2185. https://doi.org/10.3390/nano11092185
Vilímová I, Šišková K. Distinctly Different Morphologies of Bimetallic Au-Ag Nanostructures and Their Application in Submicromolar SERS-Detection of Free Base Porphyrin. Nanomaterials. 2021; 11(9):2185. https://doi.org/10.3390/nano11092185
Chicago/Turabian StyleVilímová, Iveta, and Karolína Šišková. 2021. "Distinctly Different Morphologies of Bimetallic Au-Ag Nanostructures and Their Application in Submicromolar SERS-Detection of Free Base Porphyrin" Nanomaterials 11, no. 9: 2185. https://doi.org/10.3390/nano11092185