Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Rice Cultivation and Experiment Setup
2.3. Elements Analysis
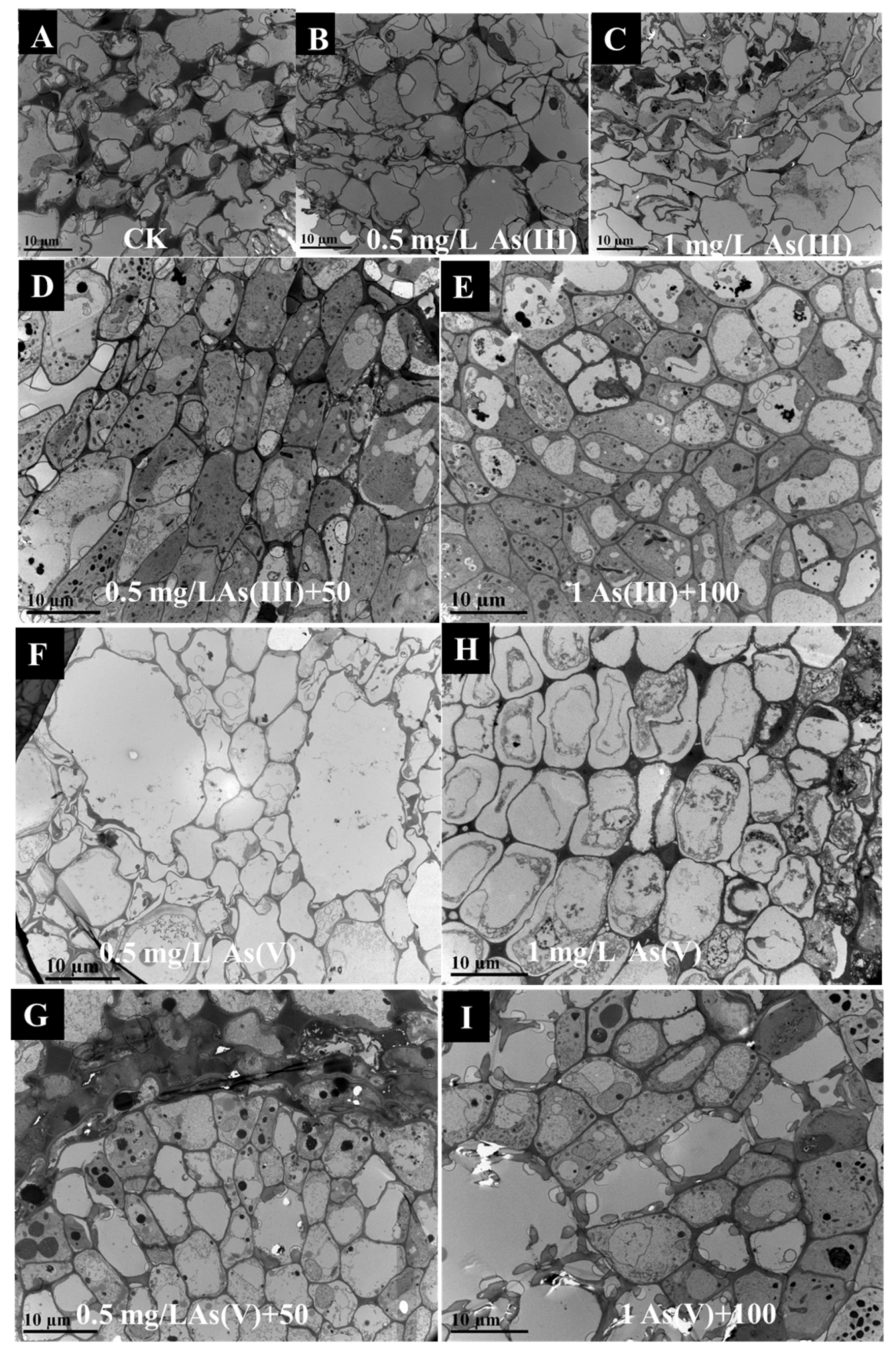
2.4. The Observation of CuO NPs and Rice Roots
2.5. Real-Time Quantitative PCR
2.6. Data Analysis
3. Results
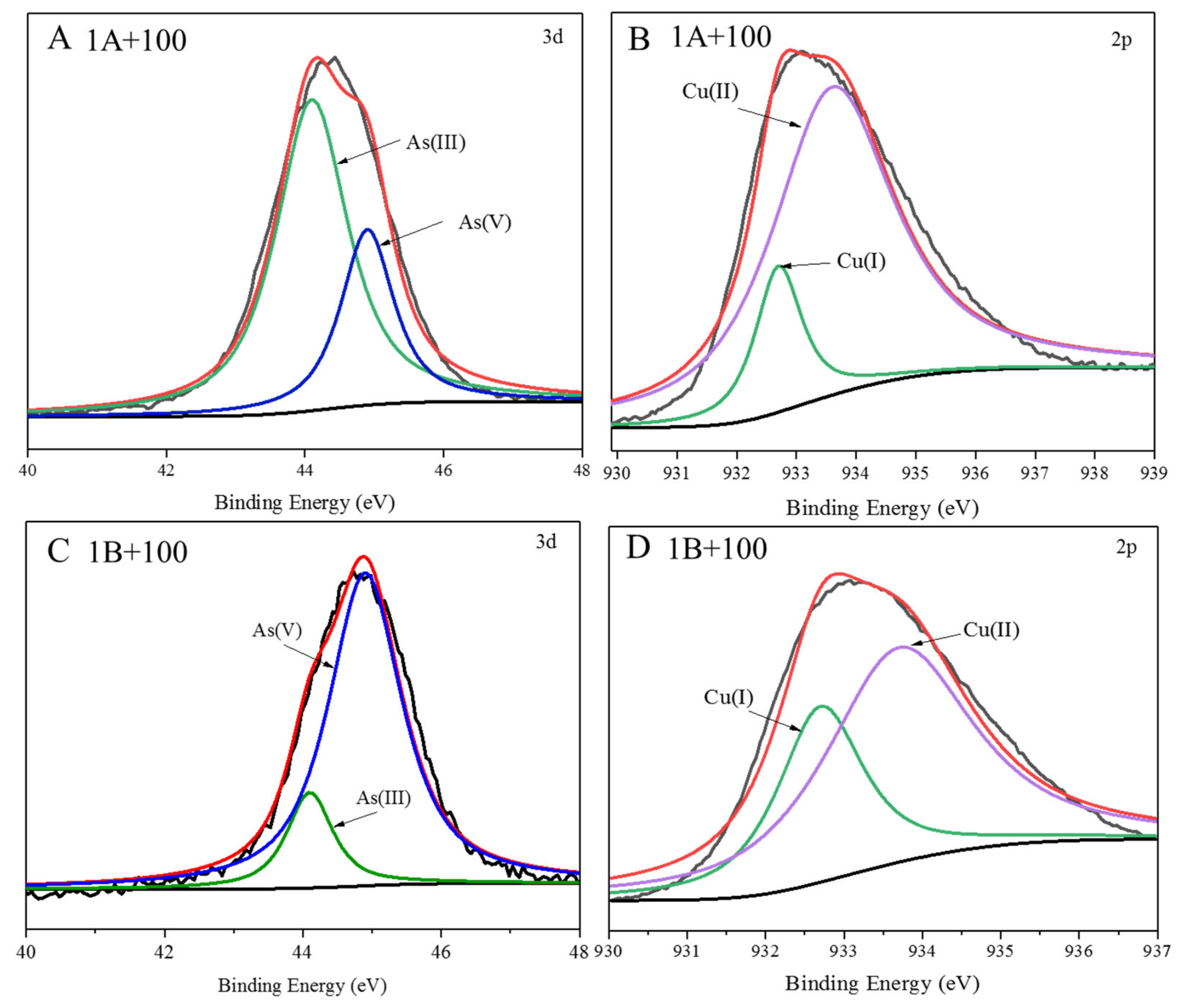
3.1. Adsorption of As by CuO NPs
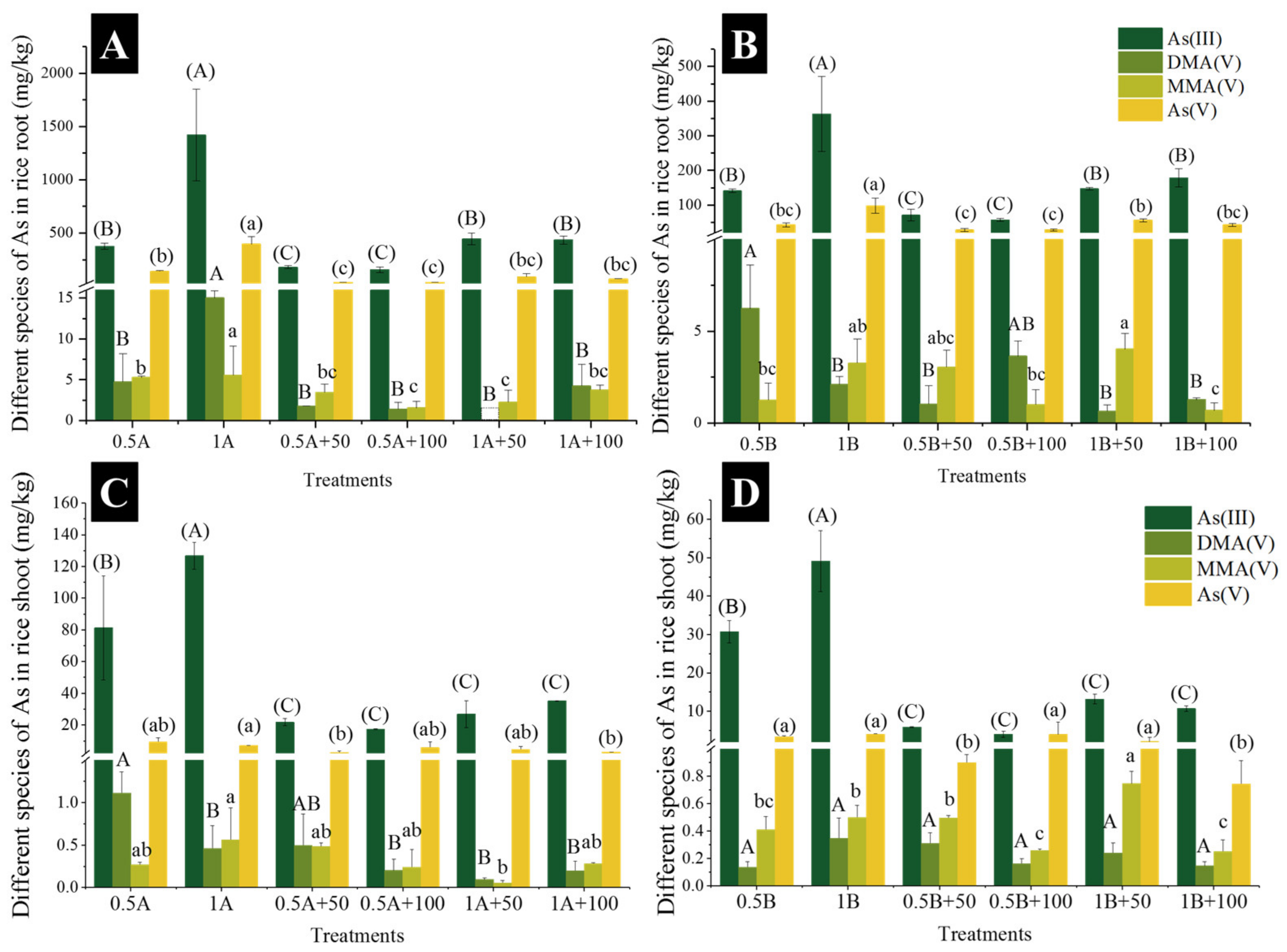
3.2. Accumulation and Speciation of As in Rice
3.3. Morphology of Rice Root Cell
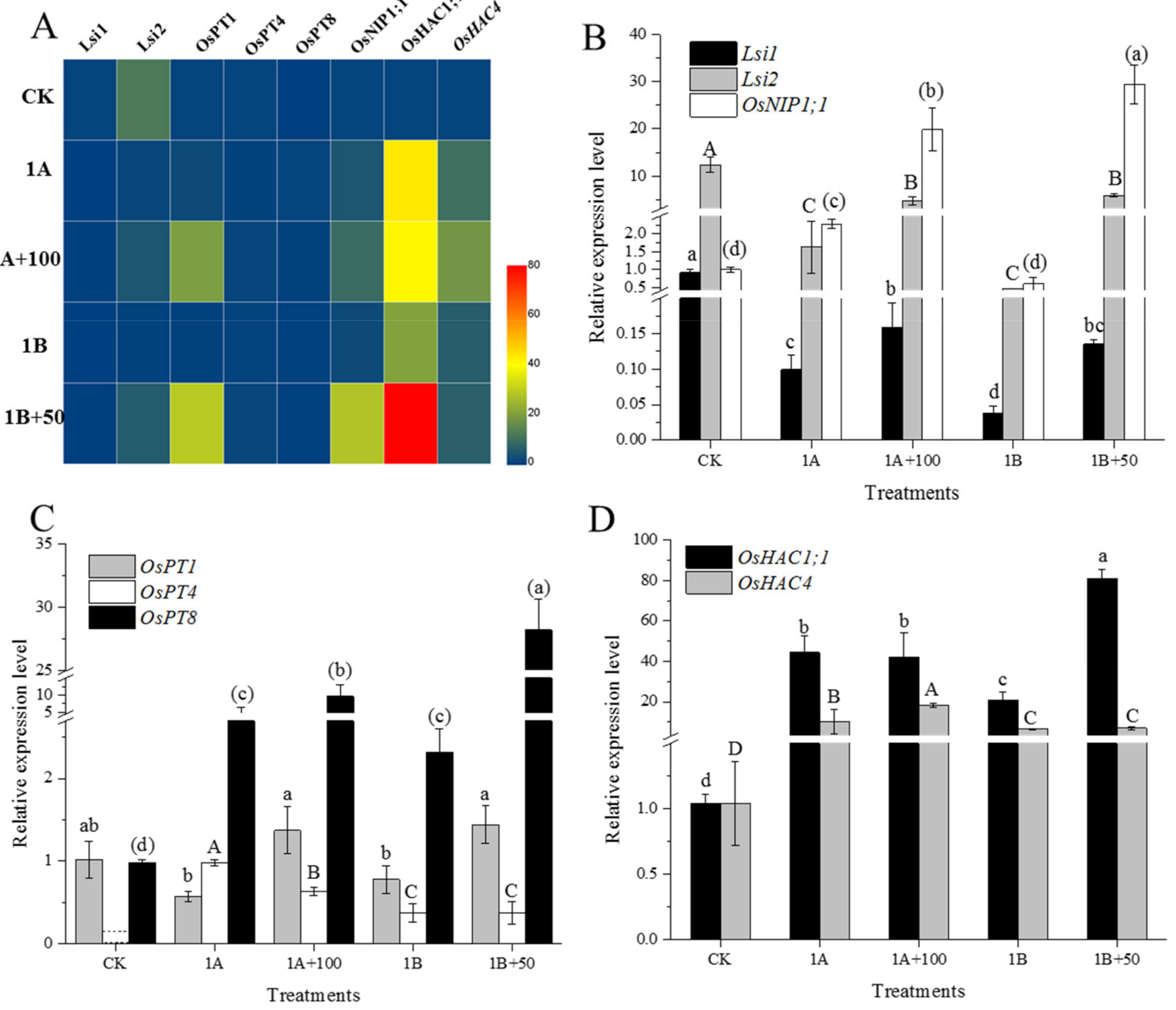
3.4. Relative Expression of As Related Genes in Root
4. Discussion
4.1. CuO NPs Decreased As(III) Accumulation in Rice
4.2. CuO NPs Regulated the Expression of As Related Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capek, I. Dispersions, novel nanomaterial sensors and nanoconjugates based on carbon nanotubes. Adv. Colloid Interface Sci. 2009, 150, 63–89. [Google Scholar] [CrossRef]
- Chen, J.N.; Mao, S.Y.; Xu, Z.F.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne ralstonia solanacearum. RSC Adv. 2019, 9, 3788–3799. [Google Scholar] [CrossRef] [Green Version]
- El-Garhy, H.A.S.; Elsisi, A.A.; Mohamed, S.A.; Morsy, O.M.; Osman, G.; Abdel-Rahman, F.A. Transcriptomic changes in green bean pods against grey mould and white rot diseases via field application of chemical elicitor nanoparticles. IET Nanobiotechnol. 2020, 14, 574–583. [Google Scholar] [CrossRef]
- Ochoa, L.; Zuverza-Mena, N.; Medina-Velo, I.A.; Flores-Margez, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper oxide nanoparticles and bulk copper oxide, combined with indole-3-acetic acid, alter aluminum, boron, and iron in pisum sativum seeds. Sci. Total Environ. 2018, 634, 1238–1245. [Google Scholar] [CrossRef]
- Banitalebi, G.; Mosaddeghi, M.R.; Shariatmadari, H. Evaluation of physico-chemical properties of biochar-based mixtures for soilless growth media. J. Mater. Cycles Waste 2021, 23, 950–964. [Google Scholar] [CrossRef]
- Gomez, A.; Narayan, M.; Zhao, L.J.; Jia, X.R.; Bernal, R.A.; Lopez-Moreno, M.L.; Peralta-Videa, J.R. Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J. Hazard. Mater. 2021, 401, 123385. [Google Scholar] [CrossRef]
- Liu, J.; Wolfe, K.; Potter, P.M.; Cobb, G.P. Distribution and speciation of copper and arsenic in rice plants (oryza sativa japonica ’koshihikari’) treated with copper oxide nanoparticles and arsenic during a life cycle. Environ. Sci. Technol. 2019, 53, 4988–4996. [Google Scholar] [CrossRef]
- Wang, X.X.; Sun, W.J.; Ma, X.M. Differential impacts of copper oxide nanoparticles and copper(ii) ions on the uptake and accumulation of arsenic in rice (oryza sativa). Environ. Pollut. 2019, 252, 967–973. [Google Scholar] [CrossRef]
- Wang, S.L.; Liu, H.Z.; Zhang, Y.X.; Xin, H. The effect of cuo nps on reactive oxygen species and cell cycle gene expression in roots of rice. Environ. Toxicol. Chem. 2015, 34, 554–561. [Google Scholar] [CrossRef]
- Peng, C.; Tong, H.; Shen, C.S.; Sun, L.J.; Yuan, P.; He, M.; Shi, J.Y. Bioavailability and translocation of metal oxide nanoparticles in the soil-rice plant system. Sci. Total Environ. 2020, 713, 136662. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, S.K.; Tang, Z.; Liu, G.D.; Moore, K.L.; Maathuis, F.J.M.; Miller, A.J.; McGrath, S.P.; Zhao, F.J. The nodulin 26-like intrinsic membrane protein osnip3;2 is involved in arsenite uptake by lateral roots in rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Chatterjee, S.; Moogouei, R.; Gupta, D.K. Arsenic accumulation in rice and probable mitigation approaches: A review. Agronomy 2017, 7, 67. [Google Scholar] [CrossRef]
- Fontenot, E.B.; DiTusa, S.F.; Kato, N.; Olivier, D.M.; Dale, R.; Lin, W.Y.; Chiou, T.J.; Macnaughtan, M.A.; Smith, A.P. Increased phosphate transport of arabidopsis thaliana pht1;1 by site-directed mutagenesis of tyrosine 312 may be attributed to the disruption of homomeric interactions. Plant Cell Environ. 2015, 38, 2012–2022. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.Y.; Liu, X.; Sun, S.B.; Xu, G.H.; Liu, Y.G.; Chen, Y.S.; Ma, L.N.Q. Knocking out ospt4 gene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [CrossRef]
- Wang, P.T.; Zhang, W.W.; Mao, C.Z.; Xu, G.H.; Zhao, F.J. The role of ospt8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J. Exp. Bot. 2016, 67, 6051–6059. [Google Scholar] [CrossRef]
- Shi, J.Y.; Ye, J.; Fang, H.X.; Zhang, S.; Xu, C. Effects of copper oxide nanoparticles on paddy soil properties and components. Nanomaterials 2018, 8, 839. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.M.; Shi, S.L.; Wang, L.; Tang, Z.; Lv, T.T.; Zhu, X.L.; Ding, X.M.; Wang, Y.F.; Zhao, F.J.; Wu, Z.C. Oshac4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol. 2017, 215, 1090–1101. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wang, J.U.; Rui, M.M.; Yang, L.J.; Shen, J.; Chu, H.W.; Song, S.Y.; Chen, Y. Osftip7 determines metallic oxide nanoparticles response and tolerance by regulating auxin biosynthesis in rice. J. Hazard. Mater. 2021, 403, 123946. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wolfe, K.; Perrotta, B.; Cobb, G.P. Mobility of arsenic in the growth media of rice plants (oryza sativa subsp. Japonica. ’Koshihikari’) with exposure to copper oxide nanoparticles in a life-cycle greenhouse study. Sci. Total. Environ. 2021, 774, 145620. [Google Scholar] [CrossRef]
- Nazarian, M.; Ghanati, F. The role of melatonin in reinforcement of antioxidant system of rice plant (Oryza sativa L.) under arsenite toxicity? Plant Physiol. Rep. 2020, 25, 395–404. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Prasad, S.M. Arsenate and arsenite-induced inhibition and recovery in two diazotrophic cyanobacteria nostoc muscorum and anabaena sp.: Study on time-dependent toxicity regulation. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Feng, R.W.; Guo, J.K.; Wang, R.G.; Xu, Y.M.; Fan, Z.L.; Mo, L.Y. Interactions between selenite and different forms of antimony and their effects on root morphology of paddy rice. Plant Soil 2017, 413, 231–242. [Google Scholar] [CrossRef]
- Xu, J.Y.; Li, H.B.; Liang, S.; Luo, J.; Ma, L.N.Q. Arsenic enhanced plant growth and altered rhizosphere characteristics of hyperacaimulator pteris vittata. Environ. Pollut. 2014, 194, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Ranjan, P.; Quaff, A.R. Cost-effective synthesis and characterization of cuo nps as a nanosize adsorbent for as (iii) remediation in synthetic arsenic-contaminated water. J. Environ. Health Sci. 2020, 18, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.J.; Reddy, K.J.; Singh, N.; Singh, R.P.; Mukherjee, S. Removal of arsenic from groundwater in west bengal, india using cuo nanoparticle adsorbent. Environ. Earth Sci. 2015, 73, 3593–3601. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Chaudhry, S. Copper oxide nanoparticles for the removal of divalent nickel ions from aqueous solution. Toxin Rev. 2020. [Google Scholar] [CrossRef]
- Zeng, L.Q.; Wan, B.; Huang, R.X.; Yan, Y.P.; Wang, X.M.; Tan, W.F.; Liu, F.; Feng, X.H. Catalytic oxidation of arsenite and reaction pathways on the surface of cuo nanoparticles at a wide range of phs. Geochem. Trans. 2018, 19, 12. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Majumdar, A.; Kumar, J.S.; Srivastava, S. Arsenic in rice agro-ecosystem: Solutions for safe and sustainable rice production. Front. Sustain. Food Syst. 2020, 4, 53. [Google Scholar] [CrossRef]
- Cui, J.H.; Li, Y.D.; Jin, Q.; Li, F.B. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Copper oxide nanoparticles and arsenic interact to alter seedling growth of rice (oryza sativa japonica). Chemosphere 2018, 206, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B 2011, 87, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.J. Decreasing arsenic accumulation in rice by overexpressing osnip1;1 and osnip3;3 through disrupting arsenite radial transport in roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, T.; Islam, M.R.; Duan, G.L.; Uraguchi, S.; Fujiwara, T. Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter ospt1 is involved in as accumulation in shoots of rice. Soil Sci. Plant Nutr. 2013, 59, 580–590. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Xu, T.Q.; Zeng, L.T.; Cheng, D.; Yang, M.; Luo, J.; Lian, X.M. Ospt4 contributes to arsenate uptake and transport in rice. Front. Plant Sci. 2017, 8, 2197. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.Y.; Tian, Z.H.; Yang, X.L.; Liu, B.H.; Yang, J.; Lin, H.H. The role of osnla1 in regulating arsenate uptake and tolerance in rice. J. Plant Physiol. 2019, 236, 15–22. [Google Scholar] [CrossRef]
- Shi, S.L.; Wang, T.; Chen, Z.; Tang, Z.; Wu, Z.C.; Salt, D.E.; Chao, D.Y.; Zhao, F.J. Oshac1;1 and oshac1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol. 2016, 172, 1708–1719. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Shi, J.; Jiang, X.; Wu, H. Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice. Nanomaterials 2021, 11, 2228. https://doi.org/10.3390/nano11092228
Wu Q, Shi J, Jiang X, Wu H. Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice. Nanomaterials. 2021; 11(9):2228. https://doi.org/10.3390/nano11092228
Chicago/Turabian StyleWu, Qianhua, Jiyan Shi, Xiaohan Jiang, and Hanxin Wu. 2021. "Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice" Nanomaterials 11, no. 9: 2228. https://doi.org/10.3390/nano11092228
APA StyleWu, Q., Shi, J., Jiang, X., & Wu, H. (2021). Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice. Nanomaterials, 11(9), 2228. https://doi.org/10.3390/nano11092228





