Efficiency of Wood-Dust of Dalbergia sisoo as Low-Cost Adsorbent for Rhodamine-B Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.2.1. Preparation of Wood Powder
2.2.2. Preparation of AC by the Carbonization Method
2.2.3. Characterization Technique of the As-Prepared AC (Db-s)
2.2.4. Methodology of Dye Adsorption
3. Results and Discussion
3.1. Proximate Analysis
3.2. TGA/DSC
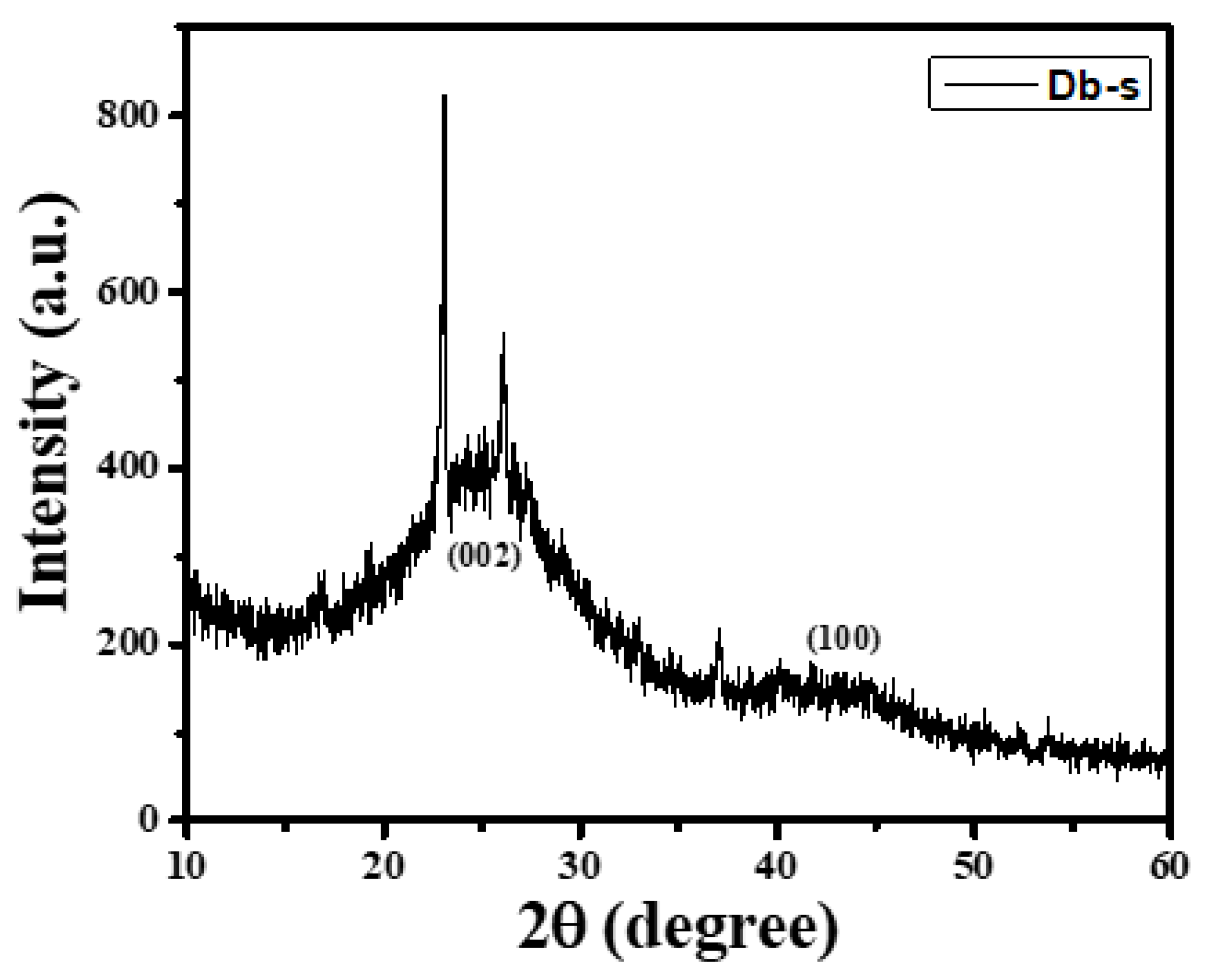
3.3. XRD Measurement
3.4. Raman Scattering Analysis
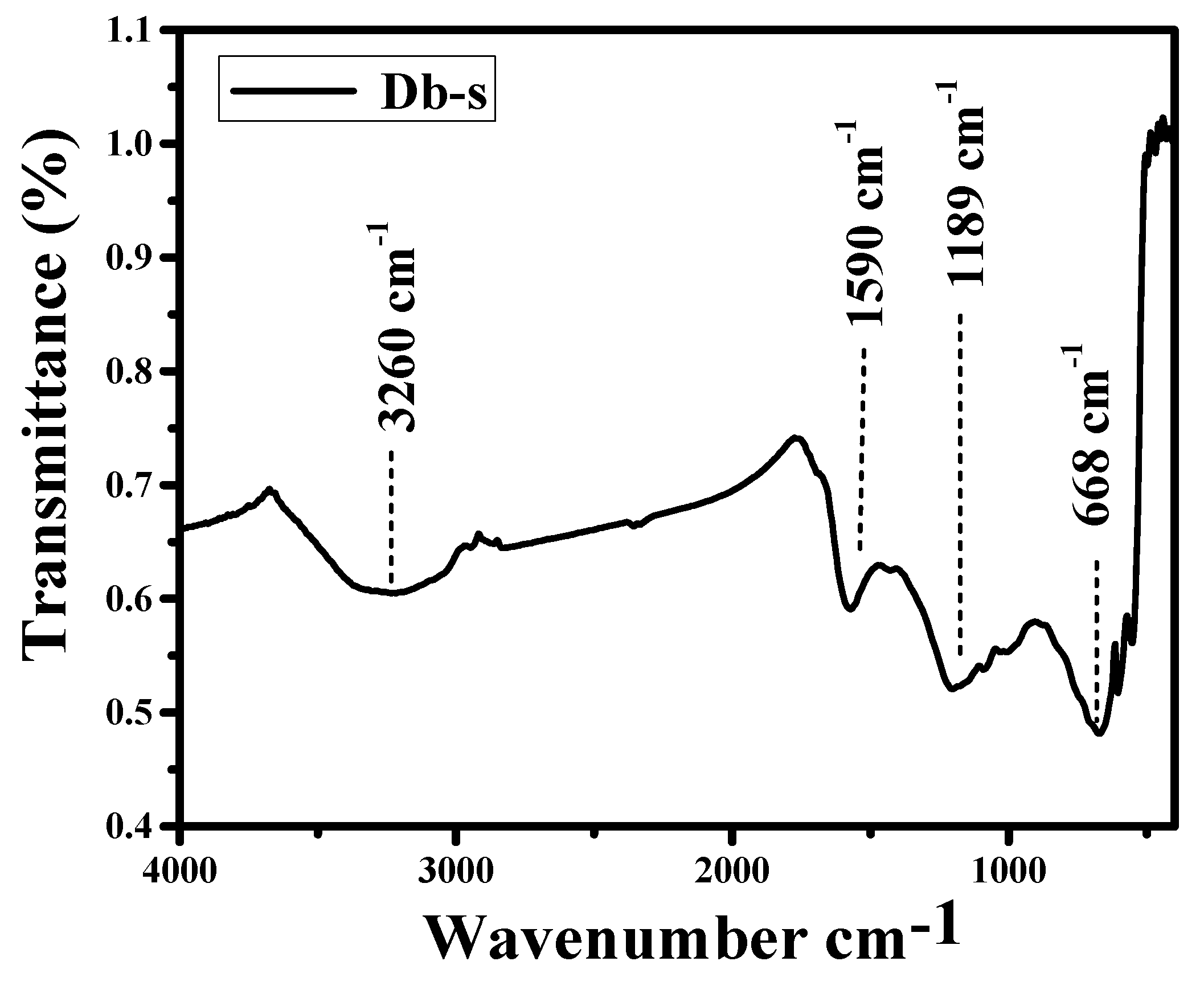
3.5. FTIR Analysis
3.6. N2 Adsorption/Desorption Isotherm/Brunauer–Emmett–Teller (BET) Theory
3.7. SEM Analysis
3.8. Effects of Variable Parameters
3.8.1. Effect of pH and Contact Time on the Adsorption of RhB
3.8.2. Optimization of the Dose of AC (Db-s)
4. Conclusions
- AC was successfully prepared from the agro waste of Dalbergia sisoo by activating with H3PO4 followed by carbonization at 400 °C.
- BET measurements showed the formation of a mesoporous structure with an active surface area of 1376 m2 g−1, a pore volume of 1.2 cm3 g−1, and a pore size of 4.06 nm.
- AC (Db-s) was found to possess the best adsorption capacity at pH 8.5 and a dose of 0.030 g. Under this condition, 98.4% of 20 ppm of RhB dye was removed from an aqueous solution within 5 min.
- The results revealed that laboratory prepared phosphoric acid AC derived from agro waste such as wood dust of Dalbergia sisoo can be converted into value-added materials for the removal of toxic dyes such as RhB.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated rice husk-based activated carbon. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Üner, O.; Gökara, G.; Bayrak, Y. Adsorption of cationic dyes on activated carbon obtained from waste Elaeagnus stone. Adsorpt. Sci. Technol. 2016, 34, 512–525. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Cai, Y.; Yang, G.; Liu, Y.; Zeng, G.; Zhou, Y.; Li, S.; Wang, J.; Zhang, S.; Fang, Y.; et al. Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl. Surf. Sci. 2014, 314, 746–753. [Google Scholar] [CrossRef]
- Lacerda, V.D.S.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Bascones, M.S.; Gracia, L.M.N.; Martín-Ramos, P.; Martín-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manag. 2015, 155, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laasri, L.; Elamrani, M.K.; Cherkaoui, O. Removal of two cationic dyes from a textile effluent by filtration-adsorption on wood sawdust. Environ. Sci. Pollut. Res. 2007, 14, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, A.G. Adsorption of Rhodamine B dye from aqueous solution on Irvingia gabonensis biomass: Kinetics and thermodynamics studies. S. Afr. J. Chem. 2015, 68, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B.L. Remediation of Rhodamine B Dye from Aqueous Solution Using Casuarina equisetifolia Cone Powder as a Low-Cost Adsorbent. Adv. Phys. Chem. 2016, 2016, 9497378. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.B.L.; Priyantha, N.; Fang, X.Y.; Mohamad Zaidi, N.A.H. Artocarpusodoratissimus peel as a potential adsorbent in environmental remediation to remove toxic Rhodamine B dye. J. Mater. Environ. Sci. 2017, 8, 494–502. [Google Scholar]
- Bello, O.S.; Lasisi, B.M.; Adigun, O.J.; Ephraim, V. Scavenging Rhodamine B dye using moringa oleifera seed pod. Chem. Speciat. Bioavailab. 2017, 29, 120–134. [Google Scholar] [CrossRef] [Green Version]
- Rasapoor, M.; Young, B.; Asadov, A.; Brar, R.; Sarmah, A.K.; Zhuang, W.-Q.; Baroutian, S. Effects of biochar and activated carbon on biogas generation: A thermogravimetric and chemical analysis approach. Energy Convers. Manag. 2020, 203, 112221. [Google Scholar] [CrossRef]
- Suiuay, C.; Sudajan, S.; Katekaew, S.; Senawong, K.; Laloon, K. Production of gasoline-like-fuel and diesel-like-fuel from hard-resin of Yang (Dipterocarpus alatus) using a fast pyrolysis process. Energy 2019, 187, 115967. [Google Scholar] [CrossRef]
- Senturk, H.B.; Ozdes, D.; Duran, C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 2010, 252, 81–87. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Yagmur, E.; Gokce, Y.; Tekin, S.; Semerci, N.I.; Aktas, Z. Characteristics and comparison of activated carbons prepared from oleaster (Elaeagnus angustifolia) fruit using KOH and ZnCl2. Fuel 2020, 267, 117232. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, E.; Cordero, T.; Rodriguez-Mirasol, J.; Cotoruelo, L.; Rodriguez, J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors. Water Res. 2004, 38, 3043–3050. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. Incorporation of nanostructured manganese dioxide into carbon nanofibers and its electrochemical performance. Mater. Lett. 2012, 72, 18–21. [Google Scholar] [CrossRef]
- Shrestha, D.; Maensiri, S.; Wongpratat, U.; Lee, S.W.; Nyachhyon, A.R. Shorea robusta derived activated carbon decorated with manganese dioxide hybrid composite for improved capacitive behaviors. J. Environ. Chem. Eng. 2019, 7, 103227. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Rodriguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. J. Collids Surf. Physicochem. Eng. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Shi, K.; Ren, M.; Zhitomirsky, I. Activated carbon-coated carbon nanotubes for energy storage in supercapacitors and capacitive water purification. ACS Sustain. Chem. Eng. 2014, 2, 1289–1298. [Google Scholar] [CrossRef]
- Kondrat, S.; Perez, C.R.; Presser, C.R.; Gogotsi, Y.; Kornyshev, A.A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 2012, 5, 6474. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Three-Dimensional Nano-Architecture of BiFeO3 anchored TiO2 Nanotube Arrays for Electrochemical Energy Storage and Solar Energy Conversion. J. ACS Sustain. Chem. Eng. 2015, 3, 2254–2263. [Google Scholar] [CrossRef]
- Tawfik, A.S.; Gaddafi, I.D. Adsorptive desulfurization of dibenzothiophene from fuels by rubber tyres-derived carbons: Kinetics and isotherms evaluation. Proc. Safe. Environ. Prot. 2016, 102, 9–19. [Google Scholar]
- Arivoli, S.; Viji, J.M.; Rajachandrasekar, T. Cobalt Adsorption on a Low Cost Carbon-Kinetic, Equilibrium and Mechanistic Studies. Mat. Sci. Res. India 2006, 3, 241–250. [Google Scholar]
- Doumic, L.I.; Soares, P.A.; Ayude, M.A.; Cassanello, M.; Boaventura, R.A.; Vilar, V.J. Enhancement of a solar photo-Fenton reaction by using ferrioxalate complexes for the treatment of a synthetic cotton-textile dyeing wastewater. Chem. Eng. J. 2015, 277, 86–96. [Google Scholar] [CrossRef]
- Stavropoulos, G.; Zabaniotou, A. Production and characterization of activated carbons from olive-seed waste residue. Microporous Mesoporous Mater. 2005, 82, 79–85. [Google Scholar] [CrossRef]
- Sen, T.K.; Dawood, S. Review on Dye Removal from Its Aqueous Solution into Alternative Cost Effective and Non-Conventional Adsorbents. J. Chem. Process. Eng. 2013, 1, 104. [Google Scholar] [CrossRef] [Green Version]
- Namasivayam, C.; Radhika, R.; Suba, S. Uptake of dyes by a promising locally available agricultural solid waste: Coir pith. Waste Manag. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Bedmohata, M.A.; Chaudhari, A.R.; Singh, S.P.; Choudhary, M.D. Adsorption Capacity of Activated Carbon prepared by Chemical Activation of Lignin for the Removal of Methylene Blue Dye. Int. J. Adv. Res. Chem. Sci. 2015, 2, 1–13. [Google Scholar]
- Chen, Y.; Zhu, Y.; Wang, Z.; Li, Y.; Wang, L.; Ding, L.; Gao, X.; Ma, Y.; Guo, Y. Application studies of activated carbon derived from rice husks produced by chemical-thermal process—A review. Adv. Colloid Interface Sci. 2011, 163, 39–52. [Google Scholar] [CrossRef] [PubMed]







| Dye | IUPAC Name | Chemical Formula | Chemical Structure | Mol. Wt. (gmol−1) | Appearance | λmax (nm) |
|---|---|---|---|---|---|---|
| Rhodamine B | [9-(2-carboxyphenyl)-6-diethylamino3-xanthenylidene]-diethylammonium chloride | C28H31ClN2O3 |  | 479.02 | Reddish violet powder | 554 |
| Sample | Particle Size (µm) | Activating Agent | H3PO4:Wood Powder Ratio (w/w) | Preheating Temperature (°C) | Carbonization Temperature (°C) | Carbonization Time (h) | Atmosphere |
|---|---|---|---|---|---|---|---|
| AC (Db-s) | 150 | H3PO4 | 1:1 | 110 | 400 | 3 | N2 |
| S.N. | Parameter | Observed Value (%) |
|---|---|---|
| 1. | Moisture | 8.66 |
| 2. | Total ash | 1.07 |
| 3. | Volatile matter | 42.27 |
| 4. | Total carbon | 48.0 |
| Name of Sample | Specific Surface Area (m2 g−1) | Pore Size (BJH ads) (nm) | Pore Volume (BJH ads) (cm3 g−1) |
|---|---|---|---|
| AC (Db-s) | 1376 | 4.06 | 1.2 |
| S.N. | Surface Area (m2 g−1) | Number of Adsorption | Dose (g L−1) | Initial Conc.(ppm) | Adsorption Capacity (%) | D@A= |
|---|---|---|---|---|---|---|
| 1 | 1376 | A1 | 0.015 | 20 | 81.5 | 1.225 |
| 2 | A2 | 0.020 | 83.1 | 1.662 | ||
| 3 | A3 | 0.025 | 91.2 | 2.28 | ||
| 4 | A4 | 0.030 | 98.4 | 2.952 | ||
| 5 | A5 | 0.035 | 98.4 | 3.444 |
| S.N. | No. of Adsorption (N) | Dose (g L−1) | Initial Concentration (ppm) | Adsorption Capacity (%) (X) | X − μ | (X − μ)2 |
|---|---|---|---|---|---|---|
| 1 | A1 | 0.030 | 20 | 98.4 | 98.4 − 98.36 = −0.04 | 0.0016 |
| 2 | A2 | 98.2 | 98.2 − 98.36 = −0.16 | 0.0256 | ||
| 3 | A3 | 98.5 | 98.5 − 98.36 = 0.14 | 0.0196 | ||
| 4 | A4 | 98.4 | 98.4 − 98.36 = 0.04 | 0.0016 | ||
| 5 | A5 | 98.3 | 98.3 − 98.36 = 0.06 | 0.0036 |
| S.N. | Surface Area (m2 g−1) | Dose Taken (μmg L−1) | Dose Found (μmg L−1) | Mean Standard Deviation (%) | Relative Standard Deviation (%) |
|---|---|---|---|---|---|
| 1 | 1376 | 15 | 14.78 ± 0.226 | 98.5 | 1.5 |
| 2 | 20 | 19. 84 ± 0.157 | 99.2 | 0.8 | |
| 3 | 25 | 24.87 ± 0.126 | 99.5 | 0.5 | |
| 4 | 30 | 29.99 ± 0.015 | 99.9 | 0.1 | |
| 5 | 35 | 39.99 ± 0.012 | 99.9 | 0.1 |
| S.N. | Dye | Adsorbent Dose | Result | |||
|---|---|---|---|---|---|---|
| 0.020 mg | 0.025 mg | 0.030 mg | 0.035 mg | |||
| 1 | RhB | 83.1 | 91.2 | 98.4 | 98.4 | No effect after 0.035 mg |
| Surfactant | Surface Area (m2 g−1) | Dye | pH | Initial Conc. (ppm) | Dosage (g L−1) | Time (min) | % Removal |
|---|---|---|---|---|---|---|---|
| Db-s | 1376 | RhB | 8.5 | 20 | 0.020 | 5 | 83.1 |
| 0.025 | 91.2 | ||||||
| 0.030 | 98.4 | ||||||
| Com. C | 876.02 | RhB | 8.5 | 20 | 0.020 | 5 | 64.4 |
| 0.025 | 84 | ||||||
| 0.030 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, D. Efficiency of Wood-Dust of Dalbergia sisoo as Low-Cost Adsorbent for Rhodamine-B Dye Removal. Nanomaterials 2021, 11, 2217. https://doi.org/10.3390/nano11092217
Shrestha D. Efficiency of Wood-Dust of Dalbergia sisoo as Low-Cost Adsorbent for Rhodamine-B Dye Removal. Nanomaterials. 2021; 11(9):2217. https://doi.org/10.3390/nano11092217
Chicago/Turabian StyleShrestha, Dibyashree. 2021. "Efficiency of Wood-Dust of Dalbergia sisoo as Low-Cost Adsorbent for Rhodamine-B Dye Removal" Nanomaterials 11, no. 9: 2217. https://doi.org/10.3390/nano11092217





