Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
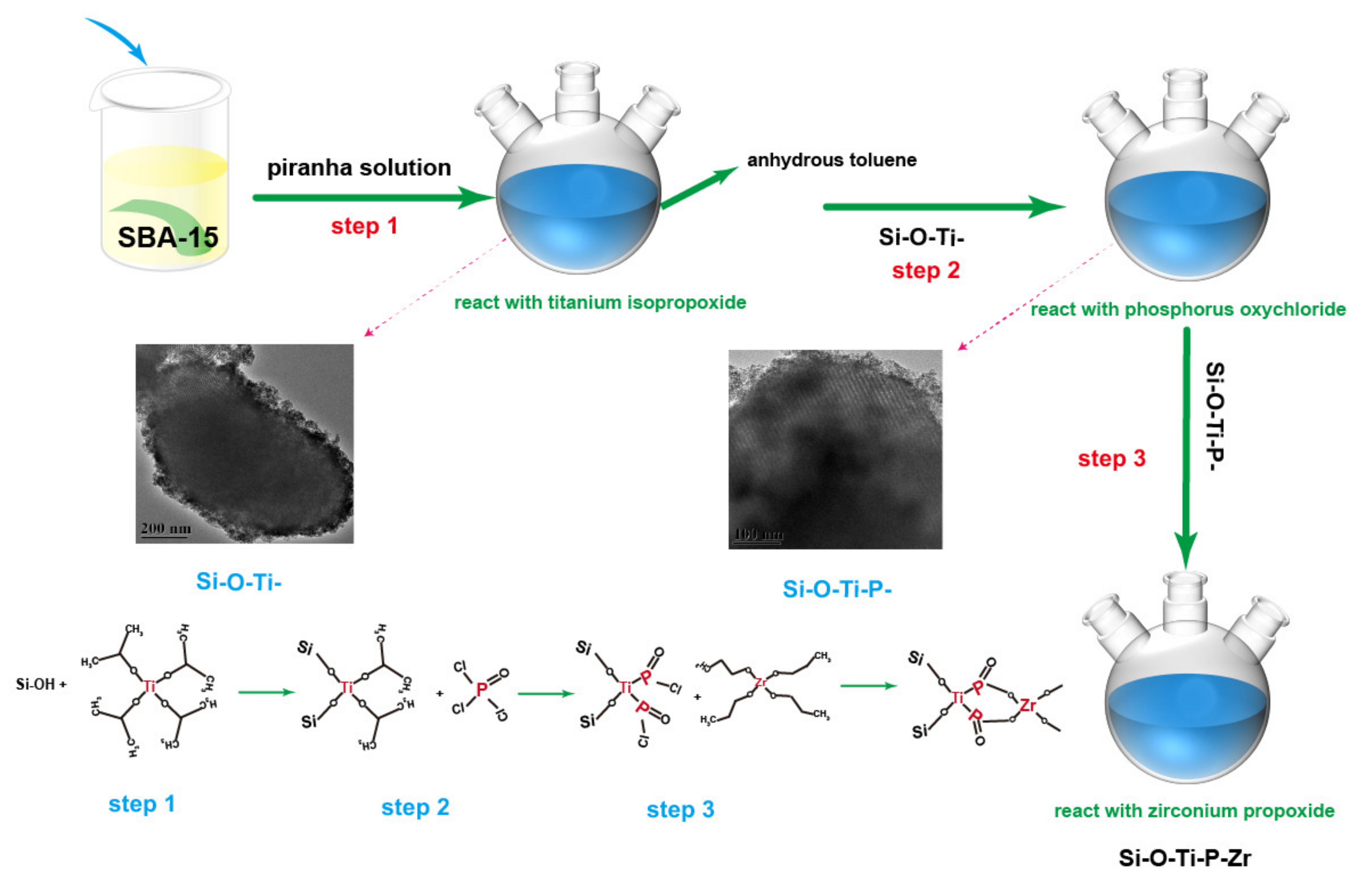
2.2. Material Synthesis Process
2.3. Characterization Methods
2.4. Ni2+ Adsorption Experiment on Zr-P-Ti-Si in Batch Experiment
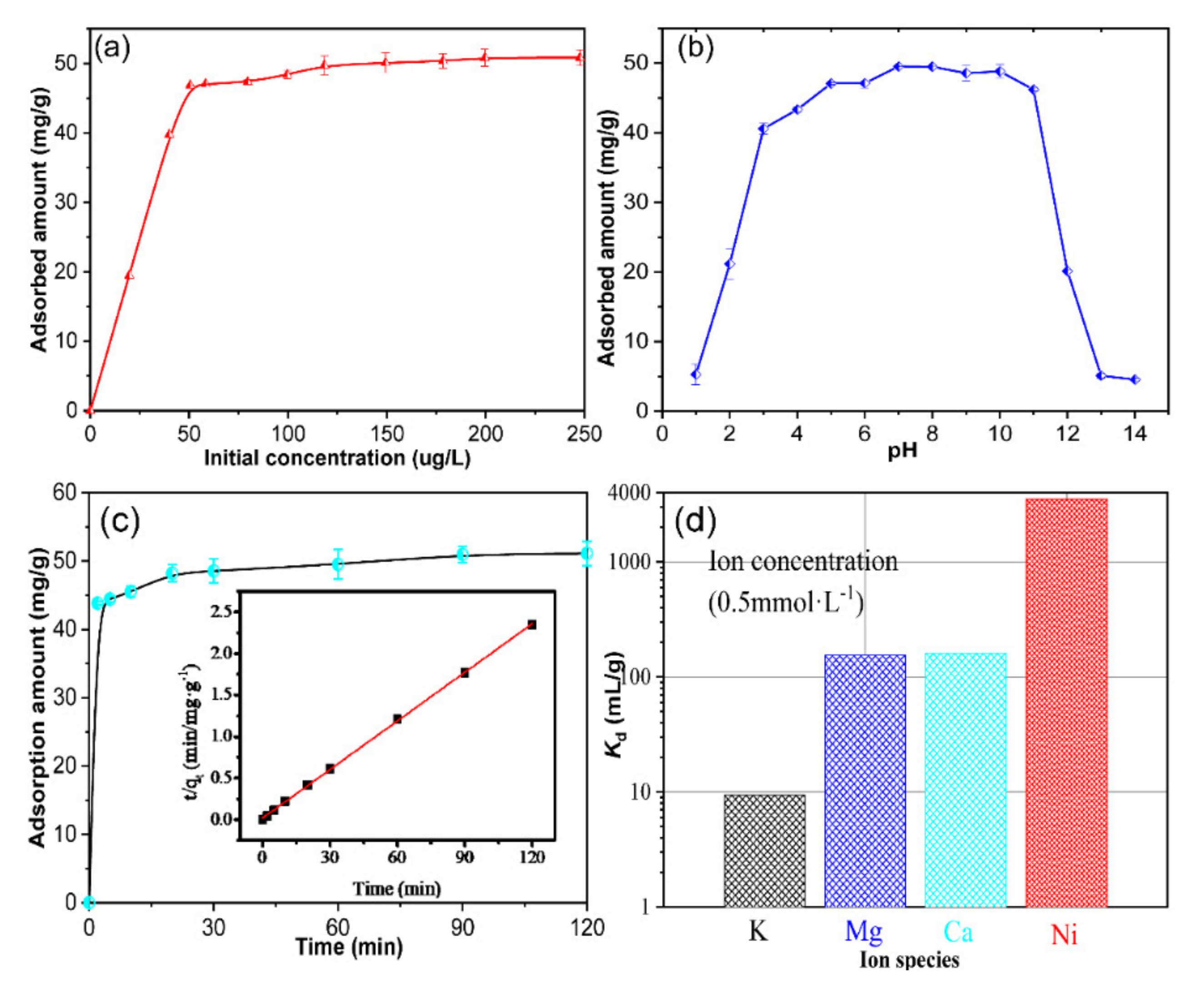
2.4.1. The Effect of the Initial Concentration of Ni Ions on the Sorption Process
2.4.2. The Effect of Contact Time on the Sorption Process
2.4.3. The Effect of pH on the Sorption Process
2.4.4. Thermodynamic Simulations of the Hydrolyzed Species of Ni Ions
2.4.5. The Affinity Measurement toward Ni Ions
3. Results and Discussion
3.1. Characterization Analysis
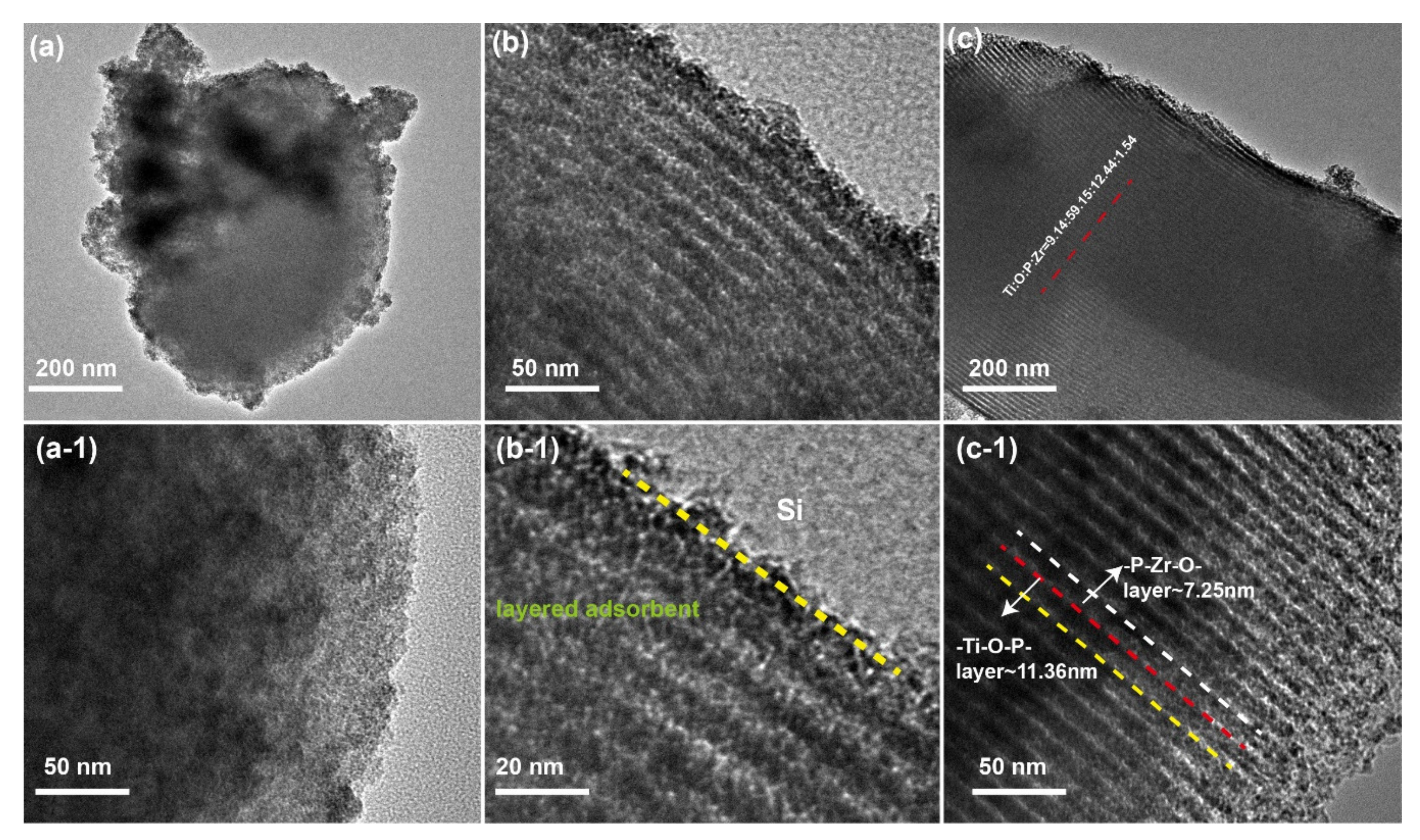
3.1.1. TEM Analysis of the SBA-15 and SiO2-Ti-P-Zr Adsorbent
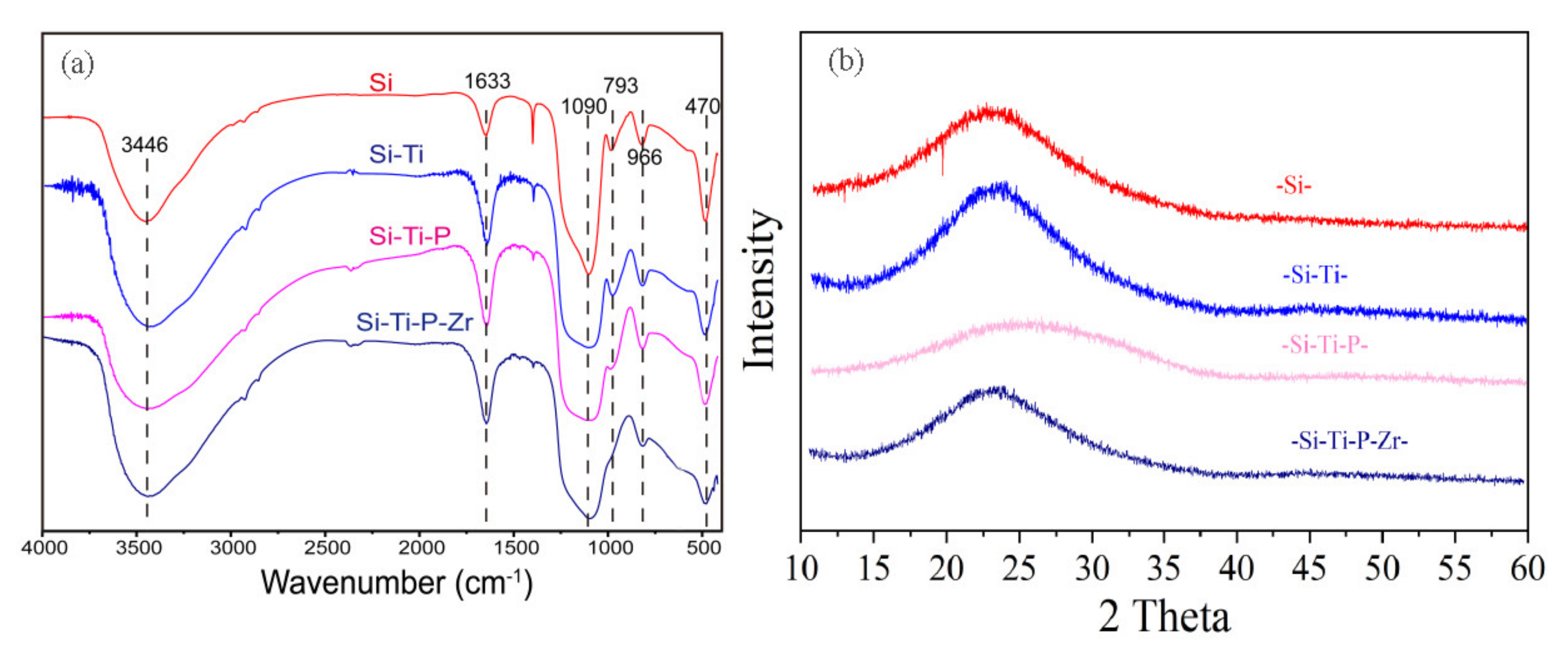
3.1.2. The FT-IR and XRD Analysis of the Formation Process of the Adsorbent
3.1.3. The Specific Surface Area and Pore Size Distribution Analysis
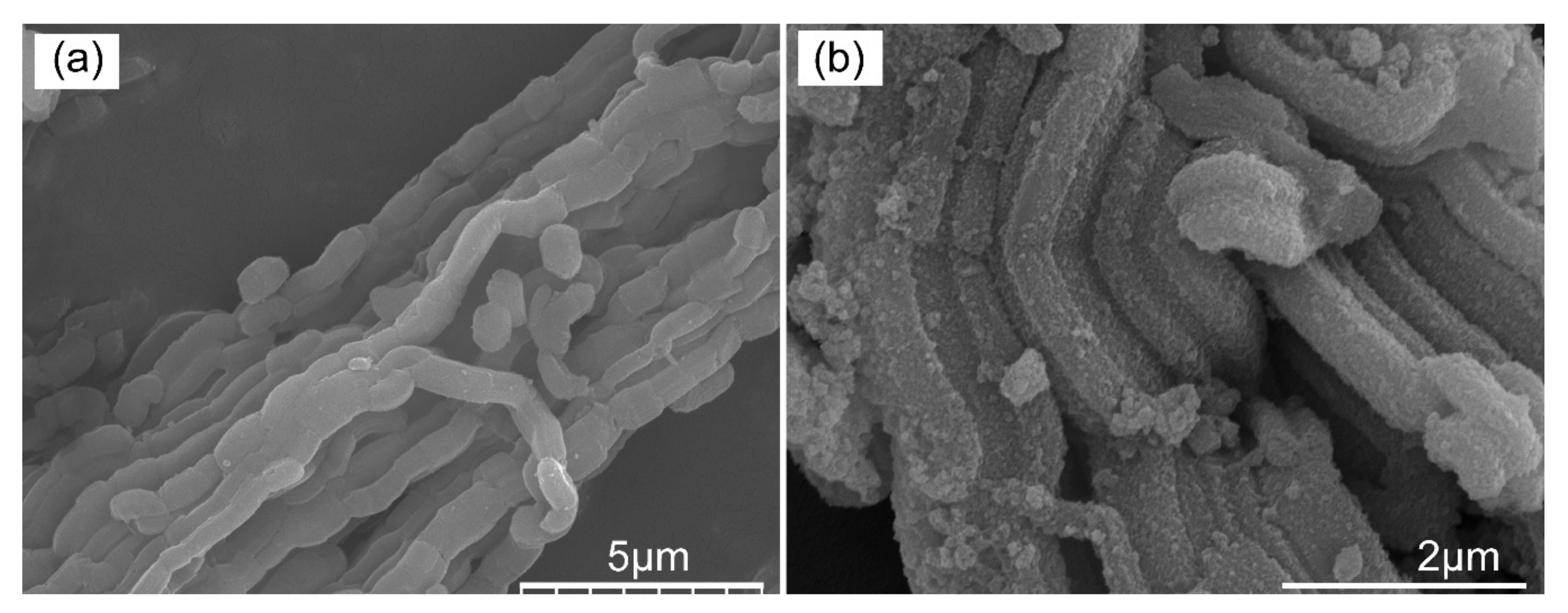
3.1.4. The Morphology and Element Mapping Analysis of the Pristine Adsorbent and Adsorbed Adsorbent
3.2. Analysis of the Hydrolyzed Species of Ni Ions by Thermodynamic Simulations Method
3.3. Analysis of the Batch Adsorption and Ion Selectivity
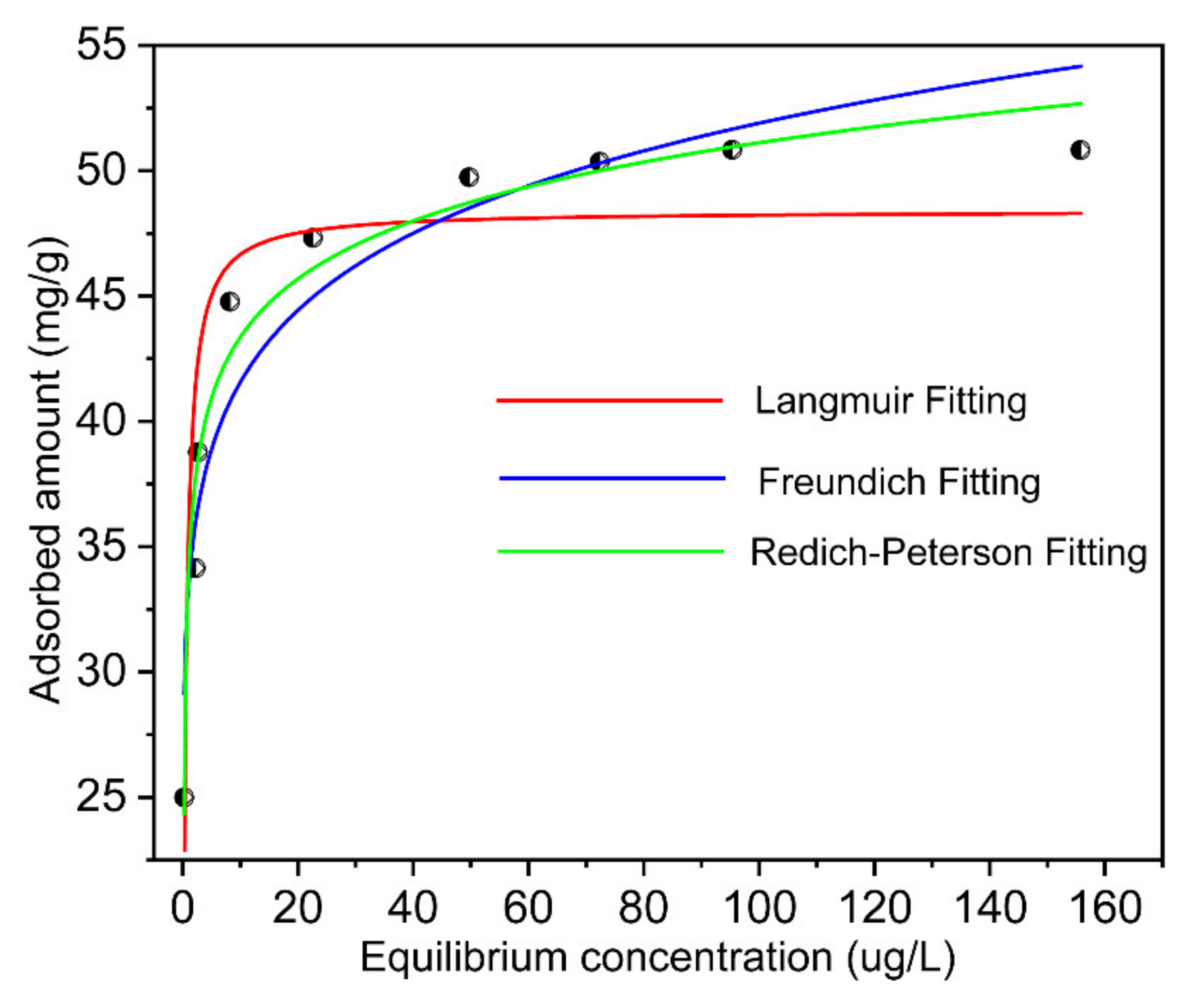
3.4. Adsorption Data Simulation by Isothermal Adsorption Models
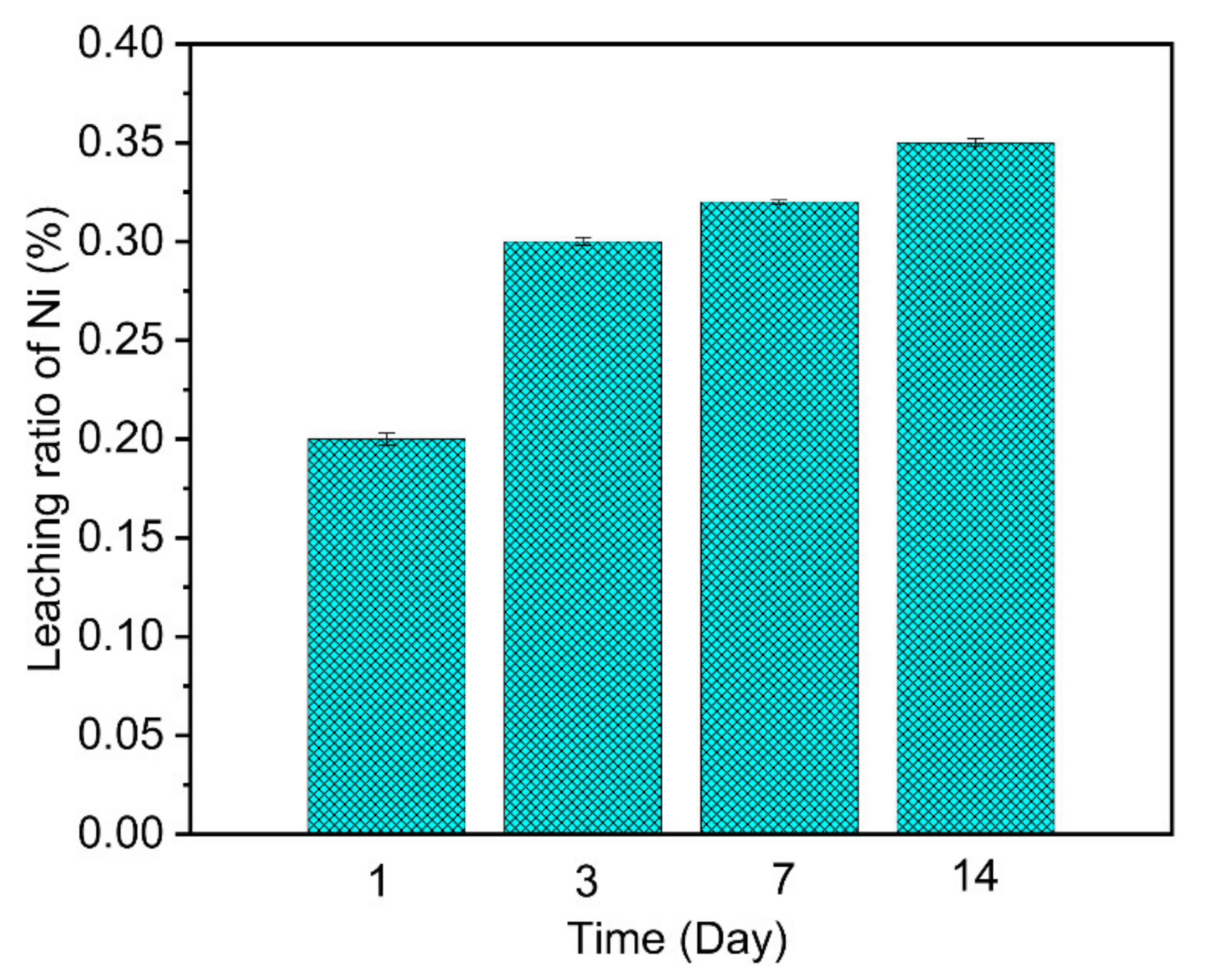
3.5. Ni Ions Leaching and the Stability of the Adsorbent Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Humood, A.N. Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: A review. Mar. Pollut. Bull. 2013, 72, 6–13. [Google Scholar]
- Liu, H.Q.; Zhao, D.P.; Dai, M.Z.; Zhu, X.F.; Qu, F.Y.; Umar, A.; Wu, X. PEDOT decorated CoNi2S4 nanosheets electrode as bifunctional electrocatalyst for enhanced electrocatalysis. Chem. Eng. J. 2021, 428, 131183. [Google Scholar] [CrossRef]
- Al-Attar, A.M. Vitamin E attenuates liver injury induced by exposure to lead, mercury, cadmium and copper in albino mice. Saudi. J. Biol. Sci. 2011, 18, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, T.; Yu, G.; Deng, S.B. Removal of low concentrations of nickel ions in electroplating wastewater using capacitive deionization technology. Chemosphere 2021, 248, 13141. [Google Scholar]
- Aggarwal, D.A.; Goyal, M.; Bansal, R.C. Adsorption of chromium by activated carbon from aqueous solution. Carbon 1999, 37, 1989–1997. [Google Scholar] [CrossRef]
- Carboni, M.C.; Abney, W.; Liu, S.; Lin, W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Wang, L.L.; Luo, F.; Dang, L.L.; Li, J.Q.; Wu, X.L.; Liu, S.J.; Luo, M.B. Ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide-and carboxyl-functionalized metal-organic framework. J. Mater. Chem. A 2015, 3, 13724–13730. [Google Scholar] [CrossRef]
- Yang, W.T.; Bai, Z.Q.; Shi, W.Q.; Yuan, L.Y.; Tian, T.Z.; Chai, F.; Wang, H.; Sun, Z. MOF-76: From a luminescent probe to highly efficient U(VI) sorption material. Chem. Commun. 2013, 49, 10415–10417. [Google Scholar] [CrossRef]
- Grabias, E.; Gladysz, P.A.; Ksiazek, A.; Majdan, M. Efficient uranium immobilization on red clay with phosphates. Environ. Chem. Lett. 2014, 12, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Misaelides, P.; Godelitsas, A.; Filippidis, A.; Charistos, D.; Anousis, I. Thorium and uranium uptake by natural zeolitic materials. Sci. Total Environ. 1995, 173, 237–246. [Google Scholar] [CrossRef]
- Attar, L.A.; Dyer, A. Sorption behaviour of uranium on birnessite, a layered manganese oxide. J. Mater. Chem. 2002, 12, 1381–1386. [Google Scholar] [CrossRef]
- Starvin, A.M.; Rao, T.P. Solid phase extractive preconcentration of uranium (VI) onto diarylazobisphenol modified activated carbon. Talanata 2004, 63, 225–232. [Google Scholar] [CrossRef]
- Ulusoy, H.I.; Simsek, S. Removal of uranyl ions in aquatic mediums by using a new material: Gallocyanine grafted hydrogel. J. Hazard. Mater. 2013, 254, 397–405. [Google Scholar] [CrossRef]
- Yue, Y.F.; Mayes, R.T.; Kim, J.P.; Fulvio, F.; Sun, X.G.; Tsouris, C.; Chen, J.H.; Brown, S.; Dai, S. Seawater uranium sorbents: Preparation from a mesoporous copolymer initiator by atom transfer radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 13458–13462. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Rong, T.L.; Du, X.Y.; Shen, Y.Y.; Shen, Y.Q. Preparation of Fe3O4 particles with unique structures from nickel slag for enhancing microwave absorption properties. Ceram. Int. 2021, 47, 18848–18857. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, R.K.; Kumar, S.; Kumar, P. Tuning in optical, magnetic and Curie temperature behaviour of nickel ferrite by substitution of monovalent K+1 ion of Ni0.8K0.2Fe2O4 nanomaterials for multifunctional applications. Phys. B 2021, 606, 412797. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S.; Barkat, M.J. The removal of uranium (VI) from aqueous solutions onto activated carbon: Kinetic and thermodynamic investigations. Colloid Interface. Sci. 2006, 296, 434–441. [Google Scholar] [CrossRef]
- Murphy, B.; Murphy, C.; Hathway, B.J. The Lewis structures of molecules, cations and anions, including oxyanions. Saudi J. Biol. Sci. 2001, 18, 395–401. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Thakkar, R.; Chudasama, U. Synthesis and characterization of zirconium titanium phosphate and its application in separation of metal ions. J. Hazard. Mater. 2009, 172, 129–137. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Khan, A.A.; Paquiza, L. Electrical behavior of conducting polymer based ‘polymeric–inorganic’ nanocomposite: Polyaniline and polypyrrole zirconium titanium phosphate. Synthetic Met. 2011, 161, 899–905. [Google Scholar] [CrossRef]
- Pan, B.C.; Zhang, Q.R.; Du, W.; Zhang, W.M.; Pan, B.J.; Zhang, Q.J.; Xu, Z.W.; Zhang, Q.X. Selective heavy metals removal from waters by amorphous zirconium phosphate: Behavior and mechanism. Water. Res. 2007, 41, 3103–3111. [Google Scholar] [CrossRef]
- Poojary, D.; Shpeizer, M.B.; Clearfield, A.J. Chem, Synthesis and x-ray powder structures of two lamellar copper arylenebis (phosphonates). Soc. Dalton. Trans. 1996, 35, 4942–4949. [Google Scholar]
- Bruque, S.; Aranda, M.A.G.; Losilla, E.R.; Olivera, P.P.; Maireles, T.P. Synthesis Optimization and Crystal Structures of Layered Metal (IV) Hydrogen Phosphates alpha-M (HPO4)2. cntdot. H2O (M = Ti, Sn, Pb). Inorg. Chem. 1995, 34, 893–899. [Google Scholar] [CrossRef]
- Ekambaram, S.; Sevov, S.C. Organically Templated Mixed-Valent TiIII/TiIV Phosphate with an Octahedral-Tetrahedral Open Framework. Angew. Chem. Int. Ed. 1999, 38, 372–380. [Google Scholar] [CrossRef]
- Yu, J.H.; Xu, R.R. Insight into the construction of open-framework aluminophosphates. Soc. Rev. 2006, 35, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Burnell, V.A.; Readman, J.E.; Tang, C.C.; Parker, J.E.; Thompson, S.P.; Hriljac, J.A. Synthesis and structural characterisation using Rietveld and pair distribution function analysis of layered mixed titanium–zirconium phosphates. J. Solid State Chem. 2010, 183, 2196–2204. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, J.W.; Wang, Q.M. Structural-property correlations of all-inorganic CsPbBr3 perovskites via synergetic controls by PbBr2, 2-mercapto-3-methyl-4-thiazoleacetic acid and water. Chem. Eng. J. 2021, 428, 131117. [Google Scholar] [CrossRef]
- Zhang, J.N.; Ma, Z.; Jiao, J.; Yin, H.F.; Yan, W.F.; Hagaman, E.W.; Yu, J.H.; Dai, S. Layer-by-Layer Grafting of Titanium Phosphate onto Mesoporous Silica SBA-15 Surfaces: Synthesis, Characterization, and Applications. Langmuir 2009, 25, 12541–12549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Li, C.M.; Zhang, Y.S.; Wang, X.P.; Yin, X.B.; Luo, N.N.; Fshin, K.A.; Wei, Y.Z. The synthesis and characterization of hydrous cerium oxide nanoparticles loaded on porous silica micro-sphere as novel and efficient adsorbents to remove phosphate radicals from water. Micropor. Mesopor. Mat. 2019, 279, 73–81. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.T.; Muhammad, Y.; Tong, Z.F.; Cui, X.M. Synthesis of highly efficient porous inorganic polymer microsphere for adsorptive removal of Pb2+ from wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zerovalent iron encapsulated chitosan nanospheres-a novel adsorbent for the removal of total inorganic arsenic from aqueous systems. Chemosphere 2012, 86, 150–155. [Google Scholar] [CrossRef]
- Li, C.M.; Wei, Y.Z.; Wang, X.P.; Yin, X.B. Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg-Al layered double hydroxide. J. Taiwan Inst. Chem. Eng. 2018, 85, 193–200. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Du, Q.; Jiao, T.F.; Pan, B.C.; Zhang, Z.X.; Sun, Q.N.; Wang, S.; Wang, F.T.; Gao, F.M. Selective removal of phosphate in waters using a novel of cation adsorbent: Zirconium phosphate (ZrP) behavior and mechanism. Chem. Eng. J. 2013, 221, 315–321. [Google Scholar] [CrossRef]
- Thakkar, R.; Chudasama, U. Synthesis, characterization and proton transport properties of a mixed material-Zirconium titanium phosphate, a tetravalent bimetallic acid salt. Electrochim. Acta 2009, 54, 2720–2726. [Google Scholar] [CrossRef]
- Liu, C.C.; Chen, L.F.; Ye, Z.X.; Li, C.M.; Yin, X.B.; Wang, X.P.; Wei, Y.Z. Pellet silica-based titanate adsorbents with high selectivity for strontium removal from synthetic radioactive solutions. J. Sol-Gel Sci. Technol. 2019, 91, 273–285. [Google Scholar] [CrossRef]
- Soner, A.H.; Sema, A.; Fikret, T. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K. Chrome waste as sorbent for the removal of arsenic (V) from aqueous solution. Environ. Technol. 1995, 16, 65–71. [Google Scholar] [CrossRef]
- Chinnaiya, N.; Sadasivam, S. Removal of arsenic (V) from aqueous solution using industrial sold waste: Adsorption rates and equilibrum studies. Ind. Eng. Chem. Res. 1998, 37, 4816–4822. [Google Scholar]
- Jossens, L.; Prausnitz, J.M.; Fritz, W. Thermodynamics of multi-solute adsorption from dilute aqueous solutions. Chem. Eng. Sci. 1978, 33, 1097–1106. [Google Scholar] [CrossRef]
- Delhia, A.; Clarence, C.; Marc, H.; Bénédicte, P.; Jerzy, Z. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar]
- Ikarashi, Y.; Mimura, H.; Nakai, T.; Niibori, Y.; Ishizaki, E.; Matsukura, M. Selective cesium uptake behavior of insoluble ferrocyanide loaded zeolites and development of stable solidifcation method. J. Ion Exch. 2014, 25, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Li, C.M.; Liu, C.C.; Chen, L.F.; Ye, Z.X.; Zhang, Y.S.; Wang, X.P.; Wei, Y.Z. Studies on the separation and in-situ sintering solidifcation of strontium by a highly-efcient titanate-based adsorbent. Micropor. Mesopor. Mater. 2019, 288, 109607. [Google Scholar] [CrossRef]
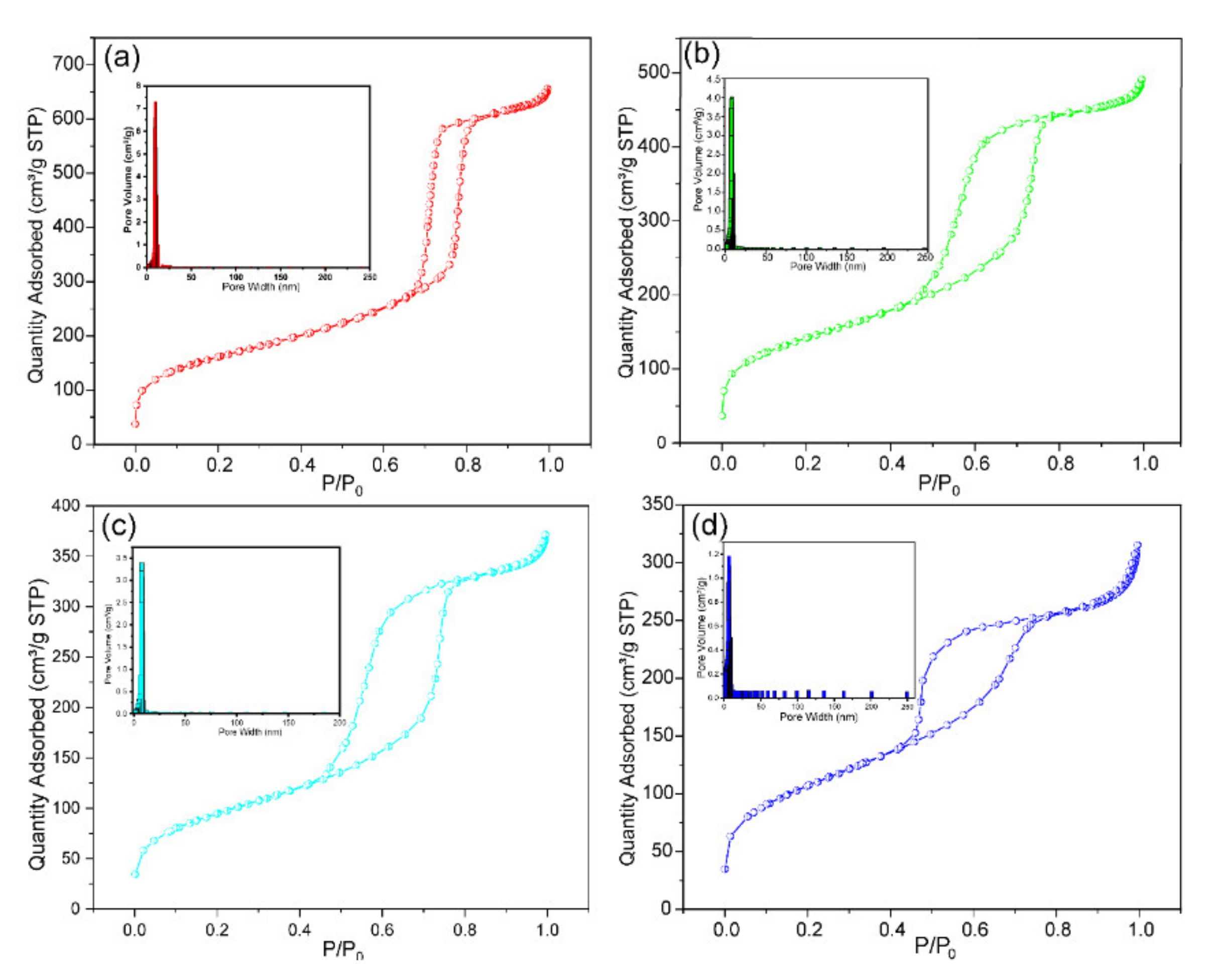



| Sample | Si Substrate | (Er.) | Si-Ti | (Er.) | Si-Ti-P | (Er.) | Si-Ti-P-Zr | (Er.) |
|---|---|---|---|---|---|---|---|---|
| BET Specific Surface Area (m2/g) | 566.991 | 0.011 | 502.598 | 0.401 | 382.295 | 0.295 | 337.881 | 0.119 |
| Pore Volume (cm3/g) | 0.903 | 0.003 | 0.679 | 0.021 | 0.434 | 0.003 | 0.506 | 0.004 |
| Average Pore Width (Å) | 70.787 | 0.213 | 59.702 | 0.298 | 49.650 | 0.350 | 66.997 | 0.287 |
| Isotherm Model | Estimated Isotherm Parameters | |||
|---|---|---|---|---|
| Langmuir | KL 2.7 | qm (mg/g) 48.4 | R2 0.82 | |
| Freundlich | KF 33.3 | n 10.3 | R2 0.91 | |
| Redlich-Peterson | A 327.3 | B 8.8 | g 0.93 | R2 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhao, J.; Zhang, Y. Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method. Nanomaterials 2021, 11, 2314. https://doi.org/10.3390/nano11092314
Li C, Zhao J, Zhang Y. Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method. Nanomaterials. 2021; 11(9):2314. https://doi.org/10.3390/nano11092314
Chicago/Turabian StyleLi, Chunmin, Jinsheng Zhao, and Yusheng Zhang. 2021. "Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method" Nanomaterials 11, no. 9: 2314. https://doi.org/10.3390/nano11092314
APA StyleLi, C., Zhao, J., & Zhang, Y. (2021). Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method. Nanomaterials, 11(9), 2314. https://doi.org/10.3390/nano11092314






