Green Afterglow of Undoped SrAl2O4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Undoped SrAl2O4 Phosphors
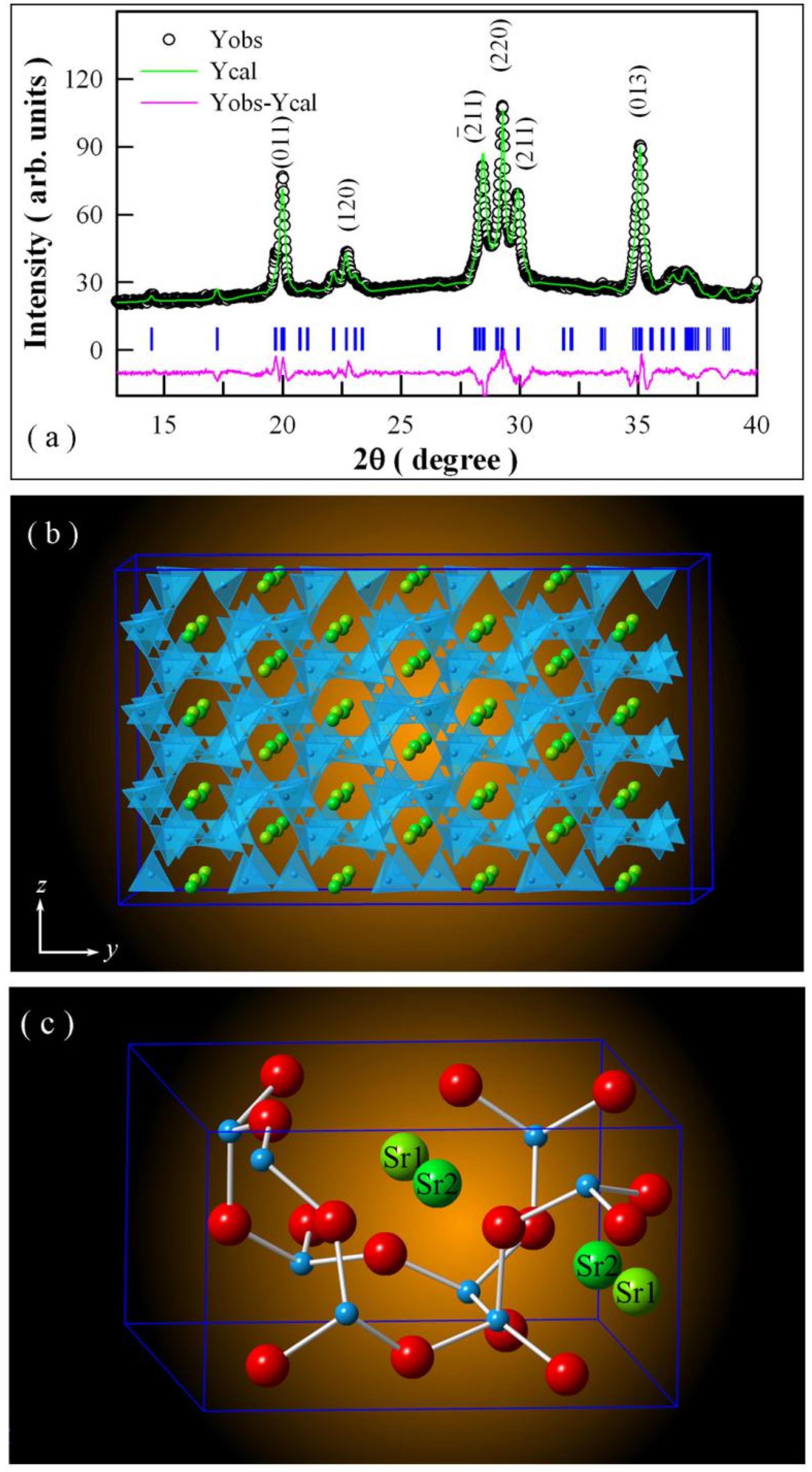
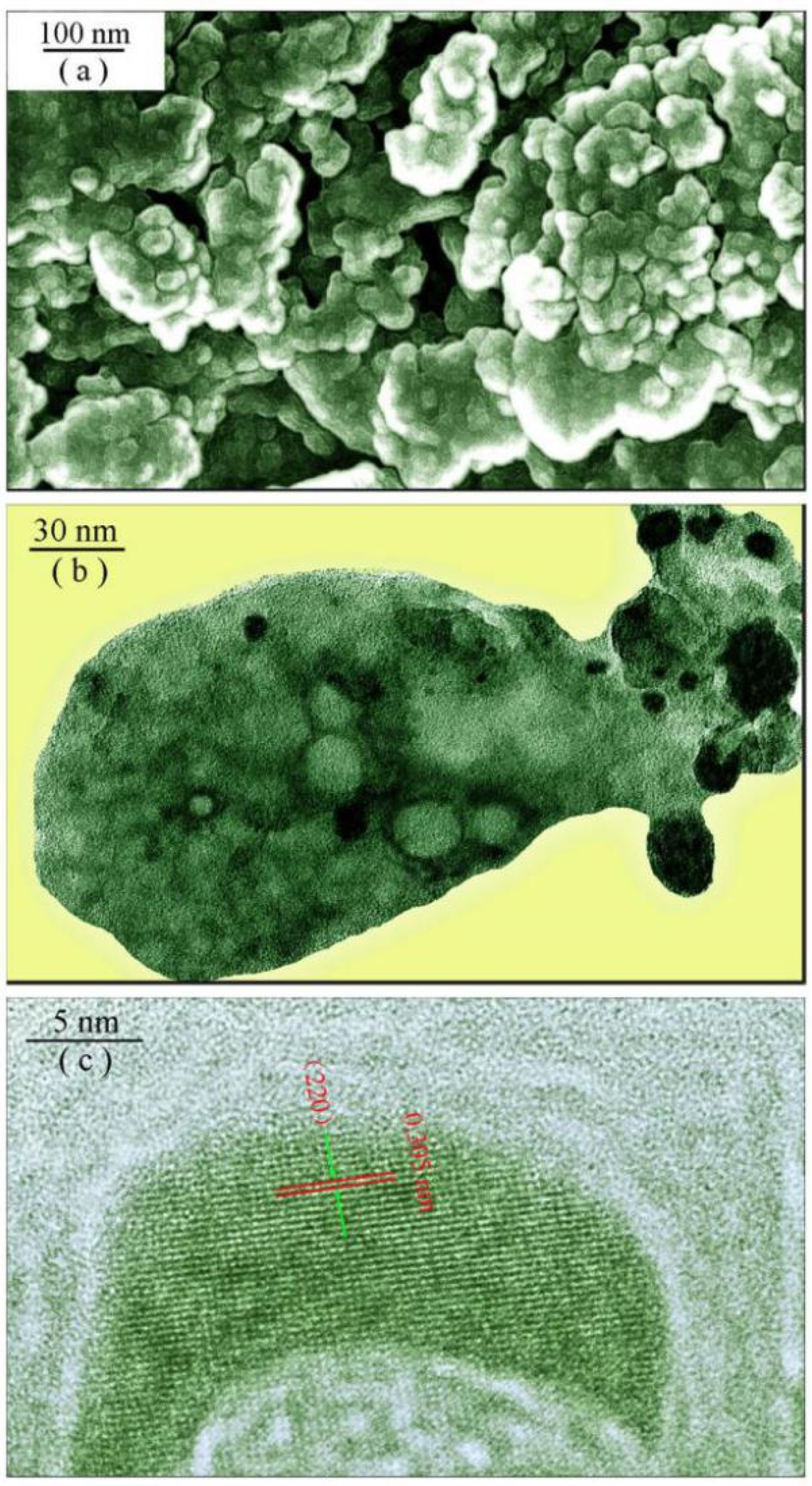
2.2. Phase and Morphology of Undoped SrAl2O4
2.3. Steady-State Photoluminescence (PL) and PL Decay Curves of Undoped SrAl2O4
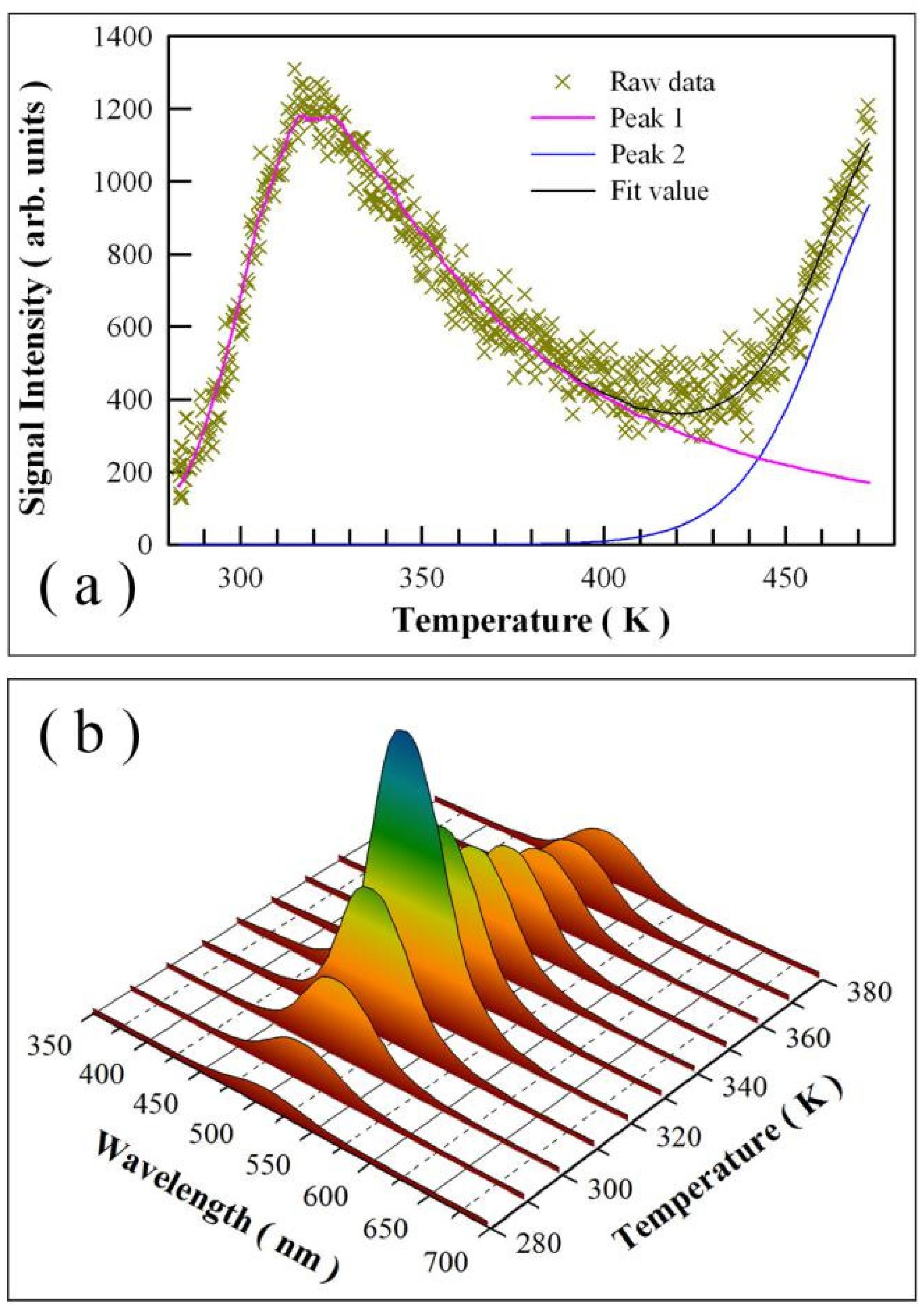
2.4. Afterglow Spectrum and Thermoluminescence (TL) Glow Curve of Undoped SrAl2O4
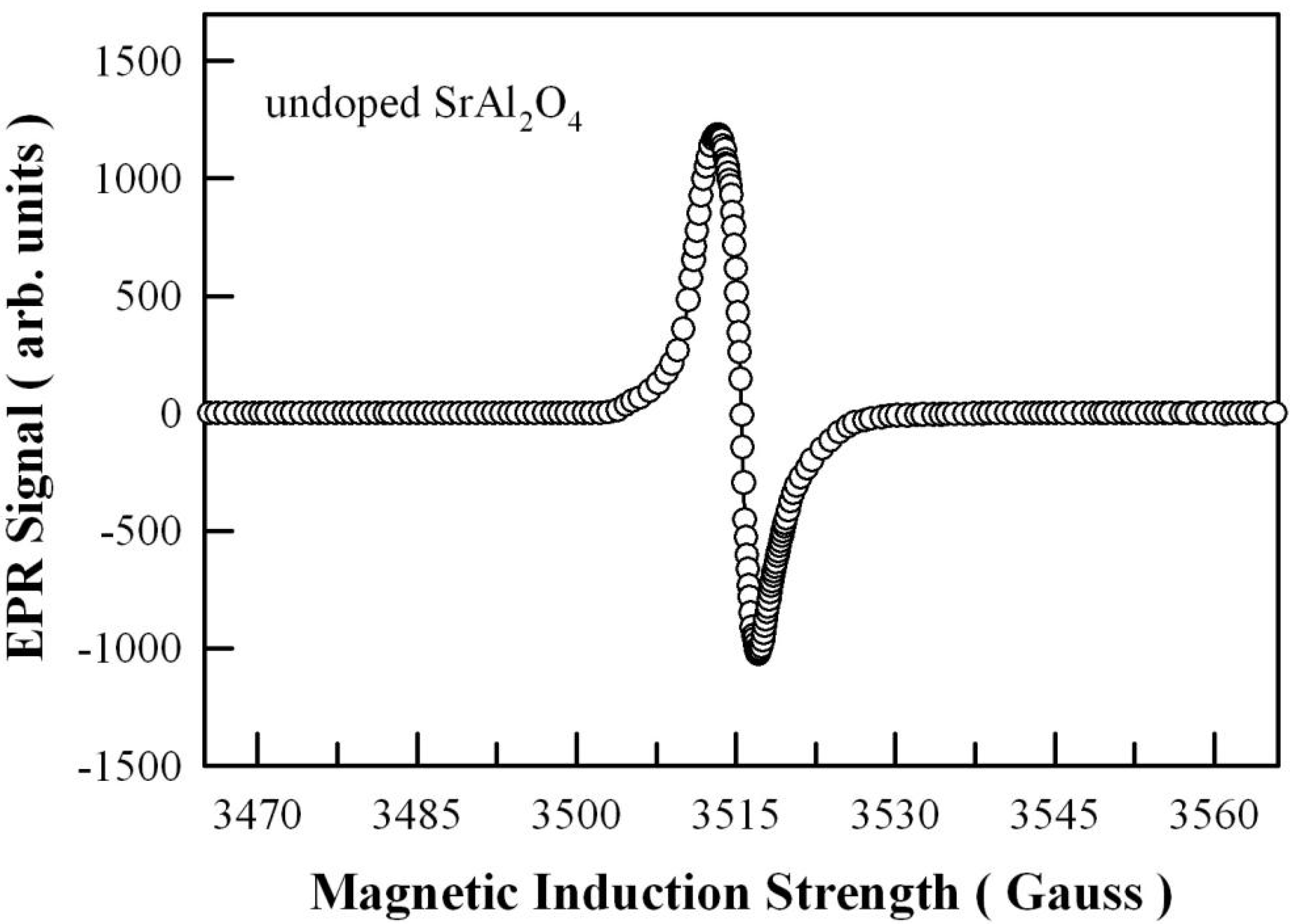
2.5. Electron Paramagnetic Resonance (EPR) Measurement of Undoped SrAl2O4
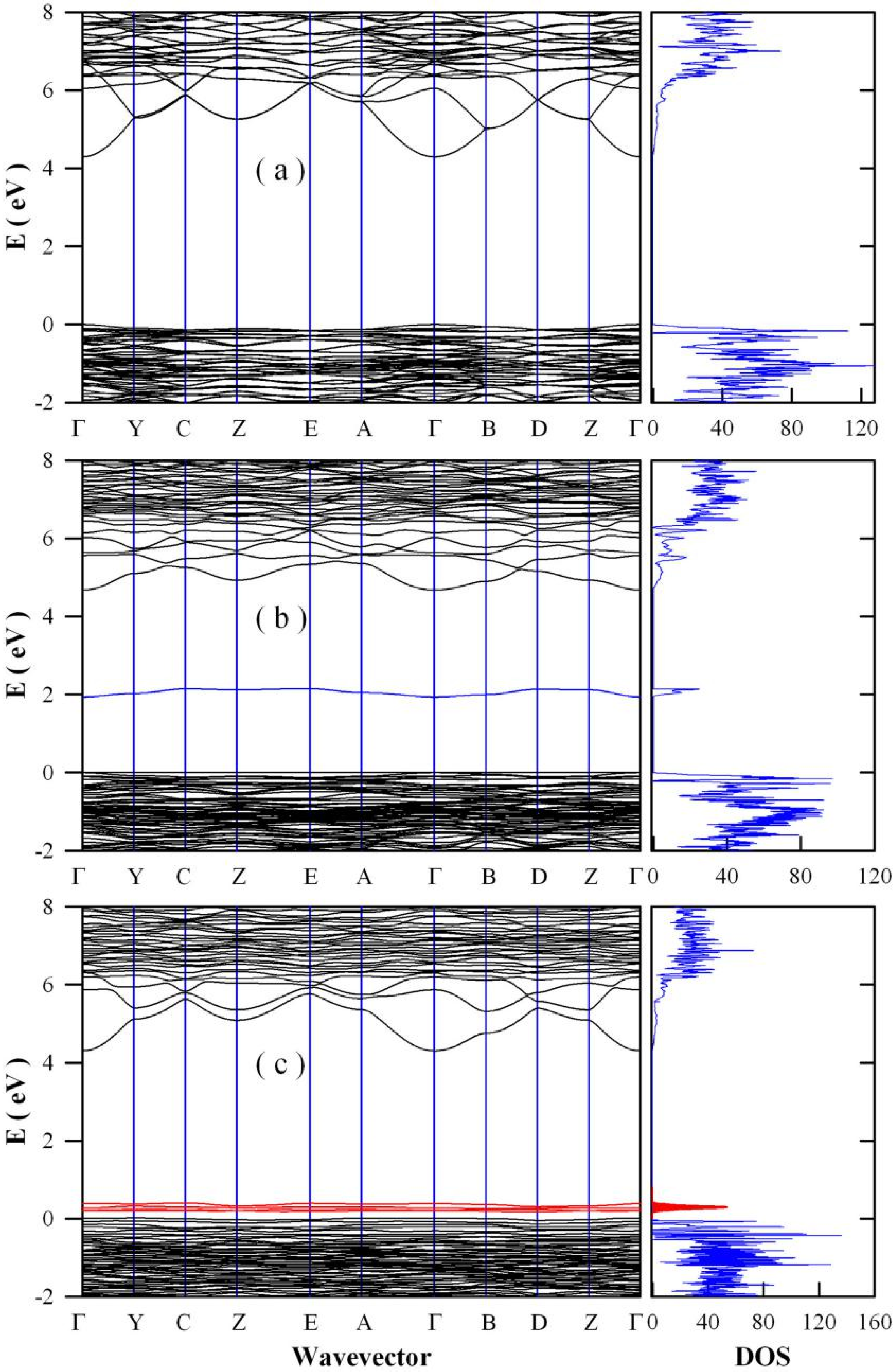
2.6. Band Structures and Densities of States of Defect-Rich SrAl2O4
3. Results and Discussion
3.1. XRD Pattern and Morphology of Undoped SrAl2O4
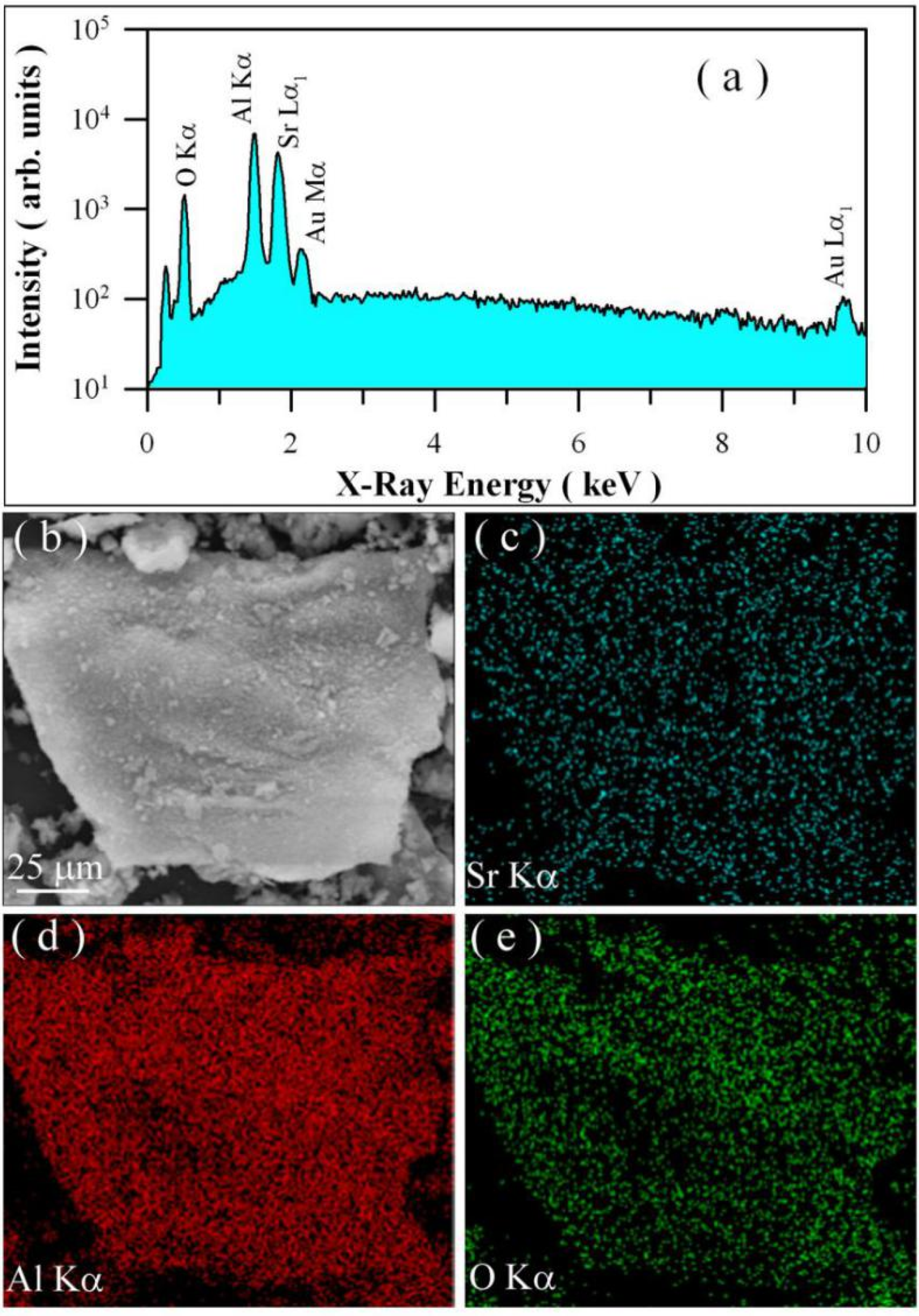
3.2. EDX Spectrum and Element Mapping of Undoped SrAl2O4
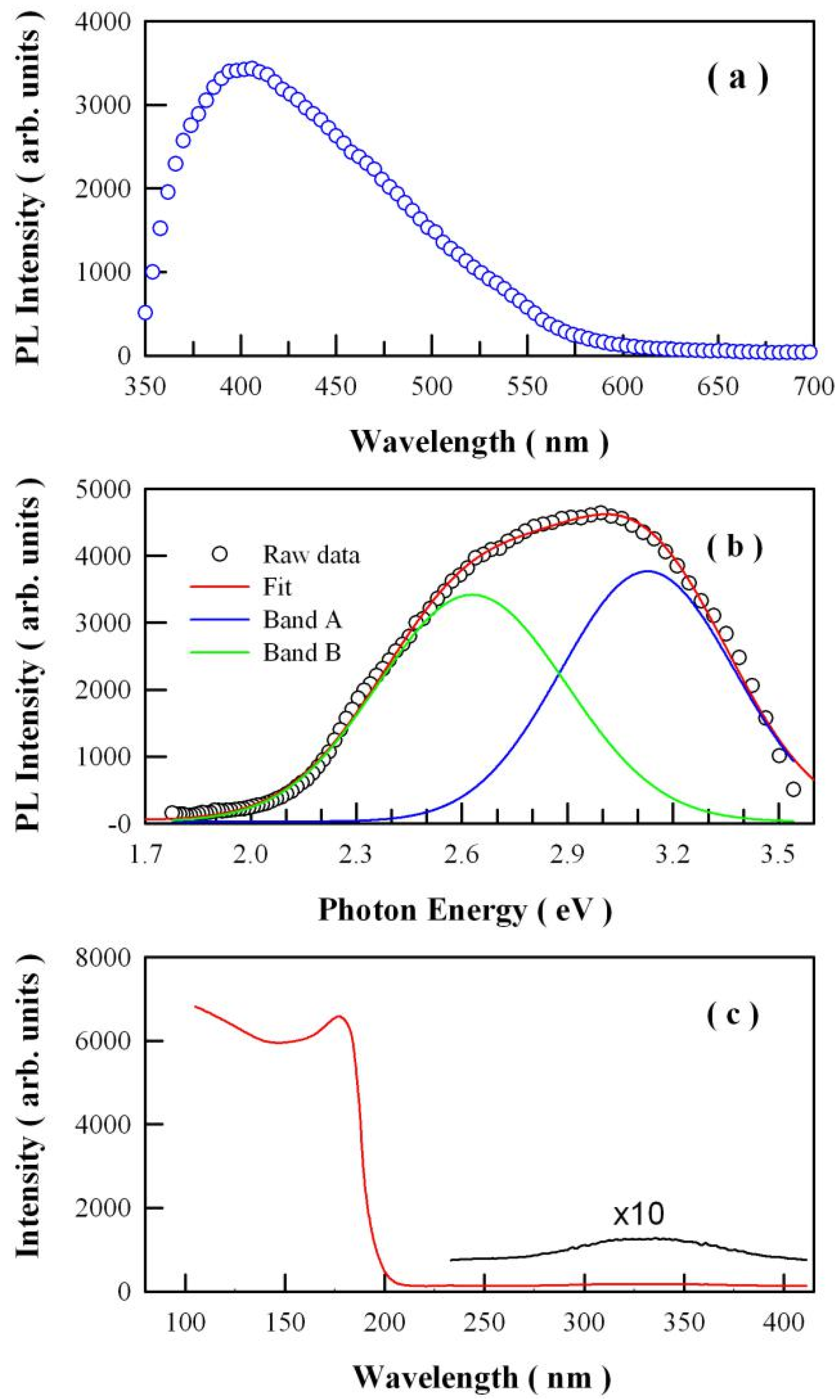
3.3. Steady-State PL Spectrum of Undoped SrAl2O4
3.4. Afterglow Spectrum and Afterglow Decay Profile of Undoped SrAl2O4
3.5. TL Glow Curve of Undoped SrAl2O4
3.6. Band Structures and Densities of States of Defect-Rich SrAl2O4
3.7. EPR Spectrum of Undoped SrAl2O4
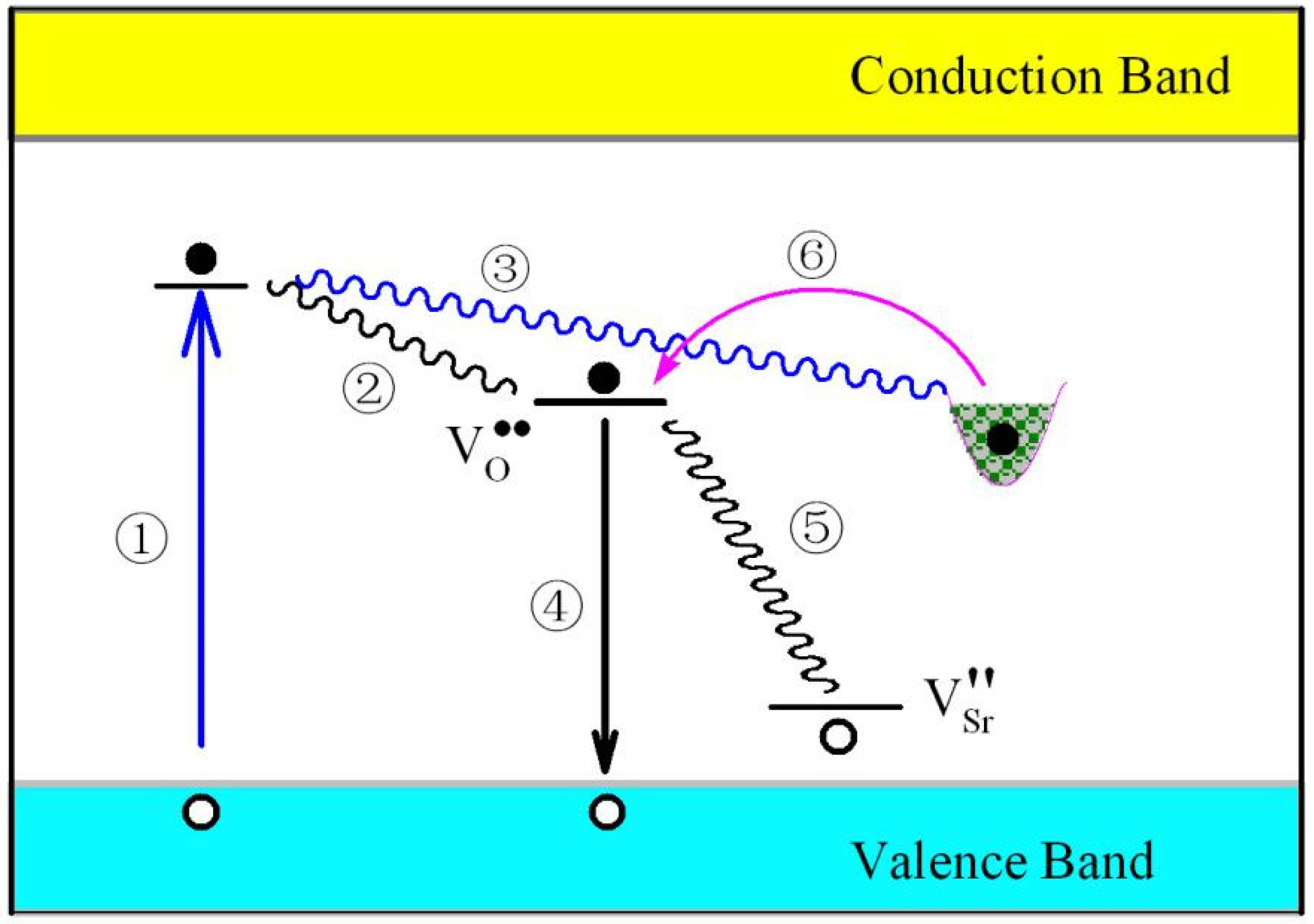
3.8. Intrinsic Defects Related PL and Afterglow Mechanisms for SrAl2O4
3.9. PL Decay Curves of Undoped SrAl2O4
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+,Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent luminescence in Eu2+-doped compounds: A review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M.; Ma, Q.L. Long afterglow of trivalent dysprosium doped strontium aluminate. J. Lumin. 2015, 160, 271–275. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhai, B.G.; Huang, Y.M. Effect of sol-gel combustion temperature on the luminescent properties of trivalent Dy doped SrAl2O4. Ceram. Int. 2015, 41, 5830–5835. [Google Scholar] [CrossRef]
- Vitola, V.; Millers, D.; Bite, I.; Smits, K.; Spustaka, A. Recent progress in understanding the persistent luminescence in SrAl2O4: Eu, Dy. Mater. Sci. Technol. 2019, 35, 1661–1677. [Google Scholar] [CrossRef]
- Wang, L.; Shang, Z.; Shi, M.; Cao, P.; Yang, B.; Zou, J. Preparing and testing the reliability of long–afterglow SrAl2O4: Eu2+, Dy3+ phosphor flexible films for temperature sensing. RSC Adv. 2020, 10, 11418. [Google Scholar] [CrossRef] [Green Version]
- Aitasalo, T.; Dereń, P.; Hölsä, J.; Jungner, H.; Krupa, J.C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J.; Stręk, W.J. Persistent luminescence phenomena in materials doped with rare earth ions. J. Solid State Chem. 2003, 171, 114. [Google Scholar] [CrossRef]
- Dorenbos, P. Mechanism of persistent luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J. Electrochem. Soc. 2005, 152, H107–H110. [Google Scholar] [CrossRef] [Green Version]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.H.; Garcia, A.; Le Mercier, T. Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+–doped SrAl2O4 with codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Ma, Q.L.; Liu, X.; Huang, Y.M. Mechanism of the prolongation of the green afterglow of SrAl2O4: Dy3+ caused by the use of H3BO3 flux. J. Lumin. 2017, 181, 78–87. [Google Scholar] [CrossRef]
- Zhai, B.G.; Huang, Y.M. Green photoluminescence and afterglow of Tb doped SrAl2O4. J. Mater. Sci. 2017, 52, 1813–1822. [Google Scholar] [CrossRef]
- Finley, E.; Tehrani, A.M.; Brgoch, J. Intrinsic defects drive persistent luminescence in monoclinic SrAl2O4:Eu2+. J. Phys. Chem. C 2018, 122, 16309–16314. [Google Scholar] [CrossRef]
- Pejakovic, D.A. Studies of the phosphorescence of polycrystalline hafnia. J. Lumin. 2010, 130, 1048–1054. [Google Scholar] [CrossRef]
- Behrh, G.K.; Isobe, M.; Massuyeau, F.; Serier-Brault, H.; Gordon, E.E.; Koo, H.-J.; Whangbo, M.-H.; Gautier, R.; Jobic, S. Oxygen-vacancy-induced midgap states responsible for the fluorescence and the long-lasting phosphorescence of the inverse spinel Mg (Mg, Sn) O4. Chem. Mater. 2017, 29, 1069–1075. [Google Scholar] [CrossRef]
- Zhai, B.G.; Huang, Y.M. Blue afterglow from undoped CaAl2O4. Europhys. Lett. 2019, 127, 17001. [Google Scholar] [CrossRef]
- Zhai, B.G.; Xu, H.; Zhuo, F.; Huang, Y.M. Annealing temperature dependent photoluminescence and afterglow of undoped CaAl2O4. J. Alloys Compd. 2020, 821, 153563. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, K.; Chen, N.; Xie, Z.; Lei, B.; Zhuang, J.; Zhang, X.; Liu, Y.; Hu, C. Room temperature long afterglow from boron oxide: A boric acid calcined product. Mater. Lett. 2020, 276, 128226. [Google Scholar] [CrossRef]
- Zhai, B.G.; Xu, H.; Zhang, Q.; Huang, Y.M. Blue photoluminescence and cyan-colored afterglow of undoped SrSO4 nanoplates. ACS Omega 2021, 6, 10129–10140. [Google Scholar] [CrossRef]
- Zhai, B.G.; Ma, Q.L.; Yang, L.; Huang, Y.M. Synthesis and optical properties of Tb-doped pentazinc dimolybdate pentahydrate. Results Phys. 2017, 7, 3991–4000. [Google Scholar] [CrossRef]
- Zhai, B.G.; Ma, Q.L.; Yang, L.; Huang, Y.M. Effects of sintering temperature on the morphology and photoluminescence of Eu3+ doped zinc molybdenum oxide hydrate. J. Nanomater. 2018, 2018, 7418508. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M.; Li, M.Y.; Yang, L.; Zhai, B.G. Eu2+ and Eu3+ doubly doped ZnWO4 nanoplates with superior photocatalytic performance for dye degradation. Nanomaterials 2018, 8, 765. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, N.; Hamada, T.; Takada, M.; Katsuki, M.; Nakagawa, M. Photoluminescence and thermoluminescence of MgSO4, CaSO4, SrSO4 and BaSO4 powder phosphors activated with Tb3+. Jpn. J. Appl. Phys. 2001, 40, 6732–6736. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.L.; Zhai, B.G.; Huang, Y.M. Rendering visible-light photocatalytic activity to undoped ZnO via intrinsic defects engineering. Catalysts 2020, 10, 1163. [Google Scholar] [CrossRef]
- Zhai, B.G.; Liu, D.; He, Y.; Yang, L.; Huang, Y.M. Tuning the photoluminescence of Eu2+ and Eu3+ co-doped SrSO4 through post annealing technique. J. Lumin. 2018, 194, 485–493. [Google Scholar] [CrossRef]
- Botterman, J.; Joos, J.J.; Smet, P.F. Trapping and detrapping in SrAl2O4: Eu, Dy persistent phosphors: Influence of excitation wavelength and temperature. Phys. Rev. B 2014, 90, 085147. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.; Bartwal, K.S. Defect structure and its relevance to photoluminescence in SrAl2O4: Eu2+, Nd3+. Phys. B Condens. Matter 2009, 404, 1714–1718. [Google Scholar] [CrossRef]
- Zhai, B.G.; Xu, H.; Huang, Y.M. Annealing temperature dependent afterglow of Tb3+ doped CaAl2O4. Opt. Mater. 2021, 112, 110739. [Google Scholar] [CrossRef]
- Zhai, B.G.; Ma, Q.L.; Huang, Y.M. Instability of the characteristic emissions of dopant Tb in ZnO hexagonal pyramids. J. Electron. Mater. 2017, 46, 947–957. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Zhou, F.F.; Shi, J.S.; Huang, Y.M. Strong photo-oxidative capability of ZnWO4 nanoplates with highly exposed {0–11} facets. Catalysts 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Mooney, J.; Kambhampati, P. Get the basics right: Jacobian conversion of wavelength and energy scales for quantitative analysis of emission spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Palilla, F.C.; Levine, A.K.; Tomkus, M.R. Fluorescent properties of alkaline earth aluminates of the type MAl2O4 activated by divalent europium. J. Electrochem. Soc. 1968, 115, 642. [Google Scholar] [CrossRef]
- Kamada, M.; Murakami, J.; Ohno, N. Excitation spectra of a long-persistent phosphor SrAl2O4: Eu, Dy in vacuum ultraviolet region. J. Lumin. 2000, 87–89, 1042–1044. [Google Scholar] [CrossRef]
- Duan, X.; Yi, L.; Zhang, X.; Huang, S. Size–dependent optical properties of nanoscale and bulk long persistent phosphor SrAl2O4: Eu2+, Dy3+. J. Nanomater. 2015, 2015, 298692. [Google Scholar] [CrossRef] [Green Version]
- Hassinen, J.; Hölsä, J.; Niittykoski, J.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Novák, P. UV–VUV spectroscopy of rare earth doped persistent luminescence materials. Opt. Mater. 2009, 31, 1751–1754. [Google Scholar] [CrossRef]
- Zhai, B.G.; Ma, Q.L.; Xiong, R.; Li, X.Z.; Huang, Y.M. Blue-green afterglow of BaAl2O4: Dy3+ phosphors. Mater. Res. Bull. 2016, 75, 1–6. [Google Scholar] [CrossRef]
- Zhai, B.G.; Yang, L.; Huang, Y.M. Intrinsic defect engineering in Eu3+ doped ZnWO4 for annealing temperature tunable photoluminescence. Nanomaterials 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Zhai, B.G.; Yang, L.; Ma, Q.L.; Huang, Y.M. Growth of ZnMoO4 nanowires via vapor deposition in air. Mater. Lett. 2017, 188, 119–122. [Google Scholar] [CrossRef]
- Nazarov, M.; Mammadova, S.; Spassky, D.; Vielhauer, S.; Abdullayeva, S.; Huseynov, A.; Jabbarov, R. SrAl2O4: Eu2+ (1%) luminescence under UV, VUV and electron beam excitation. Opt. Mater. 2018, 75, 448–452. [Google Scholar] [CrossRef]
- González, R.; Monge, M.A.; Muñoz Santiuste, J.E.; Pareja, R.; Chen, Y.; Kotomin, E.; Kukla, M.M.; Popov, A.I. Photoconversion of F-type centers in thermochemically reduced MgO single crystals. Phys. Rev. B 1999, 59, 4786–4790. [Google Scholar] [CrossRef] [Green Version]
- Kotomin, E.A.; Popov, A.I.; Stashans, A. A novel model for F+ to F photoconversion in corundum crystals. J. Phys. Condens. Matter. 1994, 6, L569–L573. [Google Scholar] [CrossRef]
- Monge, M.A.; González, R.; Muñoz Santiuste, J.E.; Pareja, R.; Chen, Y.; Kotomin, E.A.; Popov, A.I. Photoconversion and dynamic hole recycling process in anion vacancies in neutron-irradiated MgO crystals. Phys. Rev. B 1999, 60, 3787–3791. [Google Scholar] [CrossRef]
- Ma, Q.L.; Xiong, R.; Huang, Y.M. Tunable photoluminescence of porous silicon by liquid crystal infiltration. J. Lumin. 2011, 131, 2053–2057. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhai, B.G.; Huang, Y.M. Sol–gel derived ZnO/porous silicon composites for tunable photoluminescence. J. Sol–Gel Sci. Technol. 2012, 64, 110–116. [Google Scholar] [CrossRef]
- Chung, K.S.; Lee, J.I.; Kim, J.L. A computer program for the deconvolution of the thermoluminescence glow curves by employing the interactive trap model. Radiat. Meas. 2012, 47, 766–769. [Google Scholar] [CrossRef]
- Liu, B.; Gu, M.; Liu, X.; Huang, S.; Ni, C. Theoretical study of structural, electronic, lattice dynamical and dielectric properties of SrAl2O4. J. Alloys Compd. 2011, 509, 4300–4303. [Google Scholar] [CrossRef]
- Nazarov, M.; Brik, M.G.; Spassky, D.; Tsukerblat, B.; Nor Nazida, A.; Ahmad-Fauzi, M.N. Structural and electronic properties of SrAl2O4: Eu2+ from density functional theory calculations. J. Alloys Compd. 2013, 573, 6–10. [Google Scholar] [CrossRef]
- Hölsä, J.; Aitasalo, T.; Jungner, H.; Lastusaari, M.; Niittykoski, J.; Spano, G. Role of defect states in persistent luminescence materials. J. Alloys Compd. 2004, 374, 56–59. [Google Scholar] [CrossRef]

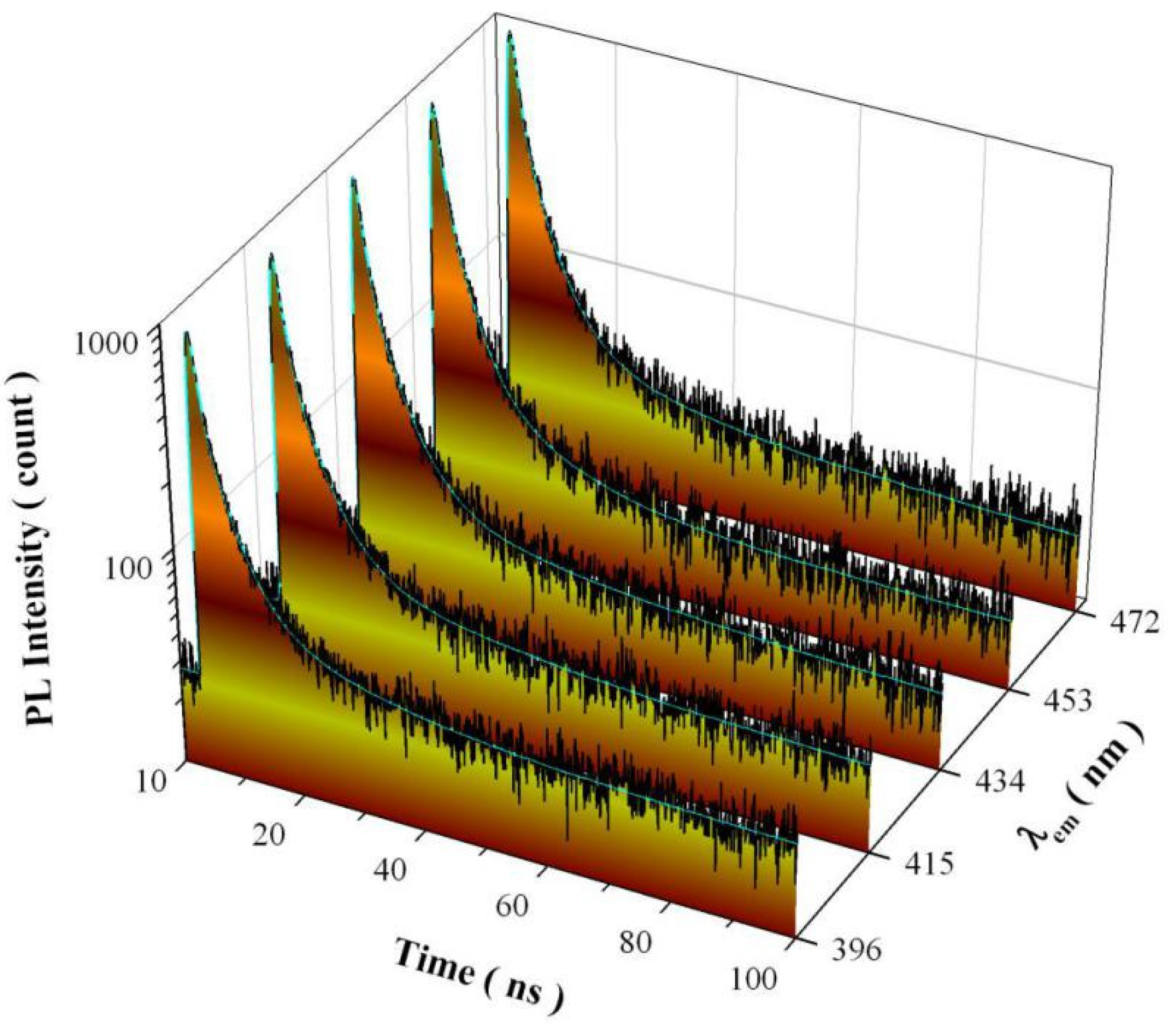
| λem (nm) | A0 | I1 | τ1 (ns) | I2 | τ2 (ns) | I3 | τ3 (ns) | τavg (ns) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 396 | 27.825 | 0.130 | 0.9140 | 0.062 | 3.7050 | 0.006 | 18.3353 | 6.49 | 0.982 |
| 415 | 25.736 | 0.125 | 1.0759 | 0.054 | 4.2216 | 0.003 | 22.6141 | 6.14 | 0.969 |
| 434 | 22.908 | 0.135 | 1.0909 | 0.056 | 4.3042 | 0.002 | 23.3102 | 5.25 | 1.026 |
| 453 | 21.369 | 0.109 | 0.7717 | 0.076 | 3.0503 | 0.008 | 11.9451 | 4.65 | 0.939 |
| 472 | 22.942 | 0.120 | 1.0084 | 0.062 | 3.9454 | 0.004 | 19.6329 | 5.92 | 1.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, B.-G.; Huang, Y.-M. Green Afterglow of Undoped SrAl2O4. Nanomaterials 2021, 11, 2331. https://doi.org/10.3390/nano11092331
Zhai B-G, Huang Y-M. Green Afterglow of Undoped SrAl2O4. Nanomaterials. 2021; 11(9):2331. https://doi.org/10.3390/nano11092331
Chicago/Turabian StyleZhai, Bao-Gai, and Yuan-Ming Huang. 2021. "Green Afterglow of Undoped SrAl2O4" Nanomaterials 11, no. 9: 2331. https://doi.org/10.3390/nano11092331





