Glutathione Disulfide as a Reducing, Capping, and Mass-Separating Agent for the Synthesis and Enrichment of Gold Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of GSSG–AuNCs and GSH–AuNCs
2.2.1. Heating Modes
2.2.2. Synthesis of GSSG–AuNCs
2.2.3. Synthesis of GSH–AuNCs
2.3. Characterization
2.4. Acetonitrile-Initiated Phase Separation
2.5. Density Functional Theory Calculation
3. Results and Discussion
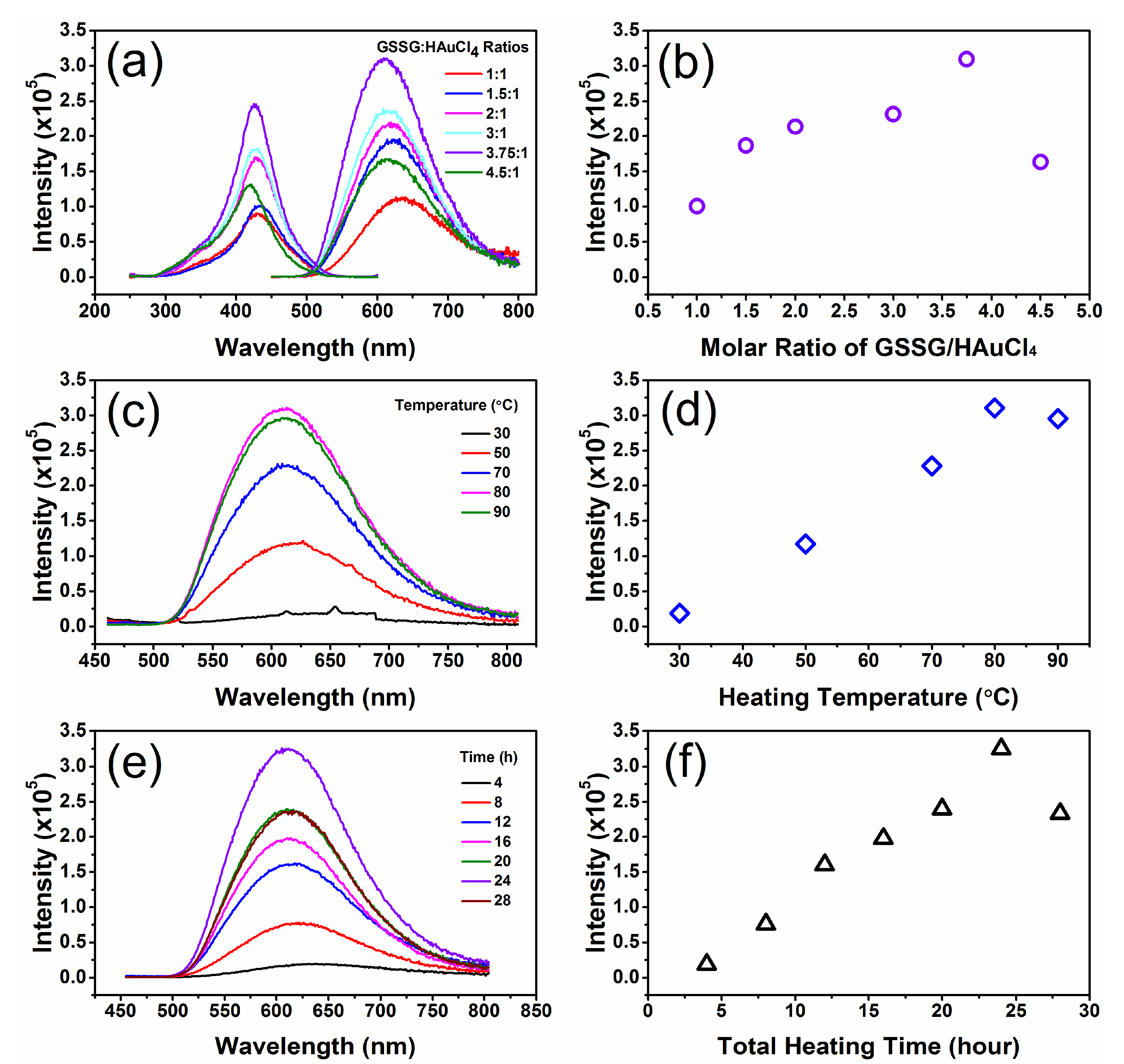
3.1. Synthesis of GSSG–AuNCs
3.2. Photophysical Properties
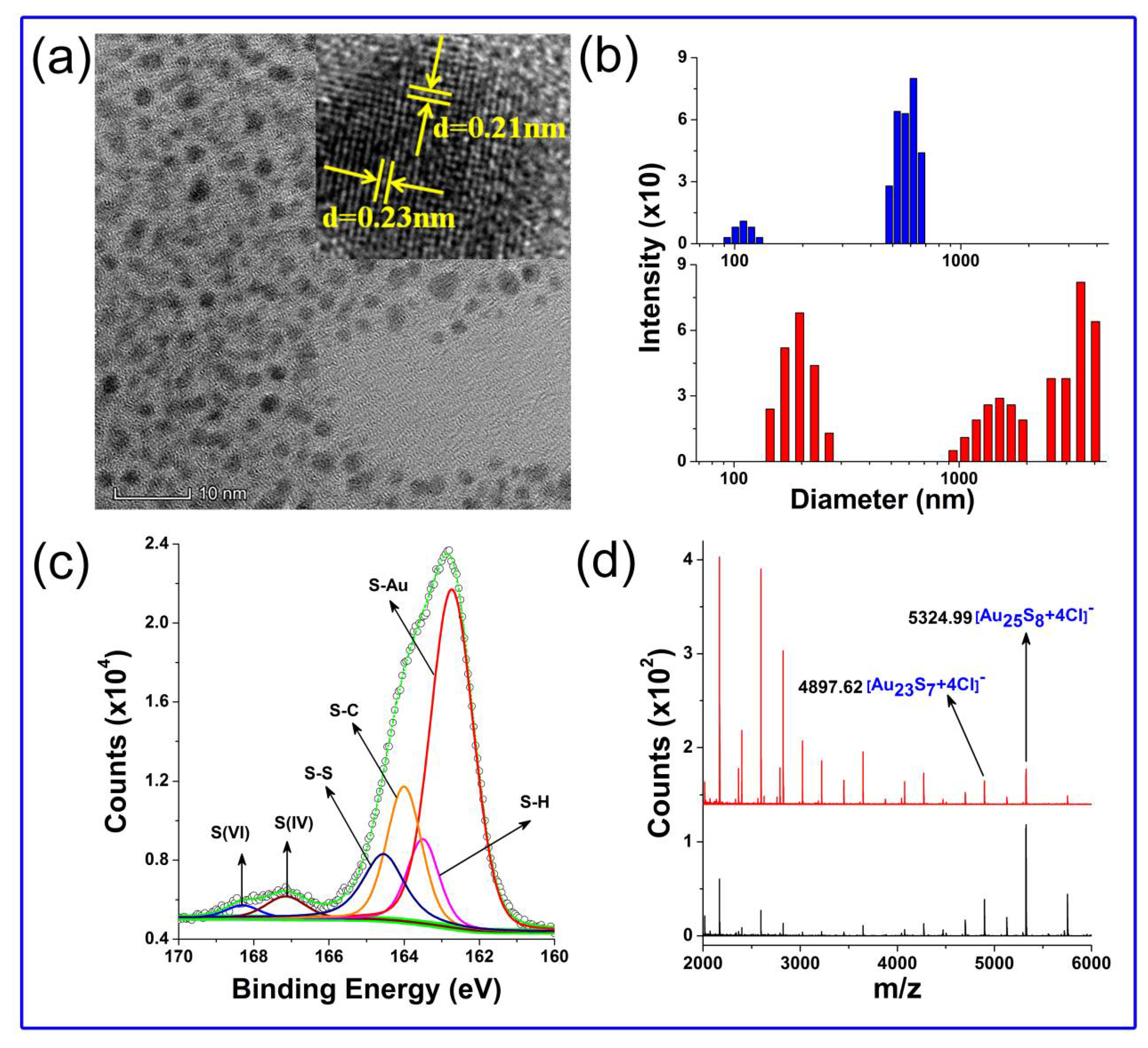
3.3. Morphological and Compositional Investigations
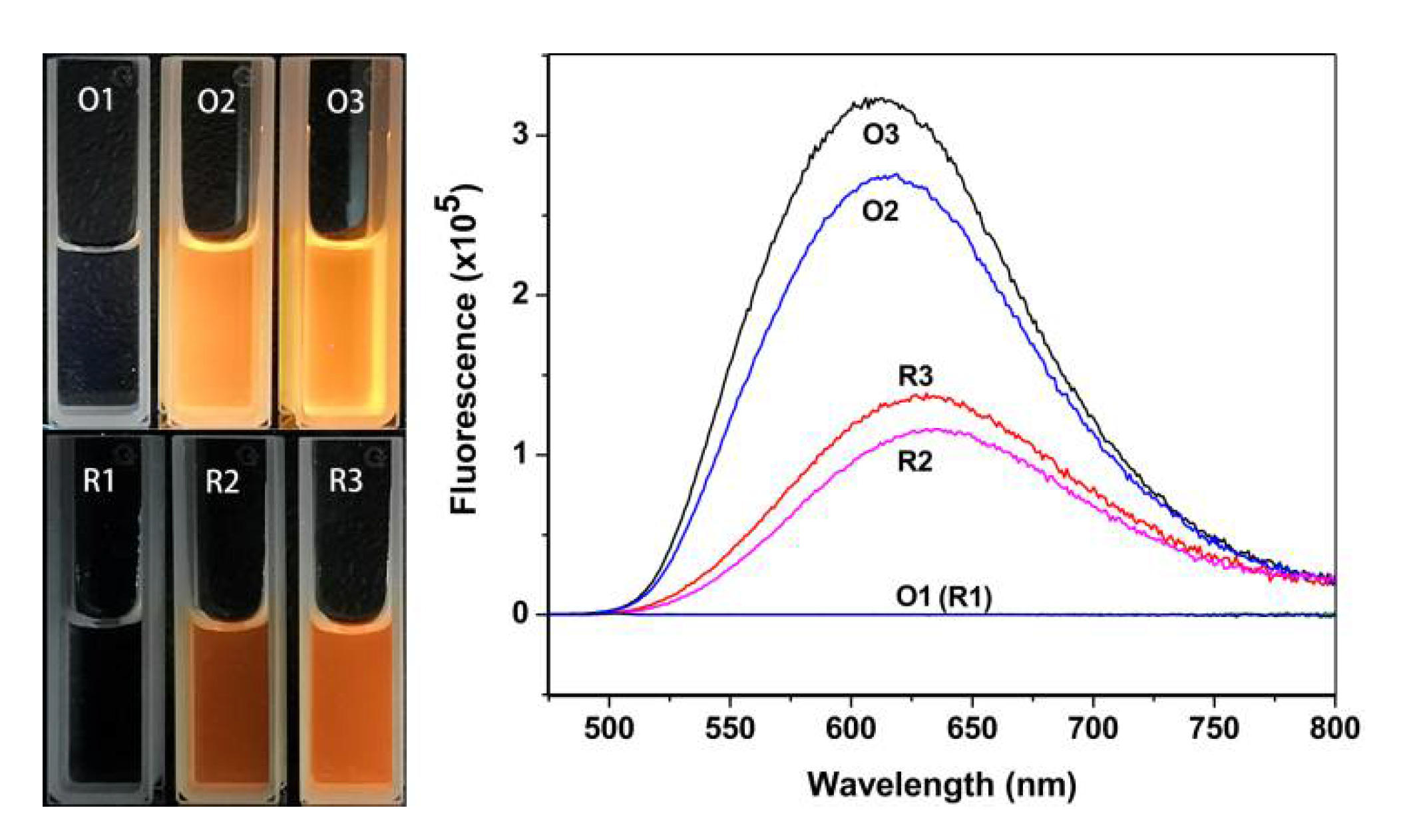
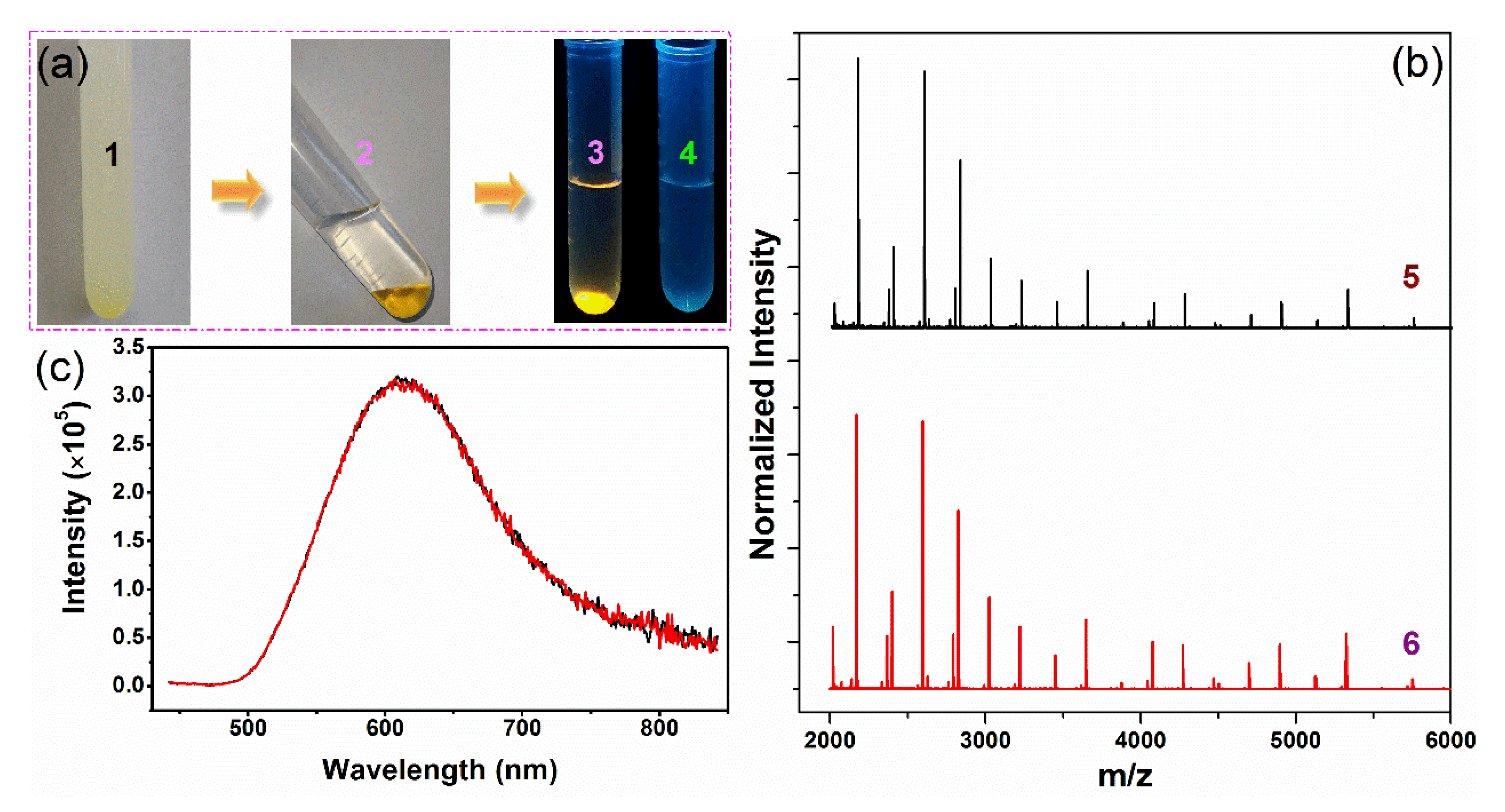
3.4. Enrichment and Redispersion of GSSG–AuNCs
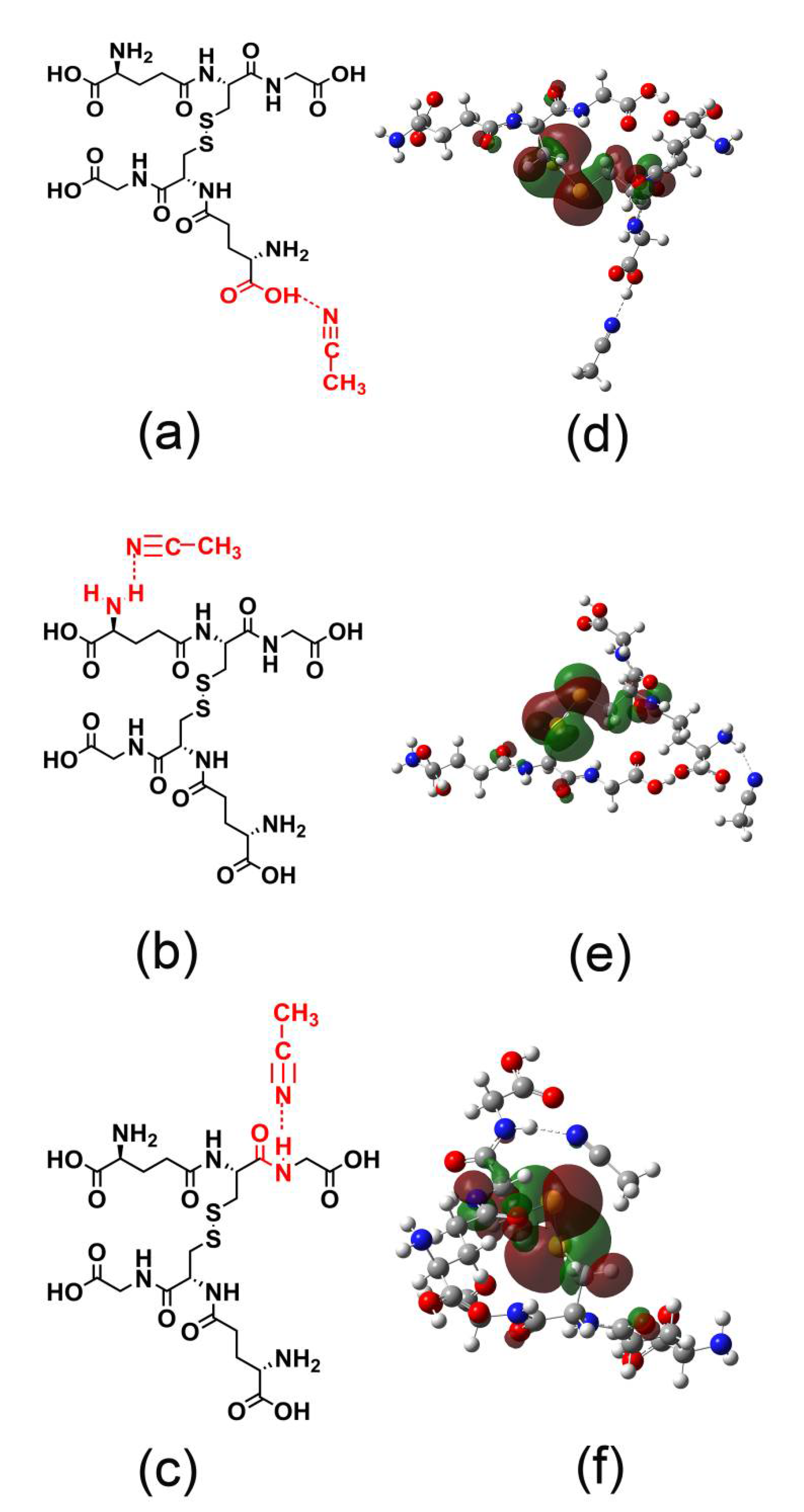
3.5. GSSG as the Mass-Separating Agent
3.6. Mechanism Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehghani, Z.; Mohammadnejad, J.; Hosseini, M. A new colorimetric assay for amylase based on starch-supported Cu/Au nanocluster peroxidase-like activity. Anal. Bioanal. Chem. 2019, 411, 3621–3629. [Google Scholar] [CrossRef]
- Kurihara, K.; Kizling, J.; Stenius, P.; Fendler, J.H. Laser and pulse radiolytically induced colloidal gold formation in water and in water-in-oil microemulsions. J. Am. Chem. Soc. 1983, 105, 2574–2579. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green Synthesis of Silver Nanoparticles Using Bilberry and Red Currant Waste Extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Iannone, A.; Lavecchia, R.; Natali, S. Green Synthesis of Gold Nanoparticles Using Kiwifruit Juice. Chem. Eng. Trans. 2020, 81, 1393–1398. [Google Scholar] [CrossRef]
- Wang, Y.A.; Nieto-Ortega, B.; Burgi, T. Transformation from Au-25(SCH2CH2CH2CH3)(18) (0) to Au-28(SCH2CH(CH3)Ph)(21) gold nanoclusters: Gentle conditions is enough. Chem. Commun. 2019, 55, 14914–14917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasaruddin, R.R.; Chen, T.K.; Yan, N.; Xie, J.P. Roles of thiolate ligands in the synthesis, properties and catalytic application of gold nanoclusters. Coord. Chem. Rev. 2018, 368, 60–79. [Google Scholar] [CrossRef]
- Liu, Z.M.; Turyanska, L.; Zamberlan, F.; Pacifico, S.; Bradshaw, T.D.; Moro, F.; Fay, M.W.; Williams, H.E.L.; Thomas, N.R. Synthesis of folic acid functionalized gold nanoclusters for targeting folate receptor-positive cells. Nanotechnology 2019, 30, 505102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Han, F. Protein coated gold nanoparticles as template for the directed synthesis of highly fluorescent gold nanoclusters. Nanotechnology 2018, 29, 165702. [Google Scholar] [CrossRef]
- Zuber, G.; Weiss, E.; Chiper, M. Biocompatible gold nanoclusters: Synthetic strategies and biomedical prospects. Nanotechnology 2019, 30, 352001. [Google Scholar] [CrossRef]
- Lee, E.S.; Cha, B.S.; Kim, S.; Park, K.S. Synthesis of Exosome-Based Fluorescent Gold Nanoclusters for Cellular Imaging Applications. Int. J. Mol. Sci. 2021, 22, 4433. [Google Scholar] [CrossRef]
- You, J.G.; Tseng, W.L. Peptide-induced aggregation of glutathione-capped gold nanoclusters: A new strategy for designing aggregation-induced enhanced emission probes. Anal. Chim. Acta 2019, 1078, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, J.P.; Zaballos-Garcia, E.; Gonzalez-Bejar, M.; Londono-Larrea, P.; Perez-Prieto, J. Adenosine monophosphate-capped gold(I) nanoclusters: Synthesis and lanthanide ion-induced enhancement of their luminescence. RSC Adv. 2016, 6, 17678–17682. [Google Scholar] [CrossRef]
- Basu, S.; Fakhouri, H.; Moulin, C.; Dolai, S.; Russier-Antoine, I.; Brevet, P.F.; Antoine, R.; Paul, A. Four orders-of-magnitude enhancement in the two-photon excited photoluminescence of homoleptic gold thiolate nanoclusters following zinc ion-induced aggregation. Nanoscale 2021, 13, 4439–4443. [Google Scholar] [CrossRef] [PubMed]
- Aizel, K.; Agache, V.; Pudda, C.; Bottausci, F.; Fraisseix, C.; Bruniaux, J.; Navarro, F.; Fouillet, Y. Enrichment of nanoparticles and bacteria using electroless and manual actuation modes of a bypass nanofluidic device. Lab Chip 2013, 13, 4476–4485. [Google Scholar] [CrossRef] [PubMed]
- Kawawaki, T.; Kataoka, Y.; Hirata, M.; Akinaga, Y.; Takahata, R.; Wakamatsu, K.; Fujiki, Y.; Kataoka, M.; Kikkawa, S.; Alotabi, A.S.; et al. Creation of High-Performance Heterogeneous Photocatalysts by Controlling Ligand Desorption and Particle Size of Gold Nanocluster. Angew. Chem. Int. Ed. 2021, 12. [Google Scholar] [CrossRef]
- Adachi, Y.; Kawasaki, H.; Nagata, T.; Obora, Y. Thiolate-protected Gold Nanoclusters Au-25(phenylethanethiol)(18): An Efficient Catalyst for the Synthesis of Propargylamines from Aldehydes, Amines, and Alkynes. Chem. Lett. 2016, 45, 1457–1459. [Google Scholar] [CrossRef]
- Brienza, M.; Manasfi, R.; Chiron, S. Relevance of N-nitrosation reactions for secondary amines in nitrate-rich wastewater under UV-C treatment. Water Res. 2019, 162, 22–29. [Google Scholar] [CrossRef]
- Xuan, S.J.; de Barros, A.O.D.; Nunes, R.C.; Ricci, E.; da Silva, A.X.; Sahid, M.; Alencar, L.M.R.; dos Santos, C.C.; Morandi, V.; Alexis, F.; et al. Radioactive gold nanocluster (198-AuNCs) showed inhibitory effects on cancer cells lines. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1214–1221. [Google Scholar] [CrossRef]
- Turcsanyi, A.; Ungor, D.; Csapo, E. Fluorescent Labeling of Hyaluronic Acid-Chitosan Nanocarriers by Protein-Stabilized Gold Nanoclusters. Crystals 2020, 10, 1113. [Google Scholar] [CrossRef]
- Fainblat, R.; Barrows, C.J.; Gamelin, D.R. Single Magnetic Impurities in Colloidal Quantum Dots and Magic-Size Clusters. Chem. Mater. 2017, 29, 8023–8036. [Google Scholar] [CrossRef]
- Huang, H.Y.; Cai, K.B.; Chen, P.W.; Lin, C.A.J.; Chang, S.H.; Yuan, C.T. Engineering Ligand-Metal Charge Transfer States in Cross-Linked Gold Nanoclusters for Greener Luminescent Solar Concentrators with Solid-State Quantum Yields Exceeding 50% and Low Reabsorption Losses. J. Phys. Chem. C 2018, 122, 20019–20026. [Google Scholar] [CrossRef]
- Knoppe, S.; Hakkinen, H.; Verbiest, T.; Clays, K. Role of Donor and Acceptor Substituents on the Nonlinear Optical Properties of Gold Nanoclusters. J. Phys. Chem. C 2018, 122, 4019–4028. [Google Scholar] [CrossRef]
- Yadav, A.; Gerislioglu, B.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.B.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.L.; et al. Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Dong, L.L.; Ahmadivand, A.; Hu, H.T.; Nordlander, P.; Halas, N.J. Monolithic Metal Dimer-on-Film Structure: New Plasmonic Properties Introduced by the Underlying Metal. Nano Lett. 2020, 20, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, J.; Luo, L.; Kuang, Y.; Sun, X. Universal Parameter Optimization of Density Gradient Ultracentrifugation Using CdSe Nanoparticles as Tracing Agents. Anal. Chem. 2016, 88, 8495–8501. [Google Scholar] [CrossRef]
- Lozano-Ramos, I.; Bancu, I.; Oliveira-Tercero, A.; Armengol, M.P.; Menezes-Neto, A.; Del Portillo, H.A.; Lauzurica-Valdemoros, R.; Borras, F.E. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J. Extracell. Vesicles 2015, 4, 27369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlesny, B.; Shiraki, T.; Janas, D. One-step sorting of single-walled carbon nanotubes using aqueous two-phase extraction in the presence of basic salts. Sci. Rep. 2020, 10, 9250. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.Y.; Leopold, K. Ligand-Assisted Extraction for Separation and Preconcentration of Gold Nanoparticles from Waters. Anal. Chem. 2012, 84, 4340–4349. [Google Scholar] [CrossRef]
- Dong, B.X.; Cheow, W.S.; Hadinoto, K. Recovery of amorphous drug-polyelectrolyte nanoparticle complex by salt-induced flocculation to circumvent the need for ultracentrifugation. Adv. Powder Technol. 2020, 31, 3102–3109. [Google Scholar] [CrossRef]
- Shaw, C.F.; Cancro, M.P.; Witkiewicz, P.L.; Eldridge, J.E. Gold(III) oxidation of disulfides in aqueous-solution. Inorg. Chem. 1980, 19, 3198–3201. [Google Scholar] [CrossRef]
- Ma, Y.J.; Yan, Z.B.; Xu, H.; Gu, H.C. The interaction of GSSG modified magnetic nanoparticles with SPC-A1 cells in vitro. Chin. Sci. Bull. 2012, 57, 3525–3531. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Dong, B.A.; Chen, B.T.; Xu, S.; Zhang, S.; Yu, W.; Xu, C.X.; Song, H.W. Glutathione modified gold nanorods with excellent biocompatibility and weak protein adsorption, targeting imaging and therapy toward tumor cells. Dalton Trans. 2013, 42, 11548–11558. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Mondal, T.; Bhattacharyya, K. Enzyme activity of alpha-chymotrypsin: Deactivation by gold nano-cluster and reactivation by glutathione. J. Colloid Interface Sci. 2017, 494, 74–81. [Google Scholar] [CrossRef]
- Ma, D.Q.; Yu, J.; Yin, W.Y.; Zhang, X.; Mei, L.Q.; Zu, Y.; An, L.J.; Gu, Z.J. Synthesis of Surface-Modification-Oriented Nanosized Molybdenum Disulfide with High Peroxidase-Like Catalytic Activity for H2O2 and Cholesterol Detection. Chem. Eur. J. 2018, 24, 15868–15878. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]
- Glusic, M.; Stare, J.; Grdadolnik, J.; Vianello, R. Binding of cadmium dication to glutathione facilitates cysteine -SH deprotonation: A computational DFT study. J. Inorg. Biochem. 2013, 119, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Zheng, J.T.; Zhang, X.G.; Chen, L.C.; Si, Y.; Huang, F.Z.; Hong, W.J. Charge transport through a water-assisted hydrogen bond in single-molecule glutathione disulfide junctions. J. Mater. Chem. C 2020, 8, 481–486. [Google Scholar] [CrossRef]
- Yao, Q.F.; Chen, T.K.; Yuan, X.; Xie, J.P. Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.N.; Yao, Q.F.; Chai, O.J.H.; Ding, N.; Xu, W.; Zang, S.Q.; Xie, J.P. Unraveling the Impact of Gold(I)-Thiolate Motifs on the Aggregation-Induced Emission of Gold Nanoclusters. Angew. Chem. Int. Ed. 2020, 59, 9934–9939. [Google Scholar] [CrossRef]
- You, J.G.; Lu, C.Y.; Kumar, A.S.K.; Tseng, W.L. Cerium(III)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale 2018, 10, 17691–17698. [Google Scholar] [CrossRef]
- Cheng, C.H.; Huang, H.Y.; Talite, M.J.; Chou, W.C.; Yeh, J.M.; Yuan, C.T. A facile method to prepare “green” nano-phosphors with a large Stokes-shift and solid-state enhanced photophysical properties based on surface-modified gold nanoclusters. J. Colloid Interface Sci. 2017, 508, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Chen, Y.S.; Kamat, P.V. Excited-State Behavior of Luminescent Glutathione-Protected Gold Clusters. J. Phys. Chem. C 2014, 118, 1370–1376. [Google Scholar] [CrossRef]
- Benito, Q.; Le Goff, X.F.; Maron, S.; Fargues, A.; Garcia, A.; Martineau, C.; Taulelle, F.; Kahlal, S.; Gacoin, T.; Boilot, J.P.; et al. Polymorphic Copper Iodide Clusters: Insights into the Mechanochromic Luminescence Properties. J. Am. Chem. Soc. 2014, 136, 11311–11320. [Google Scholar] [CrossRef]
- Zheng, T.Y.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, J. Electrochemiluminescence of Glutathione-Stabilized Au Nanoclusters Fractionated by Gel Electrophoresis in Water. Chemelectrochem 2020, 7, 1092–1096. [Google Scholar] [CrossRef]
- Mitra, A.; Ignatovich, F.; Novotny, L. Nanofluidic preconcentration and detection of nanoparticles. J. Appl. Phys. 2012, 112, 014304. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, B.B.; Wang, B.S.; He, M.; Hu, B. Phosphoric acid functionalized magnetic sorbents for selective enrichment of TiO2 nanoparticles in surface water followed by inductively coupled plasma mass spectrometry detection. Sci. Total Environ. 2020, 703, 135464. [Google Scholar] [CrossRef]
- Kosower, N.S.; Kosower, E.M.; Wertheim, B.; Correa, W.S. Diamide, a new reagent for intracellular oxidation of glutathione to disulfide. Biochem. Biophys. Res. Commun. 1969, 37, 593–596. [Google Scholar] [CrossRef]
- Mooij, W.T.M.; van Eijck, B.P.; Kroon, J. Ab initio crystal structure predictions for flexible hydrogen-bonded molecules. J. Am. Chem. Soc. 2000, 122, 3500–3505. [Google Scholar] [CrossRef]
- Narakathu, B.B.; Devadas, M.S.; Reddy, A.S.G.; Eshkeiti, A.; Moorthi, A.; Fernando, I.R.; Miller, B.P.; Ramakrishna, G.; Sinn, E.; Joyce, M.; et al. Novel fully screen printed flexible electrochemical sensor for the investigation of electron transfer between thiol functionalized viologen and gold clusters. Sens. Actuator B Chem. 2013, 176, 768–774. [Google Scholar] [CrossRef]
- Arunachalam, V.; Tummanapelli, A.K.; Vasudevan, S. The multiple dissociation constants of glutathione disulfide: Interpreting experimental pH-titration curves with ab initio MD simulations. Phys. Chem. Chem. Phys. 2019, 21, 9212–9217. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Nassar, H.F. Molecular design of mass-separating agents for separation of cyclic ethers and acetonitrile from water. J. Mol. Liq. 2019, 281, 324–331. [Google Scholar] [CrossRef]
| Approach | Nanomaterials | Time | Enrichment Factor | Recovery | Ref. |
|---|---|---|---|---|---|
| Magnet solid-phase extraction | TiO2 nanoparticles | 25 min | 400 | 91.0–102.0% | [47] |
| Nanofluidic device under electroless actuation mode | Polystyrene nanobeads | 60 min | 200 | n/a | [14] |
| Brownian ratchet-based nanofluidic system | 100 nm polystyrene beads | 17 min | 27 | n/a | [46] |
| C-18 column adsorption ligand-assisted liquid– liquid extraction | 10 nm gold nanoparticles | n/a | 250 | 68.4–99.4% | [28] |
| Ultracentrifugation | Curcumin–chitosan nanocomplexes | >20 min | n.a. | 48% | [29] |
| Acetonitrile-initiated phase separation | Gold nanoclusters | 10 min | 400 | 99.62% | This method |
| GSSG | GSH | |
|---|---|---|
| –COOH | −6.15 | −5.99 |
| –NH2 | −2.04 | −3.89 |
| –CONH | −4.46 | −3.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, J.; Meng, Z.; Ling, R.; Ren, H.; Qin, W.; Wu, Z.; Shao, N. Glutathione Disulfide as a Reducing, Capping, and Mass-Separating Agent for the Synthesis and Enrichment of Gold Nanoclusters. Nanomaterials 2021, 11, 2258. https://doi.org/10.3390/nano11092258
Zhang Q, Wang J, Meng Z, Ling R, Ren H, Qin W, Wu Z, Shao N. Glutathione Disulfide as a Reducing, Capping, and Mass-Separating Agent for the Synthesis and Enrichment of Gold Nanoclusters. Nanomaterials. 2021; 11(9):2258. https://doi.org/10.3390/nano11092258
Chicago/Turabian StyleZhang, Qianqian, Junhua Wang, Zhao Meng, Rui Ling, Hang Ren, Weidong Qin, Zhenglong Wu, and Na Shao. 2021. "Glutathione Disulfide as a Reducing, Capping, and Mass-Separating Agent for the Synthesis and Enrichment of Gold Nanoclusters" Nanomaterials 11, no. 9: 2258. https://doi.org/10.3390/nano11092258






