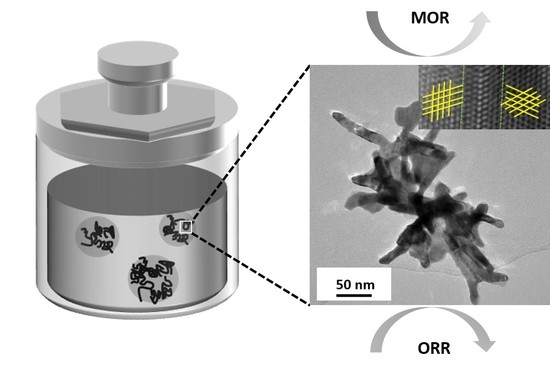
Self-Supported Defect-Rich Au-Based Nanostructures as Robust Bifunctional Catalysts for the Methanol Oxidation Reaction and Oxygen Reduction Reaction in an Alkaline Medium
Abstract
:1. Introduction
2. Materials and Methods
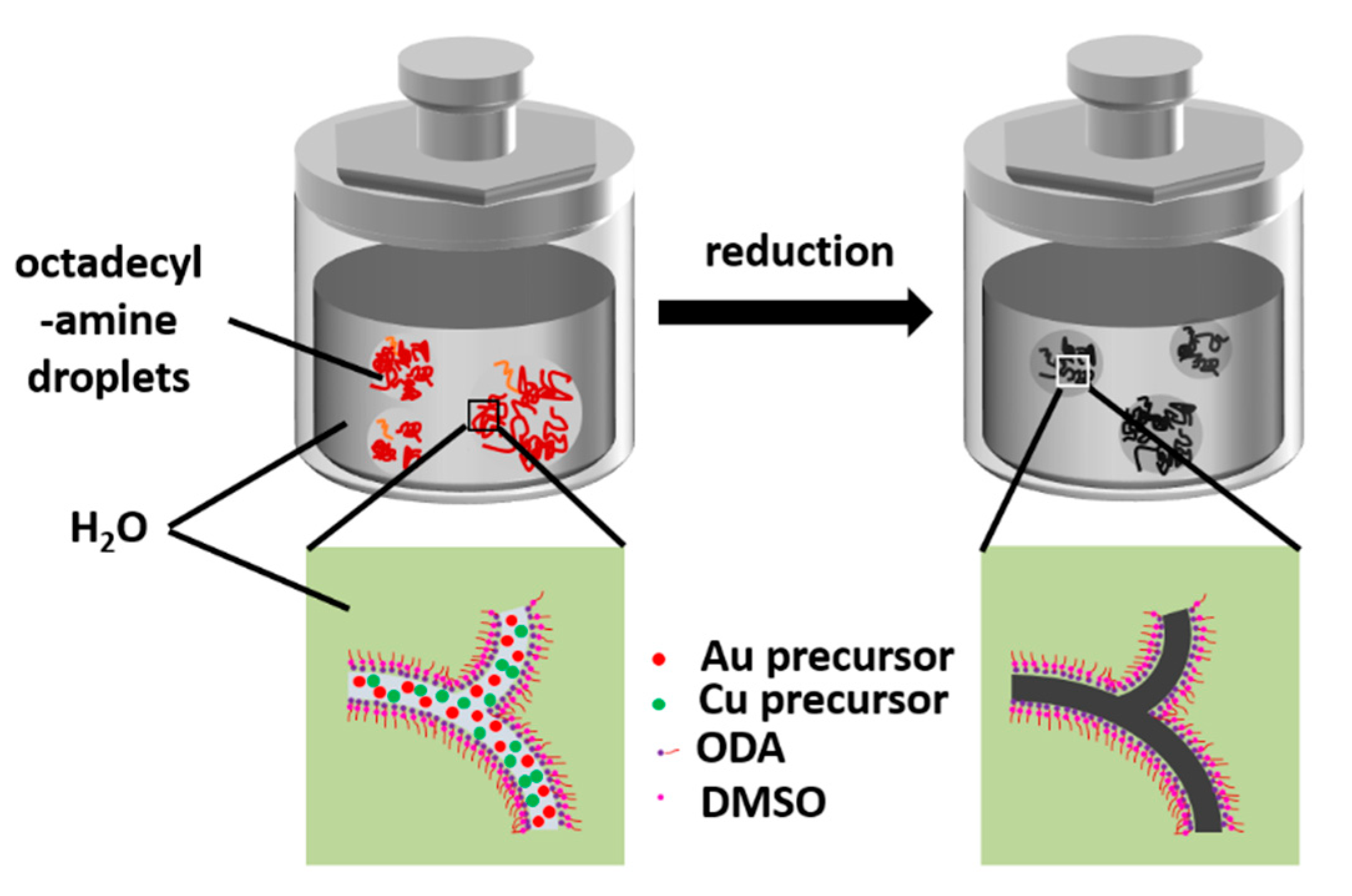
2.1. Synthesis of AuCu
2.2. Synthesis of Au/C
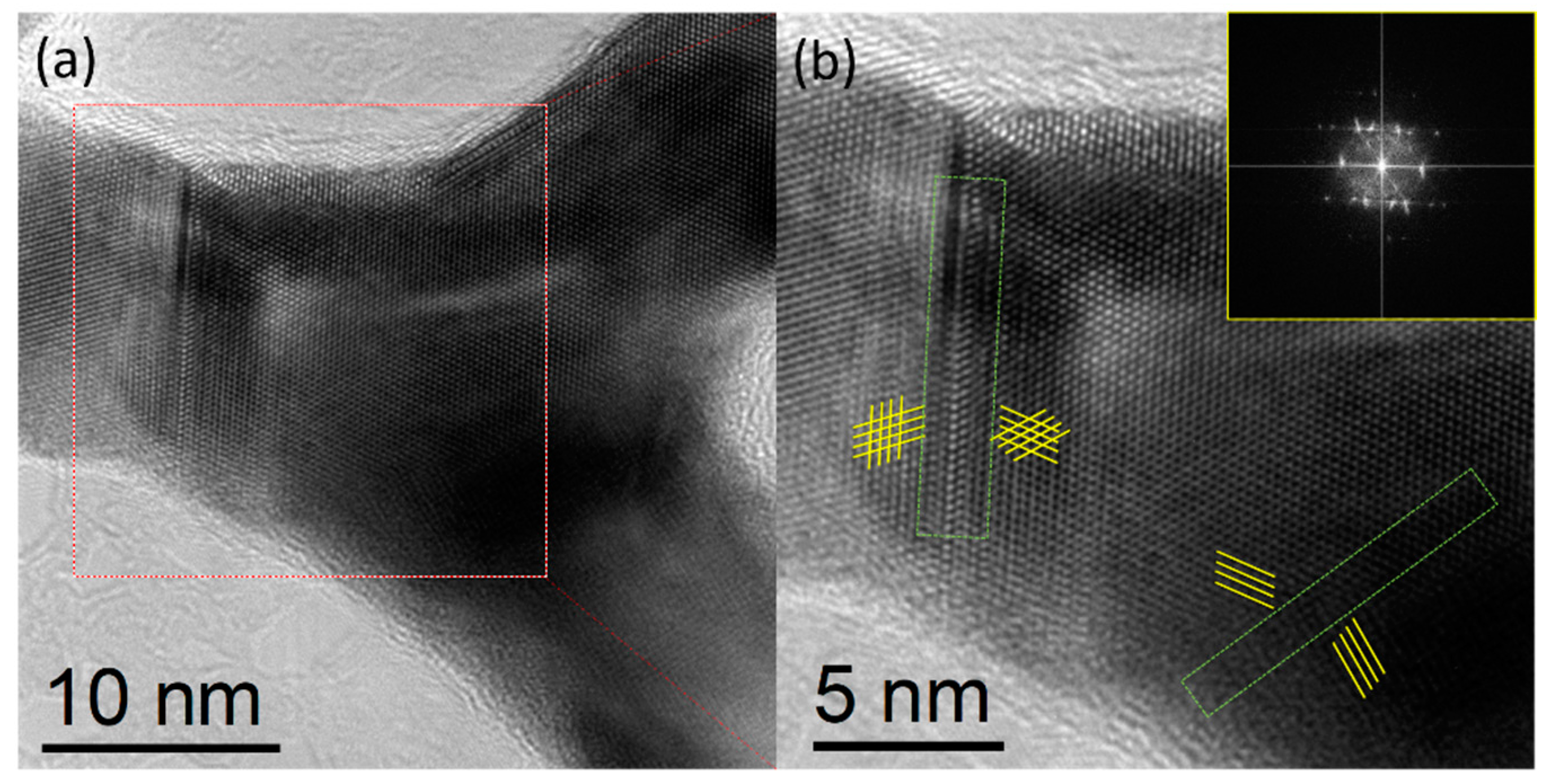
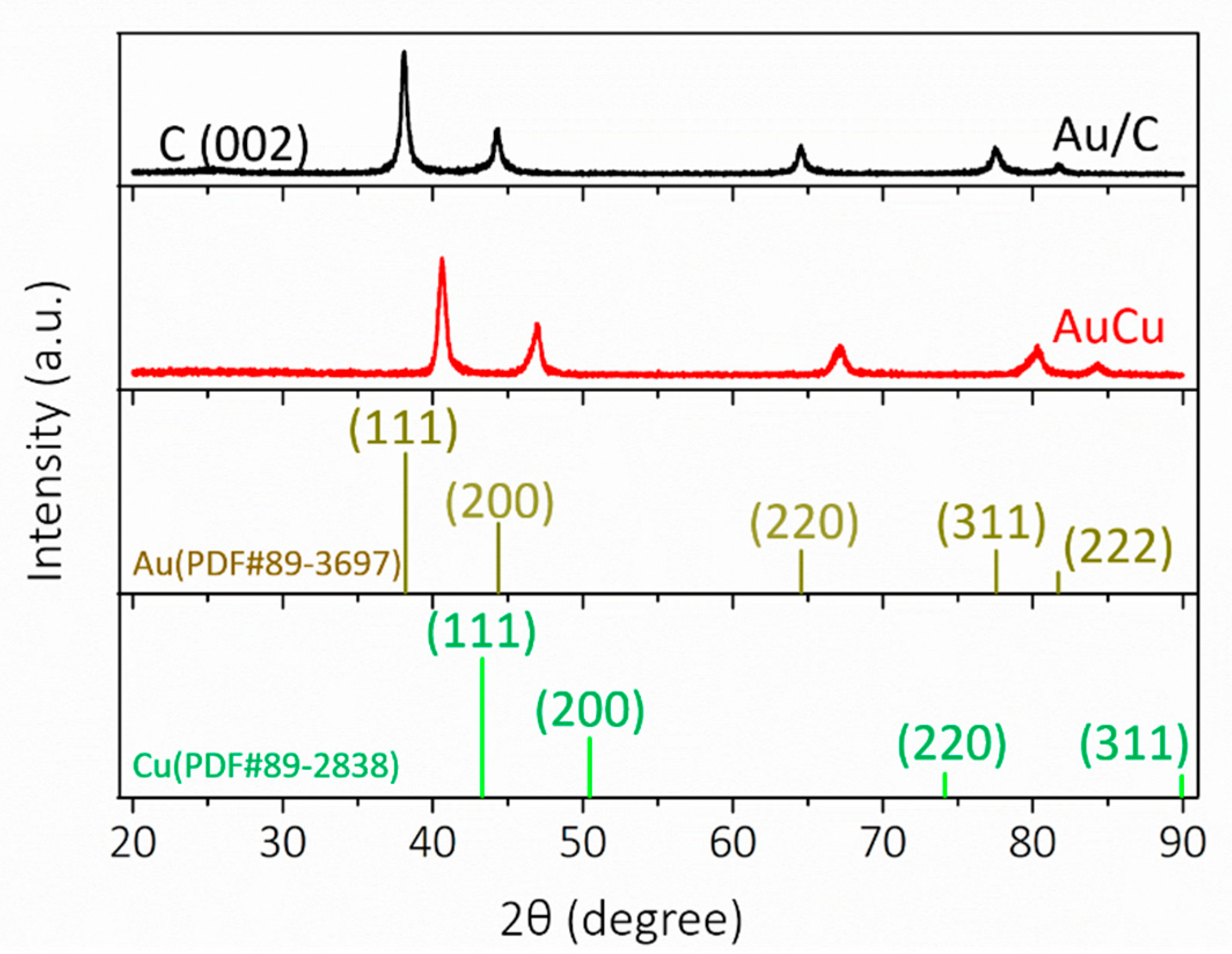
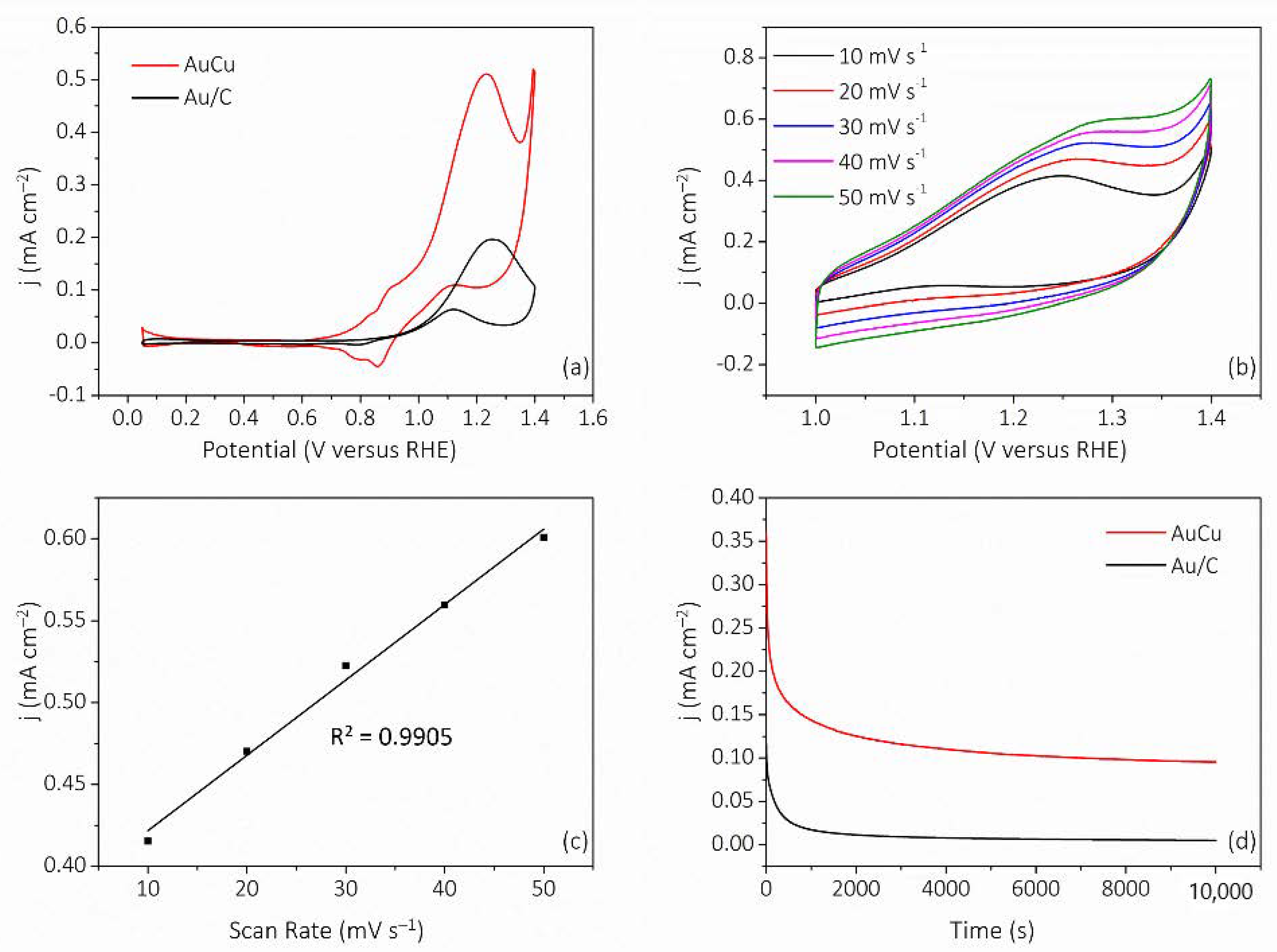
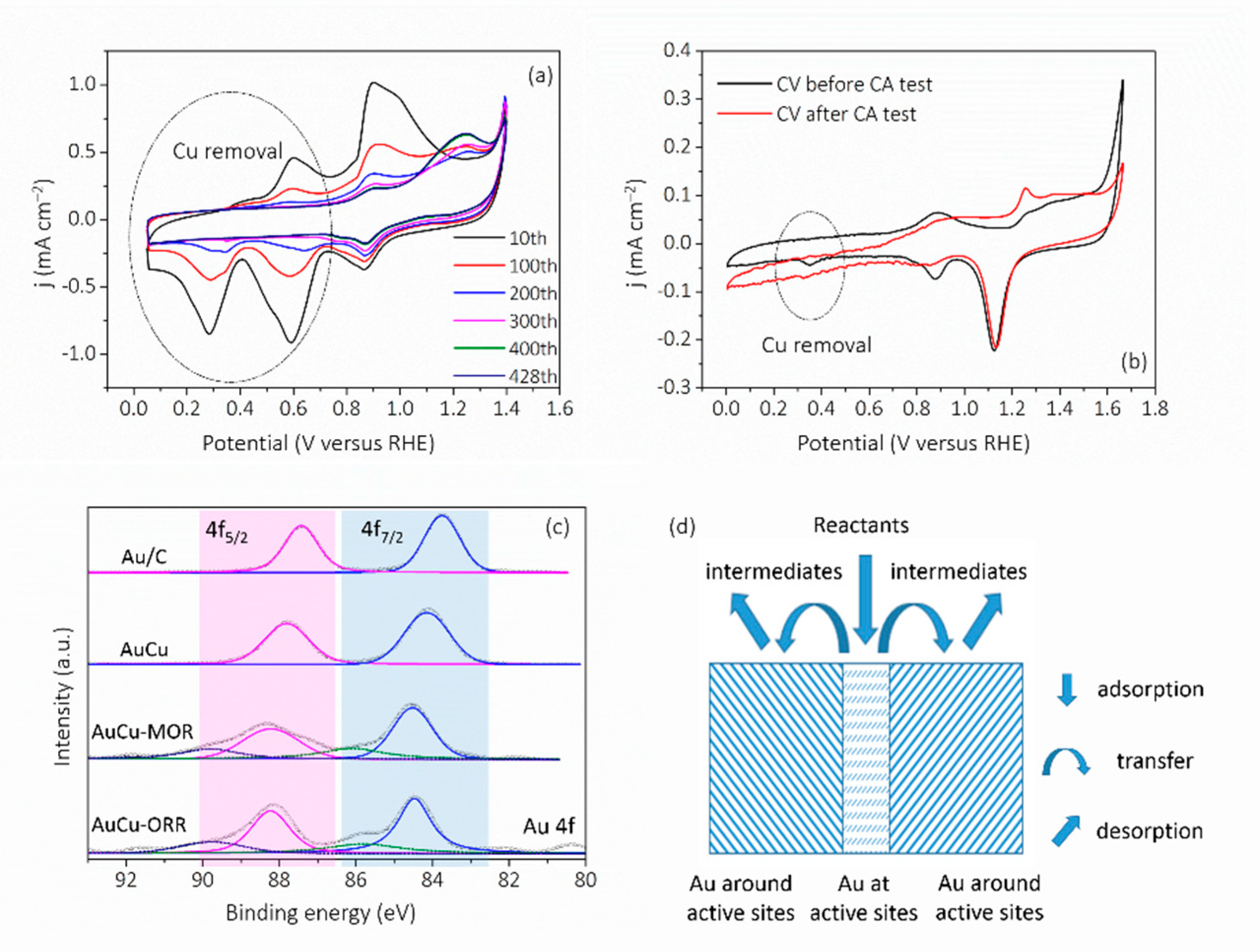
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lamy, C.; Léger, J.-M.; Srinivasan, S. Direct methanol fuel cells: From a twentieth century electrochemist’s dream to a twenty-first century emerging technology. In Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2002; pp. 53–118. [Google Scholar]
- Hamnett, A. Mechanism and electrocatalysis in the direct methanol fuel cell. Catal. Today 1997, 38, 445–457. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.; Daud, W.; Yaakub, Z. Nanocatalyst for direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2010, 35, 7957–7970. [Google Scholar] [CrossRef]
- Li, M.; Kuttiyiel, K.; Lu, F.; Gang, O.; Adzic, R.R. Platinum monolayer electrocatalysts for methanol oxidation. J. Electrochem. Soc. 2019, 166, F3300. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Qin, C.; Liang, Z.; Scida, A.; Yue, S.; Tong, X.; Adzic, R.R.; Wong, S.S. Ultrathin PtxSn1–x Nanowires for Methanol and Ethanol Oxidation Reactions: Tuning Performance by Varying Chemical Composition. ACS Appl. Nano Mater. 2018, 1, 1104–1115. [Google Scholar] [CrossRef]
- Sheng, T.; Sun, S.-G. Insight into the promoting role of Rh doped on Pt (111) in methanol electro-oxidation. J. Electroanal. Chem. 2016, 781, 24–29. [Google Scholar] [CrossRef]
- Tokarz, W.; Siwek, H.; Piela, P.; Czerwiński, A. Electro-oxidation of methanol on Pt-Rh alloys. Electrochim. Acta 2007, 52, 5565–5573. [Google Scholar] [CrossRef]
- Gong, H.; Cao, X.; Mendes, R.G.; Rummeli, M.H.; Zhang, J.; Yang, R. Self-Supported PtAuCu@Cu2O/Pt Hybrid Nanobranch as a Robust Electrocatalyst for the Oxygen Reduction Reaction. ChemElectroChem 2017, 4, 1554–1559. [Google Scholar] [CrossRef]
- Gong, H.; Cao, X.; Li, F.; Gong, Y.; Gu, L.; Mendes, R.G.; Rummeli, M.H.; Strasser, P.; Yang, R. PdAuCu Nanobranch as Self-Repairing Electrocatalyst for Oxygen Reduction Reaction. ChemSusChem 2017, 10, 1469–1474. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Li, X.; Tian, J.; Jin, C.; Yang, R. Biomass lysine-derived nitrogen-doped carbon hollow cubes via a NaCl crystal template: An efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2017, 9, 1059–1067. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Wei, C.; Sun, Z.; Zeng, K.; Shen, L.; Sun, J.; Rümmeli, M.H.; Yang, R. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction. Carbon 2021, 171, 320–328. [Google Scholar] [CrossRef]
- Yadav, A.; Li, Y.; Liao, T.W.; Hu, K.J.; Scheerder, J.E.; Safonova, O.V.; Höltzl, T.; Janssens, E.; Grandjean, D.; Lievens, P. Enhanced Methanol Electro-Oxidation Activity of Nanoclustered Gold. Small 2021, 17, 2004541. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Sun, Z.; Zhang, X.; Zhao, L.; Huang, P.; Chen, X.; Jin, H.; Sun, H.; Lian, Y.; Deng, Z.; et al. Octahedral gold-silver nanoframes with rich crystalline defects for efficient methanol oxidation manifesting a CO-promoting effect. Nat. Commun. 2019, 10, 3782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Qiu, X.; Fu, G.; Sun, J.; Huang, Z.; Sun, D.; Xu, L.; Zhou, J.; Tang, Y. Highly simple and rapid synthesis of ultrathin gold nanowires with (111)-dominant facets and enhanced electrocatalytic properties. J. Mater. Chem. A 2018, 6, 17682–17687. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Lu, Y.; An, L.; Li, X.; Xia, D.; Zhang, Z.; Li, J. Nano-Intermetallic AuCu3 Catalyst for Oxygen Reduction Reaction: Performance and Mechanism. Small 2014, 10, 2662–2669. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Jin, Y.; Lei, Y.; Johnston, R.L. One-Pot Synthesis of Dealloyed AuNi Nanodendrite as a Bifunctional Electrocatalyst for Oxygen Reduction and Borohydride Oxidation Reaction. Adv. Funct. Mater. 2017, 27, 1700260. [Google Scholar] [CrossRef]
- Gong, H.; Yang, R.; Yang, B.; Li, F.; Li, L. Boosting the catalysis of AuCuMo for oxygen reduction: Important roles of an optimized electronic structure and surface electrochemical stability. J. Alloy. Compd. 2020, 837, 155552. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Cheng, R.; Ruan, M. Hydrothermal microemulsion synthesis of stoichiometric single crystal hydroxyapatite nanorods with mono-dispersion and narrow-size distribution. Mater. Lett. 2007, 61, 1683–1687. [Google Scholar] [CrossRef]
- Gong, H.; Li, F.; Yang, Z.; Wang, Y. Understanding the role of ‘path’ for sacrificial substance migration during the fabrication of hollow nanostructures in PtPdCu system. Mater. Res. Express 2015, 2, 085008. [Google Scholar] [CrossRef]
- Hu, W.; Gu, H.; Wang, J.; Li, Y.; Wang, Z. One-step synthesis of silica hollow particles in a W/O inverse emulsion. Colloid Polym. Sci. 2013, 291, 2697–2704. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, C.; Wang, Y.; Chen, R.; Wang, S. Recent advances in defect electrocatalysts: Preparation and characterization. J. Energy Chem. 2021, 53, 208–225. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, J.; Wang, H.; Zhang, J.; Liu, X.; Zhang, P.; Chi, M.; Guo, Y.; Guo, Y.; Lu, G.; et al. Crystal Structural Effect of AuCu Alloy Nanoparticles on Catalytic CO Oxidation. J. Am. Chem. Soc. 2017, 139, 8846–8854. [Google Scholar] [CrossRef] [PubMed]
- Barbera, K.; Frusteri, L.; Italiano, G.; Spadaro, L.; Frusteri, F.; Perathoner, S.; Centi, G. Low-temperature graphitization of amorphous carbon nanospheres. Chin. J. Catal. 2014, 35, 869–876. [Google Scholar] [CrossRef]
- Pope, C.G. X-ray diffraction and the Bragg equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Electrochemical quantification of copper-based alloys using voltammetry of microparticles: Optimization of the experimental conditions. J. Solid State Electrochem. 2012, 16, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, L.; Yang, L.; Liao, F.; Sheng, M.; Jiang, B.; Shao, M. Prominent electrocatalytic methanol oxidation from cauli-flower shape gold with high-index facets. Mater. Chem. Phys. 2017, 186, 301–304. [Google Scholar] [CrossRef]
- Song, Y.; Miao, T.; Zhang, P.; Bi, C.; Xia, H.; Wang, D.; Tao, X. {331}-Faceted trisoctahedral gold nanocrystals: Synthesis, superior electrocatalytic performance and highly efficient SERS activity. Nanoscale 2015, 7, 8405–8415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, F.; Jin, Y.; Johnston, R.L. Highly active and stable AuNi dendrites as an electrocatalyst for the oxygen reduction reaction in alkaline media. J. Mater. Chem. A 2016, 4, 17828–17837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, Y.; Zhang, Y.; Song, J.; Si, Y.; Yan, J.; Ma, C.; Liu, Y.-T.; Yu, J.; Ding, B. Conductive and Elastic TiO2 Nanofibrous Aerogels: A New Concept toward Self-Supported Electrocatalysts with Superior Activity and Durability. Angew. Chem. Int. Ed. 2020, 59, 23252–23260. [Google Scholar] [CrossRef] [PubMed]
- Li, G.G.; Villarreal, E.; Zhang, Q.; Zheng, T.; Zhu, J.-J.; Wang, H. Controlled Dealloying of Alloy Nanoparticles toward Optimization of Electrocatalysis on Spongy Metallic Nanoframes. ACS Appl. Mater. Interfaces 2016, 8, 23920–23931. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, R.; Chen, M.; Zhang, Y.; Ding, Y. Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef]
- Li, X.-R.; Li, X.-L.; Xu, M.-C.; Xu, J.-J.; Chen, H.-Y. Gold nanodendrities on graphene oxide nanosheets for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 1697–1703. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Liang, X.; Xu, G.; Li, D.; Li, Y.; Zhang, N.; Chen, Y.; Jiang, X.; Gong, H. Self-Supported Defect-Rich Au-Based Nanostructures as Robust Bifunctional Catalysts for the Methanol Oxidation Reaction and Oxygen Reduction Reaction in an Alkaline Medium. Nanomaterials 2021, 11, 2193. https://doi.org/10.3390/nano11092193
Tao Y, Liang X, Xu G, Li D, Li Y, Zhang N, Chen Y, Jiang X, Gong H. Self-Supported Defect-Rich Au-Based Nanostructures as Robust Bifunctional Catalysts for the Methanol Oxidation Reaction and Oxygen Reduction Reaction in an Alkaline Medium. Nanomaterials. 2021; 11(9):2193. https://doi.org/10.3390/nano11092193
Chicago/Turabian StyleTao, Yuanyuan, Xiu Liang, Guanchen Xu, Dongwei Li, Yong Li, Na Zhang, Yingzhou Chen, Xifeng Jiang, and Hongyu Gong. 2021. "Self-Supported Defect-Rich Au-Based Nanostructures as Robust Bifunctional Catalysts for the Methanol Oxidation Reaction and Oxygen Reduction Reaction in an Alkaline Medium" Nanomaterials 11, no. 9: 2193. https://doi.org/10.3390/nano11092193