Surface Treatment of Industrial-Grade Magnetite Particles for Enhanced Thermal Stability and Mitigating Paint Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
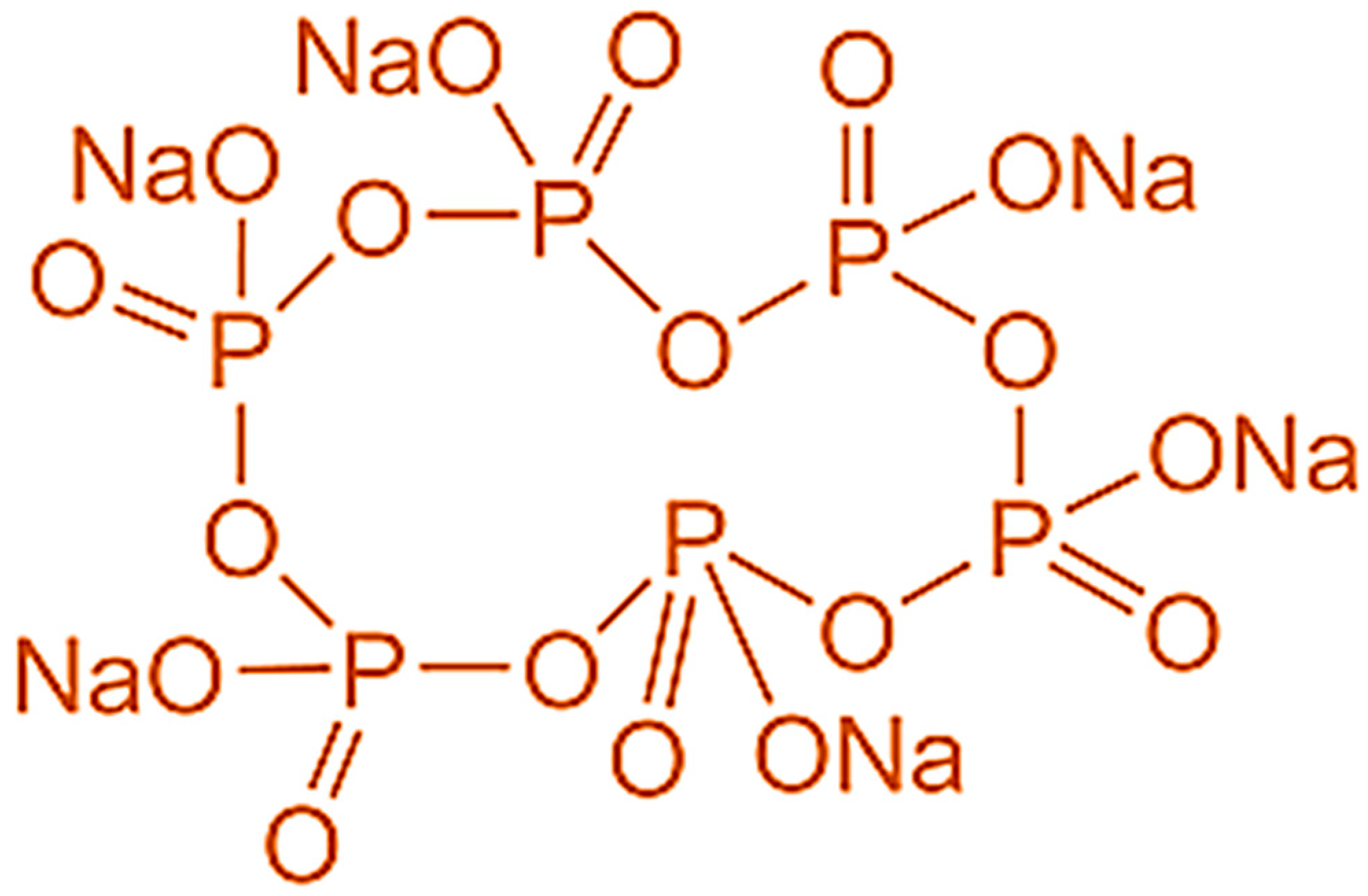
2.2. SHMP Treatment of Industrial-Grade Fe3O4
2.3. Material Characterization
2.4. Color Value Evaluation Method
3. Results and Discussions
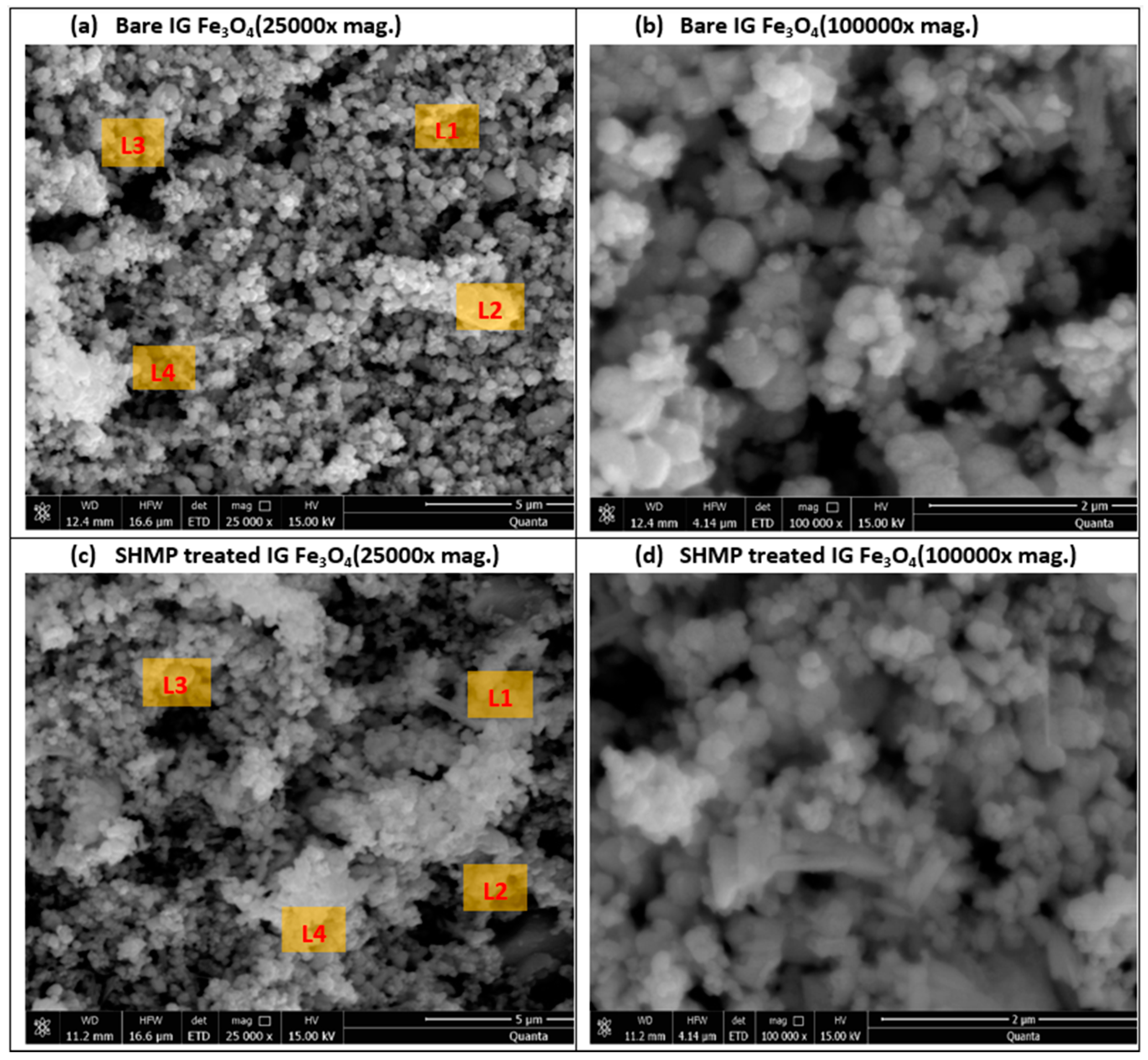
3.1. Scanning Electron Microscope and Energy Dispersive X-ray Spectroscopy Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
3.2. X-ray Diffraction Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
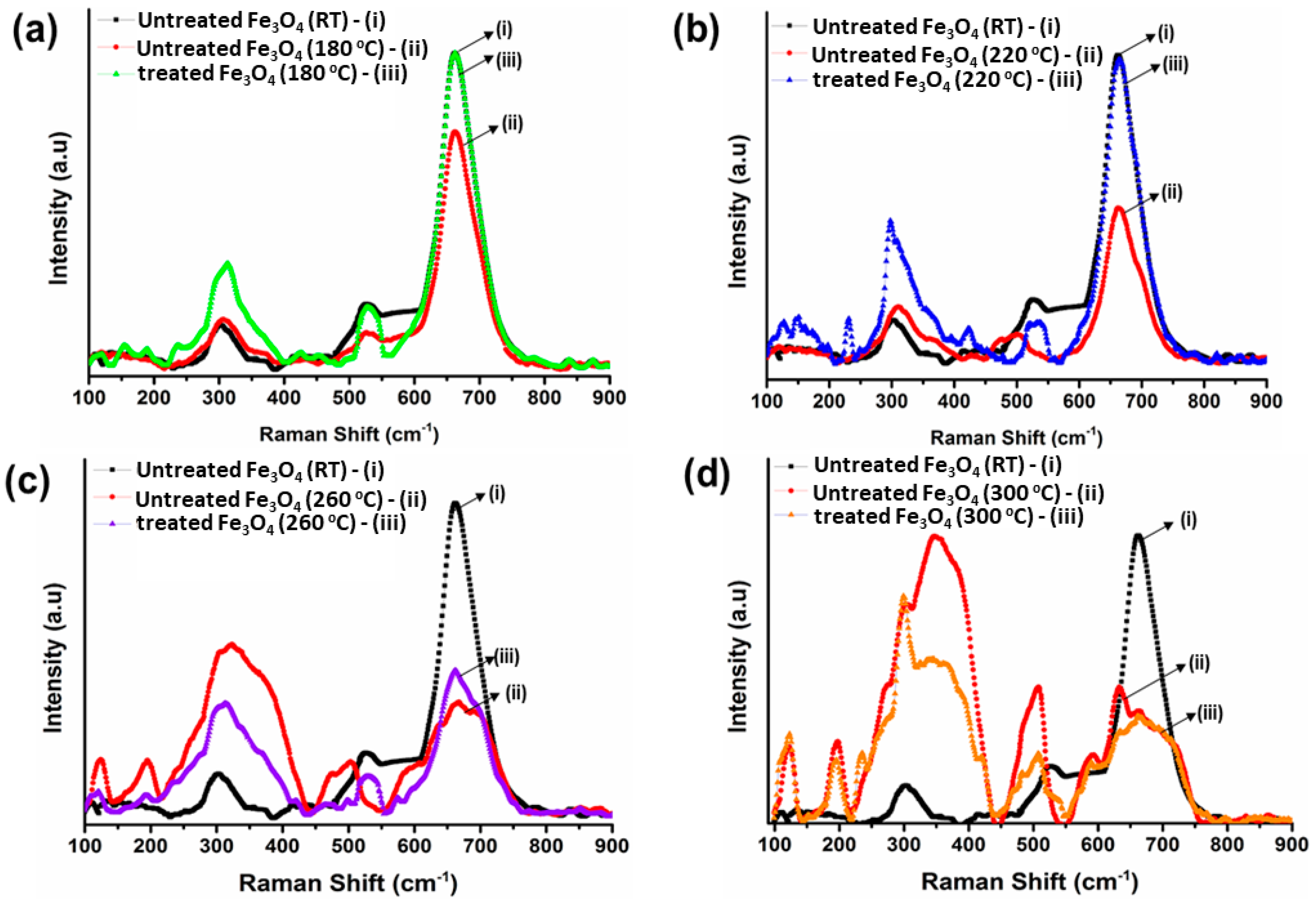
3.3. Raman Spectroscopy Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
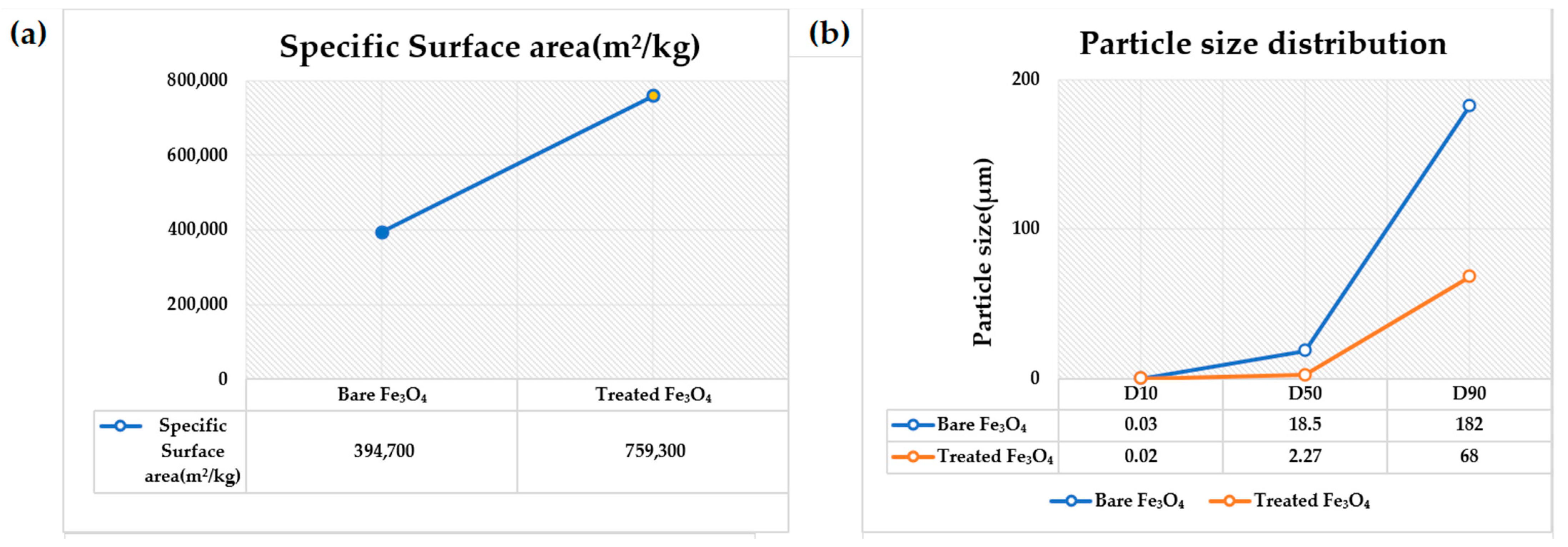
3.4. Surface Area and Particle Size Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
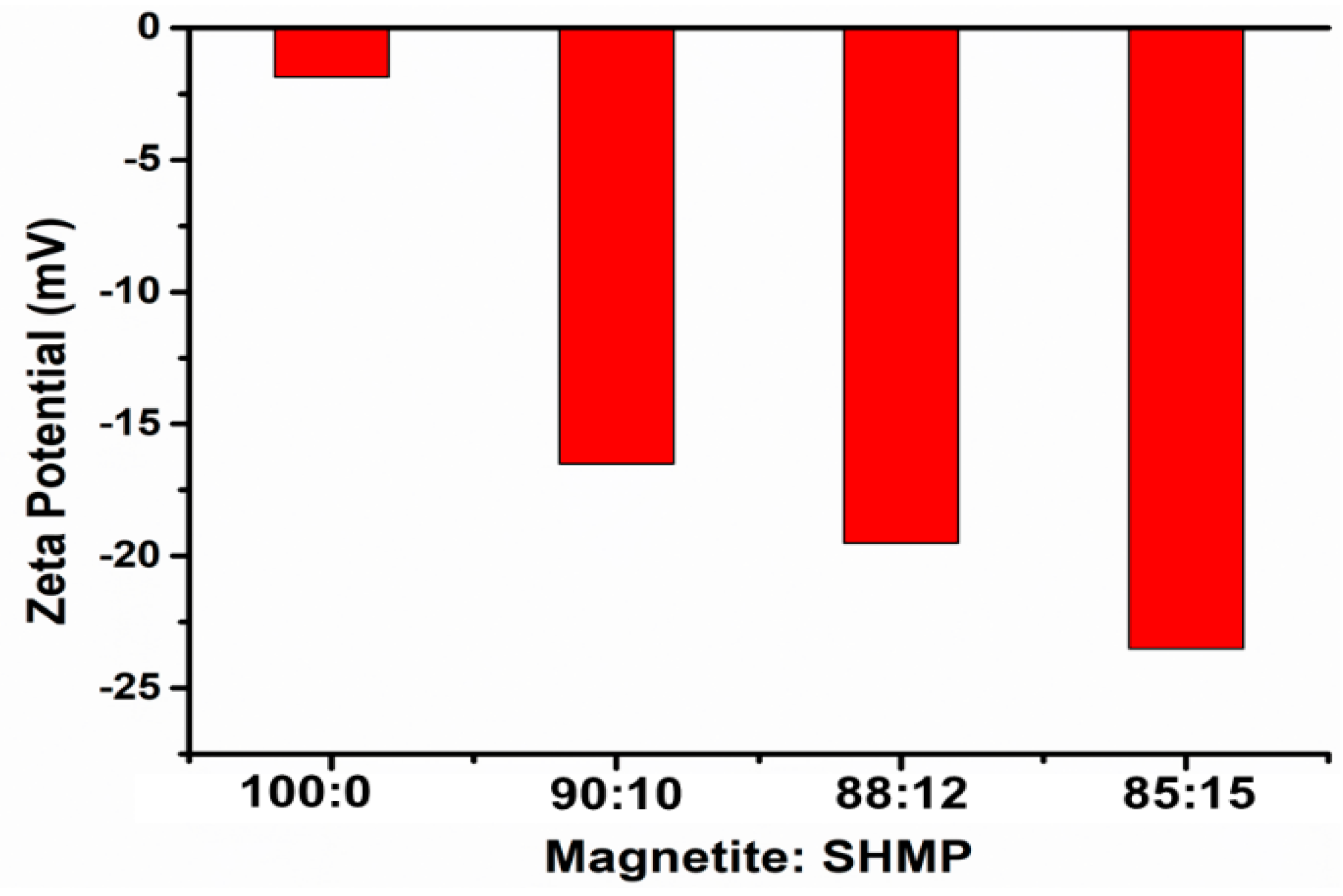
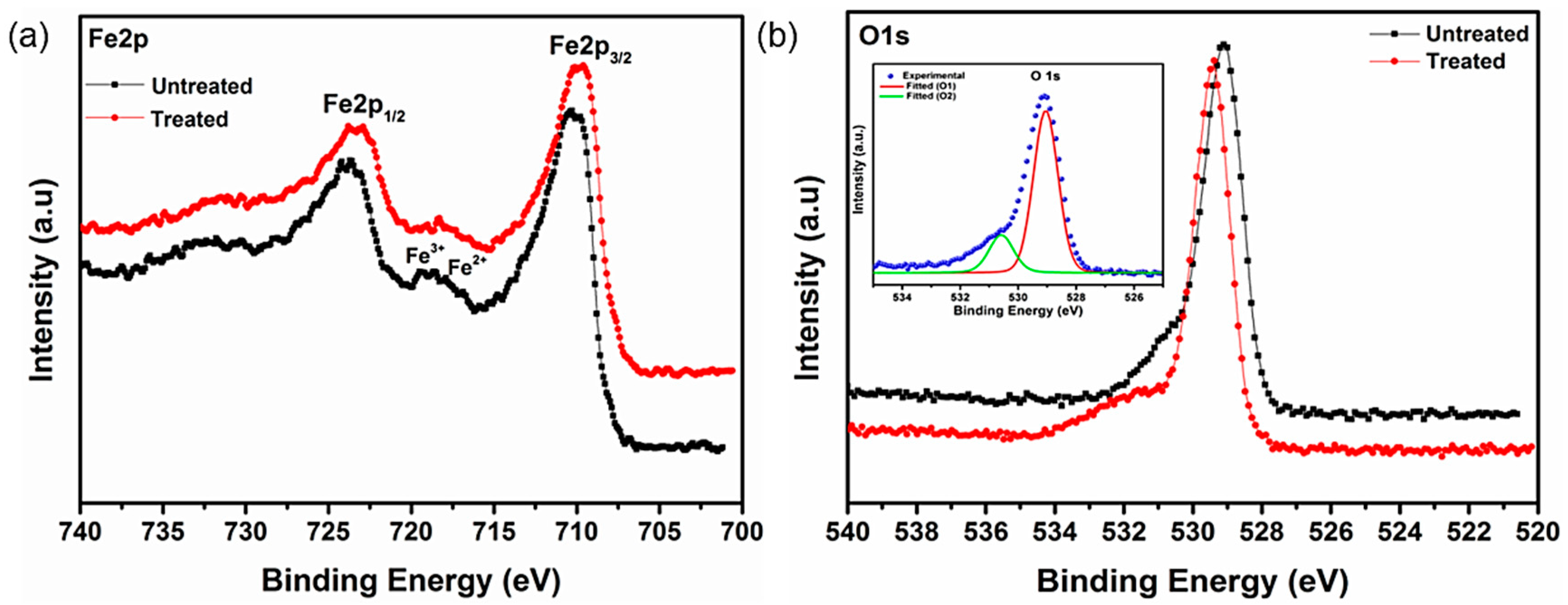
3.5. X-ray Photoelectron Spectroscopy Studies of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
3.6. Thermal Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
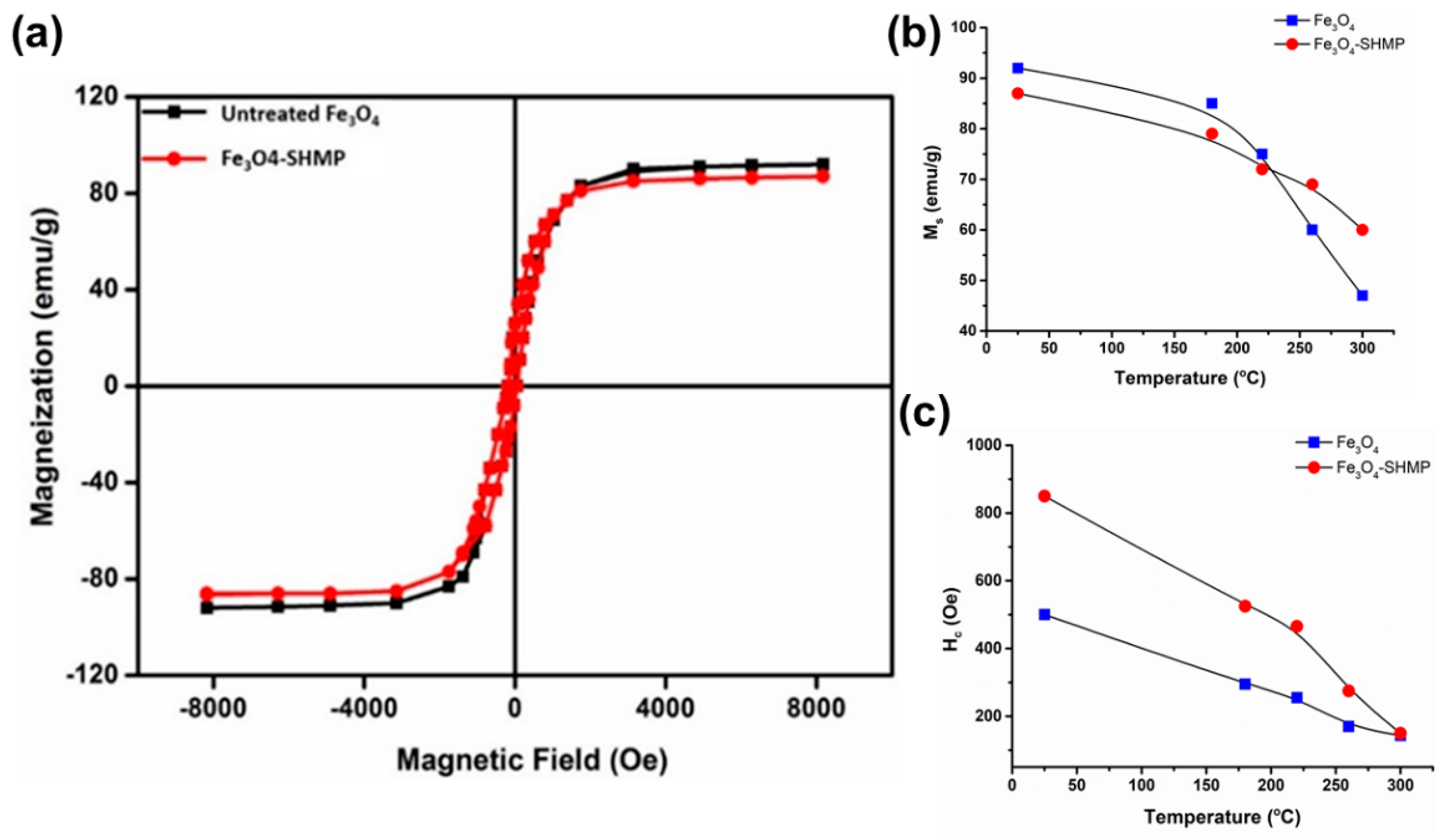
3.7. Magnetic Property Analysis of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
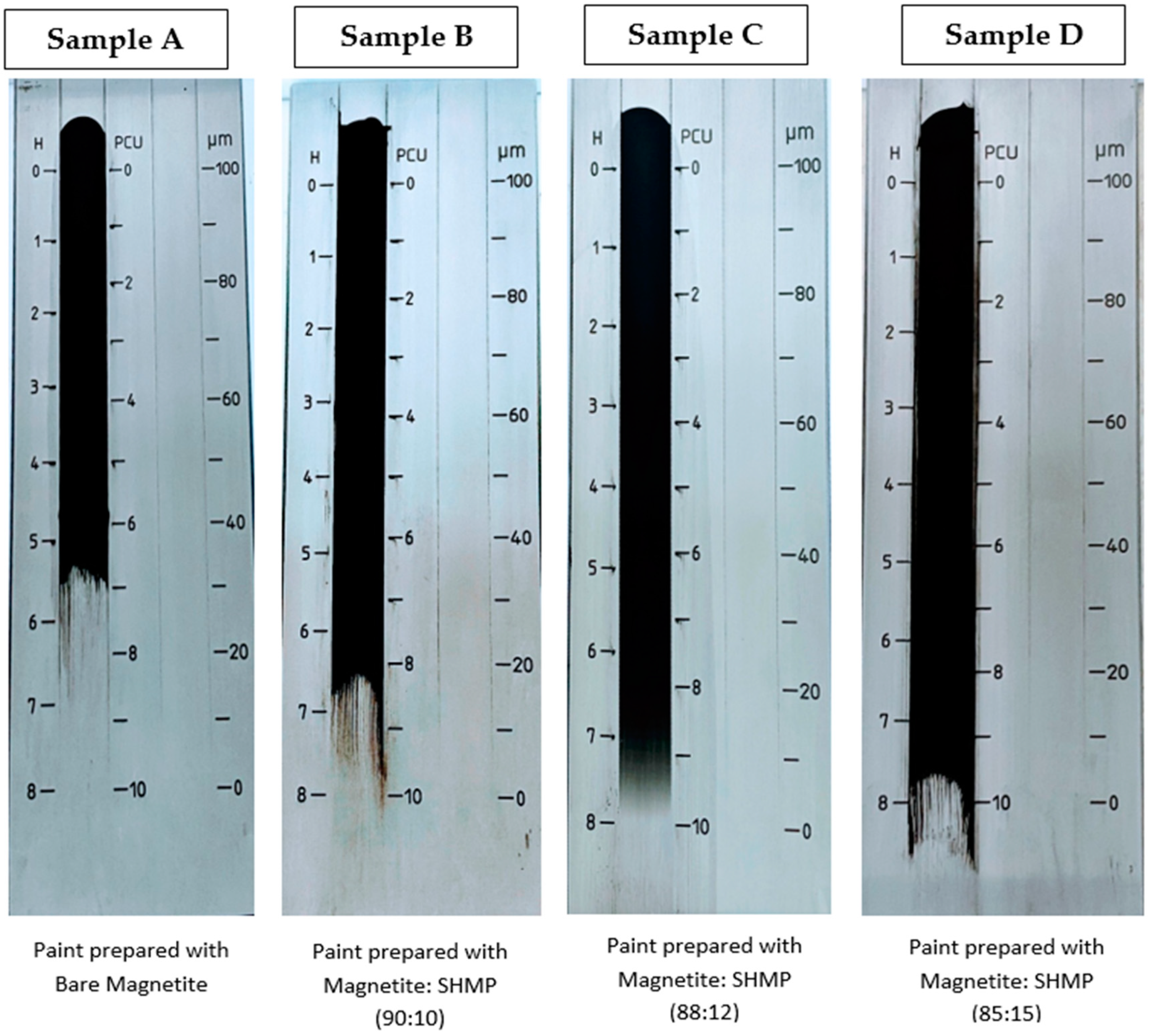
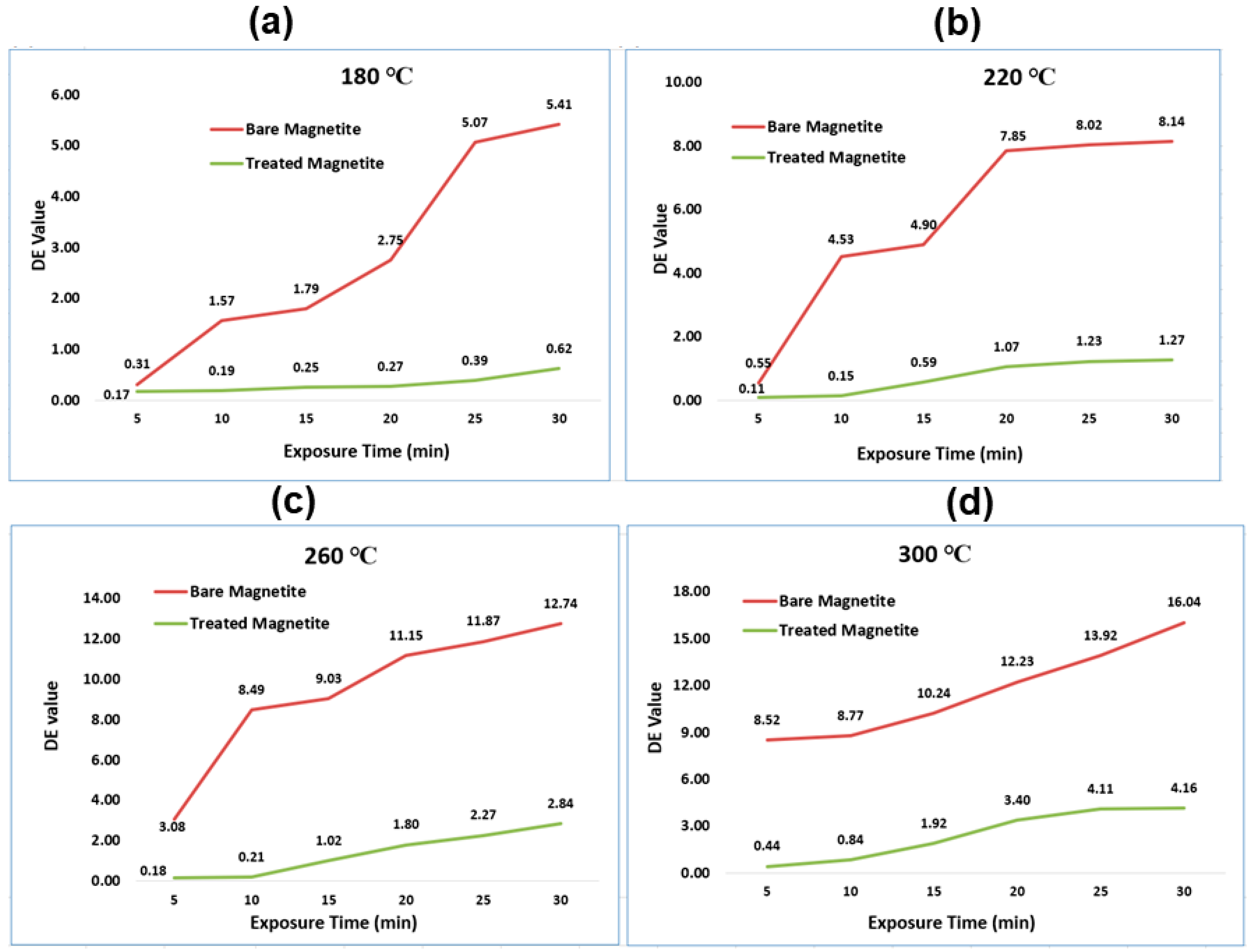
3.8. Paint Dispersion Property, and Colorimetric Study of Bare IG-Fe3O4 and SHMP-Treated Fe3O4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christianna, N.L.; Christina, A.C.; Kenneth, J.T.; Amy, C.M.; Esther, S.T.; Alan, C.W.; Mark, S.H. Energetics of Lithium Insertion into Magnetite, Defective Magnetite, and Maghemite. Chem. Mater. 2018, 30, 7922–7937. [Google Scholar]
- Böhland, T.; Brandt, K.; Brussaard, H.; Calvert, D.; Etzrodt, G.; Rieck, H.; Buxbaum, G. Coloured Pigments. Ind. Inorg. Pigment. 2005, 99–162. [Google Scholar] [CrossRef]
- Rochelle, M.C.; Udo, S. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Raj, K.; Moskowitz, R. Commercial Applications of Ferrofluids. J. Magn. Mater. 1990, 85, 233. [Google Scholar] [CrossRef]
- Muhammad, Y.R.; Mathias, A.; Lars, F.; Bernd, W. Thermal, Electrical and Magnetic Studies of Magnetite Filled Polyurethane Shape Memory Polymers. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2007, 444, 227–235. [Google Scholar]
- Eugenia, E.M.; Juan, C.C.-H.; Maria, E.C.; Moises, A.C. Thermally Stable Mesoporous Barium-Iron Mixed Oxide Phases. Mater. Lett. 2006, 60, 2119–2124. [Google Scholar]
- Cem, L.A.; Alper, E.; Merve, Y.; Necdet, A.; Seyda, B. Enhancement of Thermal Conductivity upon Application of Magnetic Field to Fe3O4 Nanofluids. J. Appl. Phys. 2011, 110, 093917. [Google Scholar]
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Coating Techniques for Biomedical Applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Dongwon, Y.; Jae-Hyun, L.; Tae-Hyun, S.; Jinwoo, C. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar]
- Don, H.; Xiaolian, S.; Shouheng, S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar]
- Yiran, T.; Xiuting, L.; Fuyan, W.; Chenjie, G.; Ziqi, Z.; Hongjie, S.; Tao, J. SERS-Based Immunoassay and Degradation of CA19-9 Mediated by Goldnanowires Anchored Magnetic–Semiconductor Nanocomposites. J. Hazard. Mater. 2021, 403, 124009. [Google Scholar]
- Yuanyuan, D.; Hongmei, L.; Ying, C.; Yiran, T.; Xiaoling, Z.; Chenjie, G.; Tao, J.; Jun, Z. Recyclable Label-Free SERS-Based Immunoassay of PSA in Human Serummediated by Enhanced Photocatalysis Arising from Ag Nanoparticles Andexternal Magnetic Field. Appl. Surf. Sci. 2020, 528, 146953. [Google Scholar]
- Orel, Z.C. Preparation of High Temperature-Resistant Selective Paints for Solar Absorbers. In Optical Materials Technology for Energy Efficiency and Solar Energy Conversion; International Society for Optics and Photonics: Bellingham, WA, USA, 1995. [Google Scholar]
- Jerome, A.C.; Keith, B.; Stuart, T.; David, M.; Paul, W.; Johann, B.; Adrian, P. Study of the Magnetite to Maghemite Transition Using Microwave Permittivity and Permeability Measurements. J. Phys. Condens. Matter 2016, 28, 106002. [Google Scholar]
- Fernández, L. Paint and Coatings Industry Worldwide-Statistics and Facts. Available online: https://www.statista.com/topics/4755/paint-and-coatings-industry/ (accessed on 12 July 2021).
- Wei, W.; Quanguo, H.; Changzhong, J. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Gerhard, B. Coloured Pigments. In Inorganic Pigments; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 92–160. [Google Scholar]
- MiningWatch Canada. Potential Toxic Effects of Chromium, Chromite Mining and Ferrochrome Production: A Literature Review; MiningWatch: Ottawa, ON, Canada, 2012. [Google Scholar]
- Mohseni, H.; Shokrollahi, H.; Sharifi, I.; Gheisari, K. Magnetic and Structural Studies of the Mn-Doped Mg-Zn Ferrite Nanoparticles Synthesized by the Glycine Nitrate Process. J. Magn. Magn Mater. 2012, 324, 3741–3747. [Google Scholar] [CrossRef]
- Xing, M.; Jeotikanta, M.; Beatty, J.; Elkins, J.; Nil, K.P.; Chalise, A.; Chen, W.; Jin, M.; Liu, J.P. Iron-Based Magnetic Nanoparticles for Multimodal Hyperthermia Heating. J. Alloy. Compd. 2021, 871, 159475. [Google Scholar] [CrossRef]
- Yudhisthira, S.; Hillel, P.; Tcipi, F.; Dina, G.; Larisa, B.; Chaim, N.S.; Gil, M. Alkyl Phosphonate/Phosphate Coating on Magnetite Nanoparticles: A Comparison with Fatty Acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Kreller, I.D.; Gibson, G.; Novak, W.; Van Loon, G.W.; Horton, H.J. Competitive Adsorption of Phosphate and Carboxylate with Natural Organic Matter on Hydrous Iron Oxides as Investigated by Chemical Force Microscopy. Colloids Surf. A 2003, 212, 249–264. [Google Scholar] [CrossRef]
- Meghann, A.W.; Jeremiah, A.J.; Jeffrey, T.K.; Nicholas, J.T. Toward the Syntheses of Universal Ligands for Metal Oxide Surfaces: Controlling Surface Functionality Through Click Chemistry. J. Am. Chem. Soc. 2006, 128, 11356–11357. [Google Scholar] [CrossRef]
- Parab, H.J.; Huang, J.H.; Lai, T.-C.; Jan, Y.-H.; Liu, R.-S.; Wang, J.-L.; Hsiao, M.; Chen, C.-H.; Hwu, Y.-K.; Tsai, D.P. Biocompatible Transferrin-Conjugated Sodium Hexametaphosphate-Stabilized Gold Nanoparticles: Synthesis, Characterization, Cytotoxicity, and Cellular Uptake. Nanotechnology 2011, 22, 395706. [Google Scholar] [CrossRef] [Green Version]
- Asha, G.; Preetam, S.; Shivakumara, C. Synthesis of BaSO4 Nanoparticles by Precipitation Method Using Sodium Hexa Metaphosphate as a Stabilizer. Solid State Commun. 2010, 150, 386–388. [Google Scholar] [CrossRef]
- Wang, N.-X.; Wang, Y.-Q.; He, X.-W.; Li, W.-Y. One-Step, and Rapid Synthesis of Composition-Tunable and Water-Soluble ZnCdS Quantum Dots. J. Nanosci. Nanotechnol. 2011, 11, 4039–4045. [Google Scholar] [CrossRef]
- Kuppayee, M.; Nachiyar, G.K.V.; Ramasamy, V. Synthesis and Characterization of Cu2+ Doped ZnS Nanoparticles Using TOPO and SHMP as Capping Agents. Appl. Surf. Sci. 2011, 257, 6779–6786. [Google Scholar] [CrossRef]
- Mehrdad, R.; Solriman, A.; Fatemeha, A.; Rammile, E. On the Heat Stability of Whey Protein: Effect of Sodium Hexametaphosphate. Int. J. Dairy Technol. 2019, 73, 46–56. [Google Scholar]
- Demand for Ecofriendly Paint Expected to Rise as Building Outlook Improves. Available online: https://www.engineeringnews.co.za/print-version/construction-demand-boosts-local-eco-friendly-paints-2017-03-10 (accessed on 31 July 2021).
- The Paints That Eat Pollutants and Heat Homes. Available online: https://ec.europa.eu/research-and-innovation/en/horizon-magazine/paints-eat-pollutants-and-heat-homes (accessed on 31 July 2021).
- Available online: https://www.averda.com/rsa/news/turning-industrial-waste-into-paints (accessed on 31 July 2021).
- Bruno, L.; Cíntia, Z.F.-P.; João, A.P.; Julio, C.P. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Jahnke, J.C.; Hornbuckle, K.C. PCB Emissions from Paint Colourants. Environ. Sci. Technol. 2019, 53, 5187–5194. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, S.G.; Mohd, A.M.; Rozan, M.Y.; Dilla, D.B. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar]
- Wangzhong, S.; Sha, H.; William, J.S.; Adah, A. Review of the Progress Toward Achieving Heat Confinement-The Holy Grail of Photothermal Therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar]
- Challa, S.S.R.K.; Faruq, M. Magnetic Nanomaterials for Hyperthermia-based Therapy and Controlled Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar]
- Veronica, I.; Thomas, R.P.; Sungho, J. Magnetic Nanoparticles for Theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar]
- Douglas, R.D.; Brewer, J.D. Acceptability of Shade Differences in Metal Ceramic Crowns. J. Prosthet. Dent. 2005, 79, 254–260. [Google Scholar] [CrossRef]
- Mansoor, F.; Akbar, M.; Iraj, K. An Investigation of Structural Phase Transformation of Monosize γ-Fe2O3 Nanoparticles Fabricated by Arc Discharge Method. Mat. Lett. 2012, 89, 140–142. [Google Scholar] [CrossRef]
- Surowiec, Z.; Miaskowski, A.; Budzyński, M. Investigation of Magnetite Fe3O4 Nanoparticles for Magnetic Hyperthermia. Nukleonika 2017, 62, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Brescia, R.; Prato, M.; Marras, S.; Manna, L.; Colombo, M. Tuning the CO Oxidation Catalytic Activity of Supported Metal–Metal Oxide Heterostructures by an Aqueous Phase Post-Treatment Process. J. Mater. Chem. A 2016, 4. [Google Scholar] [CrossRef]
- Barbosa, P.F.; Lagoeiro, L.; Scholz, R.; Graça, L.M.; Mohallem, N. Magnetite-Hematite Transformation: Correlation between Natural and Synthetic Features. Miner. Petrol. 2015, 109, 329–337. [Google Scholar] [CrossRef]
- Mohua, S.; Narendra, K.; Monojit, D.; Udayabhanu, G. Role of Chelating Compounds towards Thermal Resistance Property of Industrial Grade Goethite. Pigment. Resin Technol. 2020, 50. [Google Scholar] [CrossRef]
- Jerina, M.; Kanhu, C.B.; Neena, G.S.; Badri, N.P.; Puthusserickal, A.H. Water-Dispersible Polyphosphate Grafted Fe3O4 Nanomagnets for Cancer Therapy. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Lesia, H.; Hector, M.L.; Isamu, S. Selective Inhibition of the Oxidation of Ferrous Iron or Sulfur in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 2000, 66, 1031–1037. [Google Scholar]
- Rull, F.; Martinez-Frias, J.; Sansano, A.; Medina, J.; Edwards, H.G.M. Comparative Micro-Raman Study of the Nakhla and Vaca Muerta Meteorites. J. Raman Spectrosc. 2004, 35, 497–503. [Google Scholar] [CrossRef]
- Alasdair, P.L.; John, W.; David, J.M.; Wilhelm, V.B.; Angela, R.S.; Charmaine, G.D.W. In Situ Raman Spectroscopic Studies of the Teeth of the Chiton Acanthopleura hirtosa. J. Biol. Inorg. Chem. 1998, 3, 614–619. [Google Scholar]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. Appl. Mater. 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Jiwei, L.; Zhitao, Y.; Zhongyun, T.; Shengliang, Q.; Lixia, L. Surface Modification of Pentlandite and Serpentine with Reagents and Magnetite for Magnetic Separation. JOM 2018, 70. [Google Scholar] [CrossRef]
- Van Wazer, J.R. The Chemistry of Phosphates. J. Dent. Res. 1964, 43. [Google Scholar] [CrossRef]
- Marie-Alexandra, N.; Ulrich, S. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Chem. 2008, 139, 183–195. [Google Scholar] [CrossRef]
- Lei, H.; Qibin, L.; Fang, W. Mechanisms that Control the Adsorption–Desorption Behavior of Phosphate on Magnetite Nanoparticles: The Role of Particle Size and Surface Chemistry Characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of Surface Charge on the Colloidal Stability and In Vitro Uptake of Carboxymethyl Dextran-Coated Iron Oxide Nanoparticles. J. Nanopart Res. 2013, 15, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, R.S.C.; Amarantidis, J.; Skinner, W.M.; Prestidge, C.A.; la Vanier, L.; Grano, S.R. Surface Analytical Studies of Oxidation and Collector Adsorption in Sulfide Mineral Flotation. Appl. Phys. 2003, 85. [Google Scholar] [CrossRef]
- Itoh, F.; Satou, M.; Yamazaki, Y. Anomalous Increase of Coercivity in Iron Oxide Powder Coated with Sodium Polyphosphate. IEEE Trans. Magn. 1977, 13, 1385–1387. [Google Scholar] [CrossRef]




| Characteristic | Specification | Testing Method | IG Fe3O4 | SHMP-Treated Fe3O4 |
|---|---|---|---|---|
| Volatile matter % by mass | ≤2.5 | IS 33:1992 | 0.58 | 0.95 |
| Residue on sieve % by mass on 45 µ sieve | ≤0.5 | IS 33:1992 | 0.35 | 0.33 |
| Oil absorption | 22–27 | IS 33:1992 | 25.2 | 25.8 |
| Matter soluble in water % by mass | ≤1.0 | IS 33:1992 | 0.28 | 0.26 |
| Acidity (as H2SO4) or alkalinity (as NaOH) %age by mass | ≤0.1 | IS 33:1992 | 0.04 | 0.06 |
| pH of aqueous extract | 4–8 | IS 33:1992 | 6.89 | 7.13 |
| Total iron (as Fe2O3) % by mass | ≥79% | IS 44:1991 | 79.2 | 79.1 |
| Ferrous iron (as FeO) % by mass | ≥20% | IS 44:1991 | 20 | 20.1 |
| Carbonates (as CO2) % by mass | 0.5 | IS 33:1992 | 0.1 | 0.12 |
| Bare Fe3O4 | SHMP-Treated Fe3O4 | ||||
|---|---|---|---|---|---|
| Location (L) | O (%) | Fe (%) | O (%) | P (%) | Fe (%) |
| L1 | 20.47 | 77.89 | 32.19 | 1.01 | 65.68 |
| L2 | 18.27 | 80.14 | 26.11 | 0.88 | 71.87 |
| L3 | 15.60 | 82.78 | 22.31 | 0.90 | 75.63 |
| L4 | 18.18 | 80.43 | 28.54 | 0.80 | 69.69 |
| Mean | 18.13 | 80.31 | 27.29 | 0.90 | 70.72 |
| Sample | Exposed Temperatures | ||||
|---|---|---|---|---|---|
| 25 °C | 180 °C | 220 °C | 260 °C | 300 °C | |
| Bare IG Fe3O4 | 668 cm−1 | 673 cm−1 | 678 cm−1 | 697 cm−1 | 704 cm−1 |
| 540 cm−1 | 518 cm−1 | 515 cm−1 | 513 cm−1 | 512 cm−1 | |
| 312 cm−1 | 330 cm−1 | 334 cm−1 | 341 cm−1 | 365 cm−1 | |
| SHMP-treated Fe3O4 | 668 cm−1 | 668 cm−1 | 670 cm−1 | 674 cm−1 | 674 cm−1 |
| 540 cm−1 | 537 cm−1 | 540 cm−1 | 537 cm−1 | 520 cm−1 | |
| 312 cm−1 | 312 cm−1 | 315 cm−1 | 323 cm−1 | 312 cm−1 | |
| Sample | Temperature (°C) | Ms (emu g −1) | Mr (emu g−1) | Hc (Oe) |
|---|---|---|---|---|
| Fe3O4 | 25 | 92 | 34 | 500 |
| 180 | 85 | 32 | 295 | |
| 220 | 75 | 30 | 255 | |
| 260 | 60 | 28 | 170 | |
| 300 | 47 | 25 | 143 | |
| SHMP-treated Fe3O4 | 25 | 87 | 32 | 850 |
| 180 | 79 | 31.5 | 525 | |
| 220 | 72 | 31 | 465 | |
| 260 | 69 | 30 | 275 | |
| 300 | 60 | 29.5 | 150 |
| Bare Fe3O4 | |||||
| Color Coordinates | 25 °C | 180 °C | 220 °C | 260 °C | 300 °C |
| L* | 26.9 | 28.38 | 29.35 | 31.36 | 33.24 |
| a* | 0.02 | 1.93 | 2.69 | 6.96 | 6.24 |
| b* | 0.26 | 2.42 | 3.05 | 6.24 | 9.24 |
| SHMP-Treated Fe3O4 | |||||
| L* | 26.9 | 26.21 | 26.51 | 26.7 | 26.7 |
| a* | 0.02 | 0.01 | 0.13 | 0.22 | 0.25 |
| b* | 0.26 | 0.04 | 0.22 | 0.55 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinhababu, M.; Roy, A.; Kumar, N.; Dutta, M.; Sundaram, S.; Karazhanov, S.; Udayabhanu, G. Surface Treatment of Industrial-Grade Magnetite Particles for Enhanced Thermal Stability and Mitigating Paint Contaminants. Nanomaterials 2021, 11, 2299. https://doi.org/10.3390/nano11092299
Sinhababu M, Roy A, Kumar N, Dutta M, Sundaram S, Karazhanov S, Udayabhanu G. Surface Treatment of Industrial-Grade Magnetite Particles for Enhanced Thermal Stability and Mitigating Paint Contaminants. Nanomaterials. 2021; 11(9):2299. https://doi.org/10.3390/nano11092299
Chicago/Turabian StyleSinhababu, Mohua, Anurag Roy, Narendra Kumar, Monojit Dutta, Senthilarasu Sundaram, Smagul Karazhanov, and Gopalkrishnan Udayabhanu. 2021. "Surface Treatment of Industrial-Grade Magnetite Particles for Enhanced Thermal Stability and Mitigating Paint Contaminants" Nanomaterials 11, no. 9: 2299. https://doi.org/10.3390/nano11092299
APA StyleSinhababu, M., Roy, A., Kumar, N., Dutta, M., Sundaram, S., Karazhanov, S., & Udayabhanu, G. (2021). Surface Treatment of Industrial-Grade Magnetite Particles for Enhanced Thermal Stability and Mitigating Paint Contaminants. Nanomaterials, 11(9), 2299. https://doi.org/10.3390/nano11092299










