High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of Materials
2.2. Fabrication of Fuel Cells
2.3. Characterizations
3. Results and Discussion
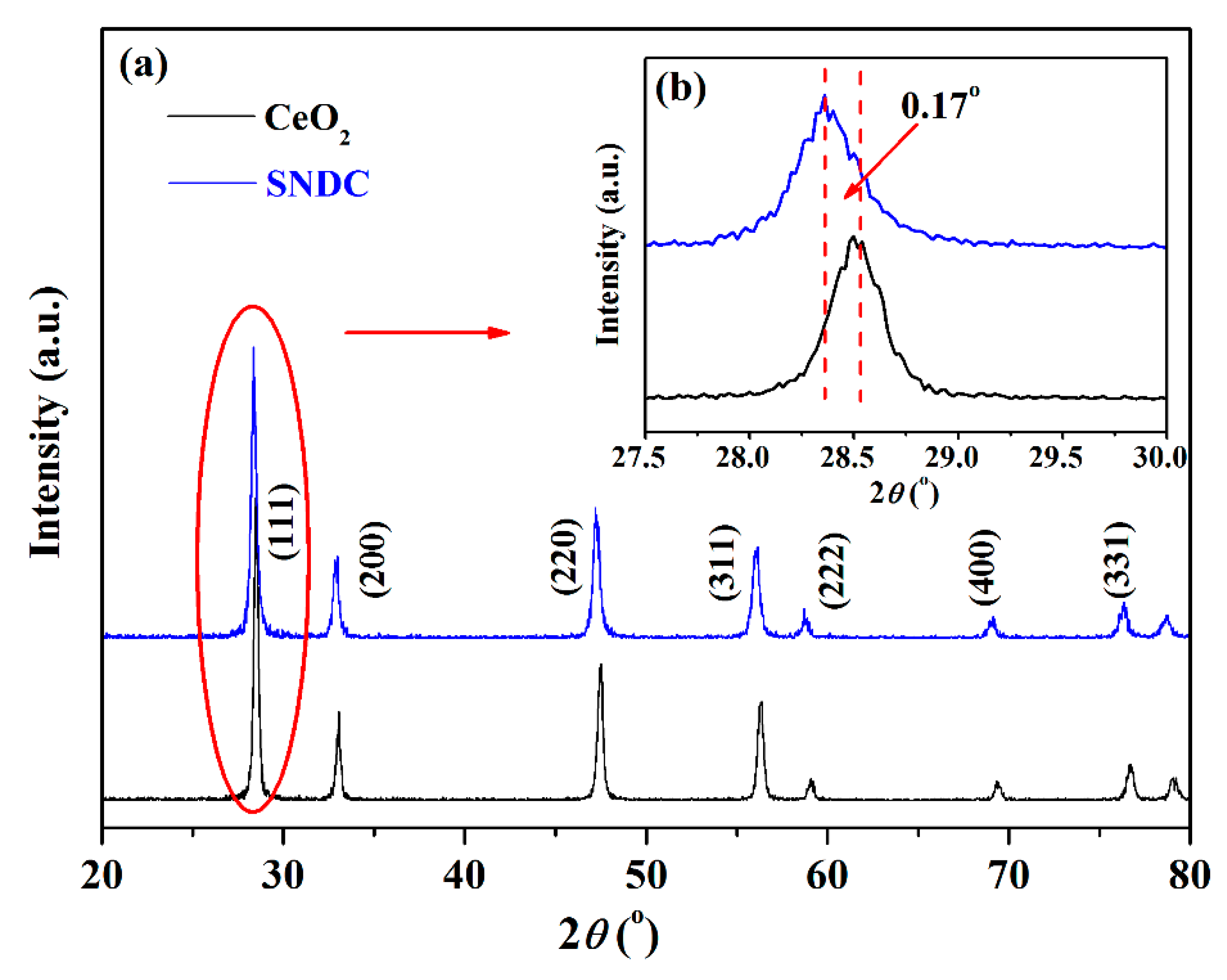
3.1. Results of XRD
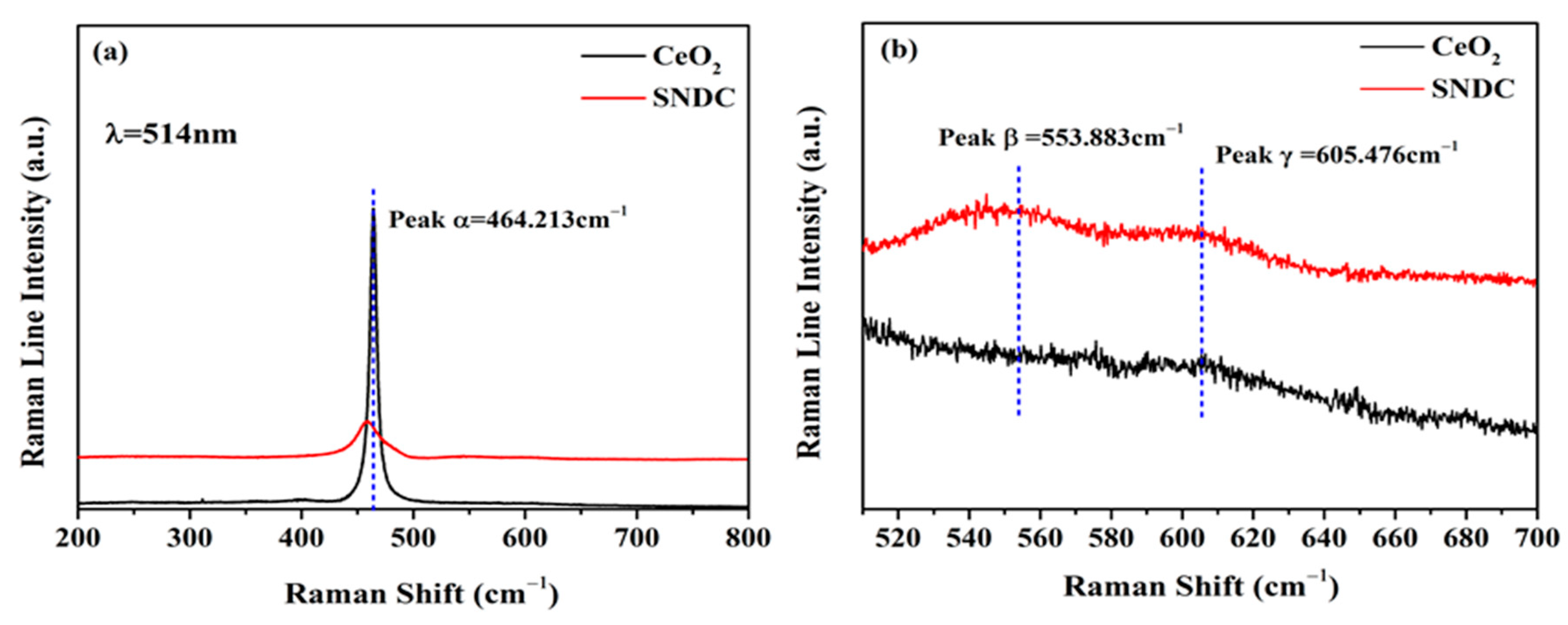
3.2. Raman Spectra Analysis
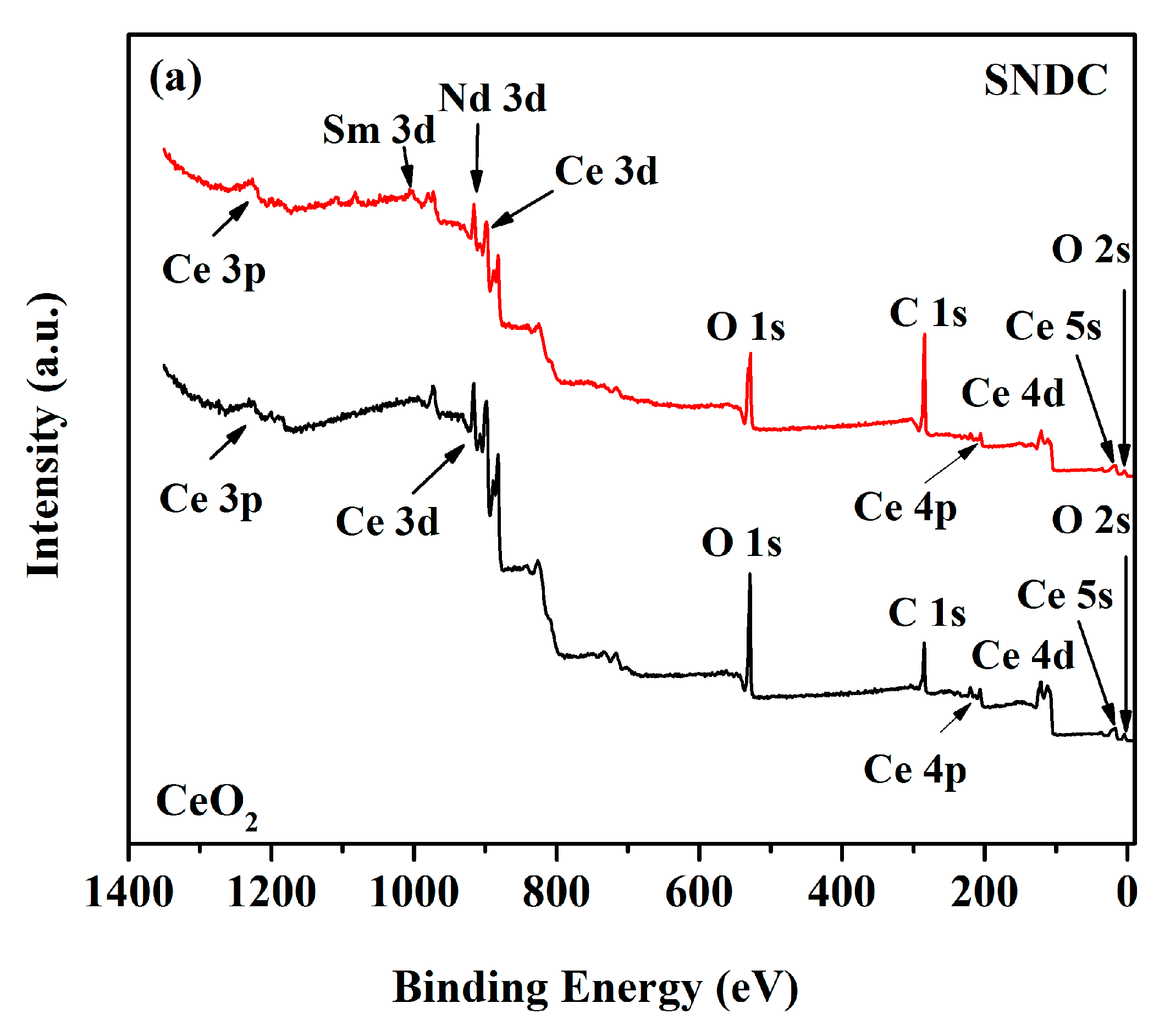
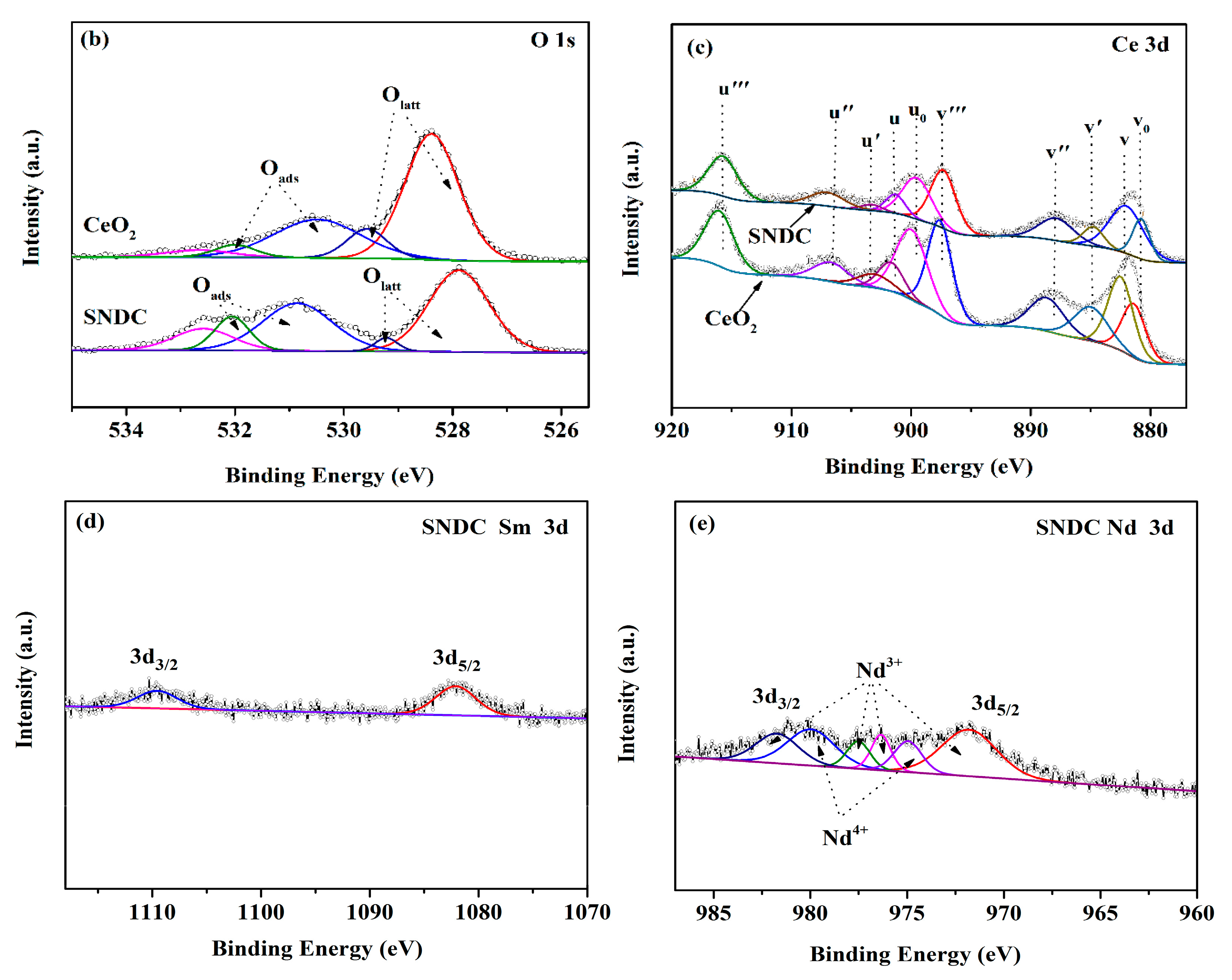
3.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
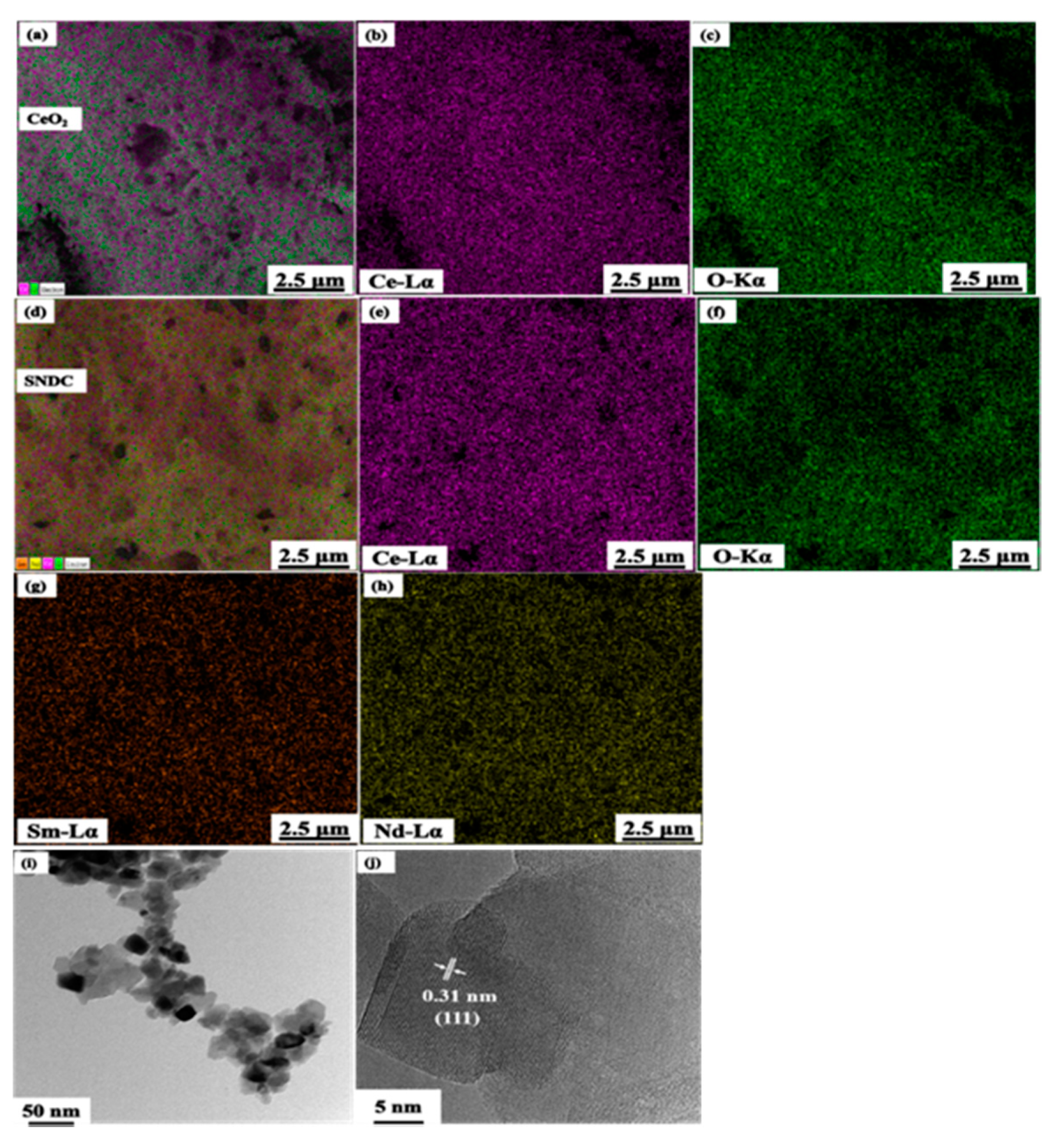
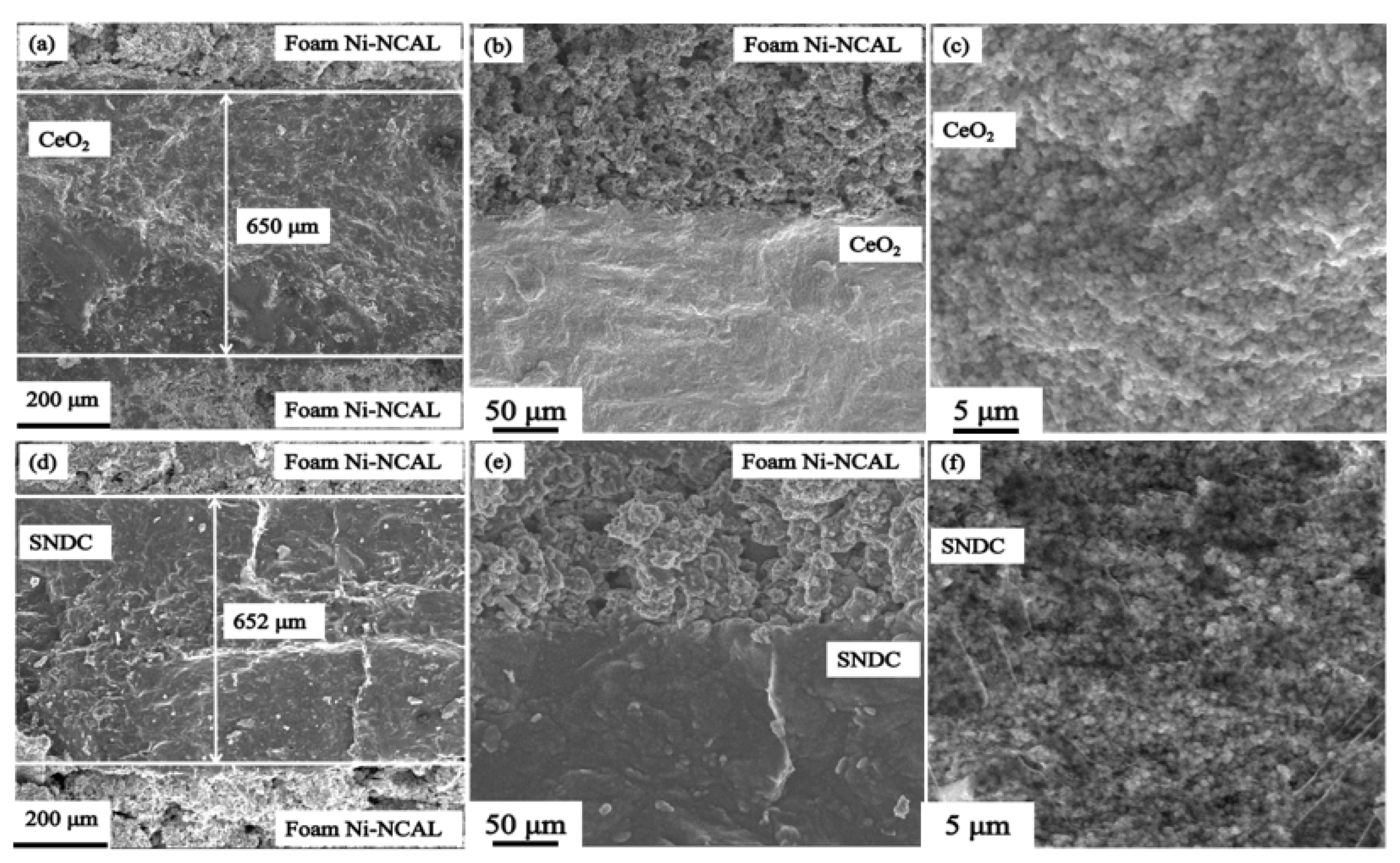
3.4. EDX and HR-TEM Analysis
3.5. Fuel Cell Performance Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loureiro, F.J.A.; Macedo, D.A.; Nascimento, R.M.; Cesário, M.R.; Grilo, J.P.F.; Yaremchenko, A.A.; Fagg, D.P. Cathodic polarisation of composite LSCF-SDC IT-SOFC electrode synthesised by one-step microwave self-assisted combustion. J. Eur. Ceram. Soc. 2019, 39, 1846–1853. [Google Scholar] [CrossRef]
- Zhao, Y.; Ju, Y.; Liu, J.; Shuai, M.; Zhang, P.; Zhu, C. Performance evaluation of BaCo0.7Nb0.2Ta0.1O3 − δ cathode for intermediate-temperature solid oxide fuel cells. Mater. Lett. 2019, 238, 301–304. [Google Scholar] [CrossRef]
- Xia, C.; Mi, Y.; Wang, B.; Lin, B.; Chen, G.; Zhu, B. Shaping triple-conducting semiconductor BaCo0.4Fe0.4Zr0.1Y0.1O3-delta into an electrolyte for low-temperature solid oxide fuel cells. Nat. Commun. 2019, 10, 1707–1715. [Google Scholar] [CrossRef]
- Hou, J.; Bi, L.; Qian, J.; Zhu, Z.; Zhang, J.; Liu, W. High performance ceria–bismuth bilayer electrolyte low temperature solid oxide fuel cells (LT-SOFCs) fabricated by combining co-pressing with drop-coating. J. Mater. Chem. A 2015, 3, 10219–10224. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.T.; Peden, C.H.F. Chemistry—Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, Y.; Akbar, M.; Jin, B.; Tu, Z.; Mushtaq, N.; Wang, B.; Qu, X.; Xia, C.; Huang, Y. A Bulk-Heterostructure Nanocomposite Electrolyte of Ce0.8Sm0.2O2-δ–SrTiO3 for Low-Temperature Solid Oxide Fuel Cells. Nano-Micro Lett. 2021, 13, 46–60. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Wang, Y.; Guo, Y.; Guo, Y. Synthesis of flower-like La or Pr-doped mesoporous ceria microspheres and their catalytic activities for methane combustion. Catal. Commun. 2010, 11, 946–950. [Google Scholar] [CrossRef]
- Katta, L.; Vinod Kumar, T.; Durgasri, D.N.; Reddy, B.M. Nanosized Ce1 − xLaxO2 − δ/Al2O3 solid solutions for CO oxidation: Combined study of structural characteristics and catalytic evaluation. Catal. Today 2012, 198, 133–139. [Google Scholar] [CrossRef]
- Shirbhate, S.; Nayyar, R.N.; Ojha, P.K.; Yadav, A.K.; Acharya, S. Exploration of Atomic Scale Changes during Oxygen Vacancy Dissociation Mechanism in Nanostructure Co-Doped Ceria: As Electrolytes for IT-SOFC. J. Electrochem. Soc. 2019, 166, F544–F554. [Google Scholar] [CrossRef]
- Rangaswamy, A.; Sudarsanam, P.; Reddy, B.M. Rare earth metal doped CeO2-based catalytic materials for diesel soot oxidation at lower temperatures. J. Rare Earths 2015, 33, 1162–1169. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Cai, Y.; Zhang, W.; Wang, B.; Zhu, B. Superionic Conductivity of Sm3+, Pr3+, and Nd3+ Triple-Doped Ceria through Bulk and Surface Two-Step Doping Approach. ACS Appl. Mater. Interfaces 2017, 9, 23614–23623. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.O.; Baker, R.T. Structural, morphological and electrical properties of Gd0.1Ce0.9O1.95 prepared by a citrate complexation method. J. Power Sources 2009, 186, 268–277. [Google Scholar] [CrossRef]
- Liu, Y.; Mushtaq, M.N.; Zhang, W.; Teng, A.; Liu, X. Single-phase electronic-ionic conducting Sm3+/Pr3+/Nd3+ triple-doped ceria for new generation fuel cell technology. Int. J. Hydrogen Energy 2018, 43, 12817–12824. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Fan, L.; Cai, Y.; Xia, C.; Liu, Y.; Raza, R.; van Aken, P.A.; Wang, H.; Zhu, B. Preparation and characterization of Sm and Ca co-doped ceria–La0.6Sr0.4Co0.2Fe0.8O3 − δsemiconductor–ionic composites for electrolyte-layer-free fuel cells. J. Mater. Chem. A 2016, 4, 15426–15436. [Google Scholar] [CrossRef]
- Banerjee, S.; Devi, P.S.; Topwal, D.; Mandal, S.; Menon, K. Enhanced Ionic Conductivity in Ce0.8Sm0.2O1.9: Unique Effect of Calcium Co-doping. Adv. Funct. Mater. 2007, 17, 2847–2854. [Google Scholar] [CrossRef]
- Rauf, S.; Zhu, B.; Shah, M.A.K.Y.; Tayyab, Z.; Attique, S.; Ali, N.; Mushtaq, N.; Asghar, M.I.; Lund, P.D.; Yang, C.P. Low-temperature solid oxide fuel cells based on Tm-doped SrCeO2-δ semiconductor electrolytes. Mater. Today Energy 2021, 20, 100661–100672. [Google Scholar] [CrossRef]
- Arabacı, A. Citrate/nitrate combustion synthesis and electrical properties of rare earth co-doped Ce0.8Gd0.2 − xNdxO1.90 electrolyte materials. Mater. Sci. Eng. B 2020, 260, 114646–114652. [Google Scholar] [CrossRef]
- Omar, S.; Wachsman, E.; Nino, J. Higher conductivity Sm3+ and Nd3+ co-doped ceria-based electrolyte materials. Solid State Ion. 2008, 178, 1890–1897. [Google Scholar] [CrossRef]
- Jaiswal, N.; Upadhyay, S.; Kumar, D.; Parkash, O. Sm3+ and Sr2+ co-doped ceria prepared by citrate–nitrate auto-combustion method. Int. J. Hydrogen Energy 2014, 39, 543–551. [Google Scholar] [CrossRef]
- Takamura, H.; Kobayashi, J.; Takahashi, N.; Okada, M. Electrical conductivity of ceria nanoparticles under high pressure. J. Electroceramics 2008, 22, 24–32. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.; Luo, Y.; He, Y.; Zhang, X.; Zhu, B.; Li, W.; Liu, X.; Ding, Y.; Li, Y.; et al. Advanced Fuel Cell Based on New Nanocrystalline Structure Gd0.1Ce0.9O2 Electrolyte. Acs Appl Mater Interfaces 2019, 11, 10642–10650. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Solis, C.; Balaguer, M.; Serra, J.M. In Situ Raman Characterization of SOFC Materials in Operational Conditions: A Doped Ceria Study. Membranes 2020, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Jampaiah, D.; Tur, K.M.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Bhargava, S.K.; Reddy, B.M. Structural characterization and catalytic evaluation of transition and rare earth metal doped ceria-based solid solutions for elemental mercury oxidation. RSC Adv. 2013, 3, 12963–12974. [Google Scholar] [CrossRef]
- Lee, Y.C.; Li, K.-D.; Lu, C.-H.; Shen, J.-H.; Teoh, L.G.; Chiang, G.W. Synthesis, Structural and Optical Characterization of Sol–Gel-Derived Y-Doped Mesoporous CeO2. J. Electron. Mater. 2013, 42, 2549–2555. [Google Scholar] [CrossRef]
- He, D.; Hao, H.; Chen, D.; Liu, J.; Yu, J.; Lu, J.; Liu, F.; Wan, G.; He, S.; Luo, Y. Synthesis and application of rare-earth elements (Gd, Sm, and Nd) doped ceria-based solid solutions for methyl mercaptan catalytic decomposition. Catal. Today 2017, 281, 559–565. [Google Scholar] [CrossRef]
- Liu, J.; Jin, F.; Yang, X.; Niu, B.; Li, Y.; He, T. YBaCo2O5+δ-based double-perovskite cathodes for intermediate-temperature solid oxide fuel cells with simultaneously improved structural stability and thermal expansion properties. Electrochim. Acta 2019, 297, 344–354. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, D.; Zeng, Q.; Jiang, Y.; Lin, B. Highly promoted performance of triple-conducting cathode for YSZ-based SOFC via fluorine anion doping. Ceram. Int. 2020, 23964–23971. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, B.; Yun, S.; Zhang, W.; Xia, C.; Afzal, M.; Cai, Y.; Liu, Y.; Wang, Y.; Wang, H. Fast ionic conduction in semiconductor CeO2-δ electrolyte fuel cells. Npg Asia Mater. 2019, 11, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hou, J.; Xi, X.; Lu, Y.; Li, M.; Fan, Y.; Wang, L.; Wang, L.; Fu, X.-Z.; Luo, J.-L. Cogeneration of ethylene and electricity in symmetrical protonic solid oxide fuel cells based on a La0.6Sr0.4Fe0.8Nb0.1Cu0.1O3—δ electrode. J. Mater. Chem. A 2020, 8, 25978–25985. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113–133116. [Google Scholar] [CrossRef]
- Borchert, H.; Borchert, Y.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Paukshtis, E.A.; Bukhtiyarov, V.I.; et al. Nanostructured, Gd-Doped Ceria Promoted by Pt or Pd: Investigation of the Electronic and Surface Structures and Relations to Chemical Properties. J. Phys. Chem. B 2005, 109, 20077–20086. [Google Scholar] [CrossRef] [PubMed]
- Shahed, S.M.F.; Hasegawa, T.; Sainoo, Y.; Watanabe, Y.; Isomura, N.; Beniya, A.; Hirata, H.; Komeda, T. STM and XPS study of CeO2(111) reduction by atomic hydrogen. Surf. Sci. 2014, 628, 30–35. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, M.; Wang, H.; He, C.; Hao, Z.; Wei, W.; Sun, Y. Facile synthesis of catalytically active CeO2 for soot combustion. Catal. Sci. Technol. 2015, 5, 1941–1952. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Q.; Zhu, Z.; Liu, X.; Zhu, B. Time-dependent performance change of single layer fuel cell with Li0.4Mg0.3Zn0.3O/Ce0.8Sm0.2O2—δ composite. Int. J. Hydrogen Energy 2014, 39, 10718–10723. [Google Scholar] [CrossRef]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and evaluation of double perovskites LnBaCoFeO 5 + δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Rivera-López, F.; Pérez, M. X-ray photoelectron spectroscopy: Surface and depth profiling studies of glasses doped with Nd and Yb ions. Surf. Interface Anal. 2012, 44, 927–930. [Google Scholar] [CrossRef]
- Shimada, H.Y.T.; Suzuki, T.; Sumi, H.; Hamamoto, K.; Fujishiro, Y. High power density cell using nanostructured Sr-doped SmCoO3 and Sm-doped CeO2 composite powder synthesized by spray pyrolysis. J. Power Sources 2016, 302, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Sun, W.; Luo, Y.; Liu, H.; Geng, S.; Yu, K.; Liu, G. Investigation of layered Ni0.8Co0.15Al0.05LiO2 in electrode for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 417–425. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhu, C.; Zhu, D.; Jia, X.; Zhang, Y.; Yu, J.; Li, X.; Yang, M. High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte. Nanomaterials 2021, 11, 2231. https://doi.org/10.3390/nano11092231
Liu J, Zhu C, Zhu D, Jia X, Zhang Y, Yu J, Li X, Yang M. High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte. Nanomaterials. 2021; 11(9):2231. https://doi.org/10.3390/nano11092231
Chicago/Turabian StyleLiu, Jiamei, Chengjun Zhu, Decai Zhu, Xin Jia, Yingbo Zhang, Jie Yu, Xinfang Li, and Min Yang. 2021. "High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte" Nanomaterials 11, no. 9: 2231. https://doi.org/10.3390/nano11092231
APA StyleLiu, J., Zhu, C., Zhu, D., Jia, X., Zhang, Y., Yu, J., Li, X., & Yang, M. (2021). High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte. Nanomaterials, 11(9), 2231. https://doi.org/10.3390/nano11092231





