Progress in Preparation of ZrB2 Nanopowders Based on Traditional Solid-State Synthesis
Abstract
:1. Introduction
2. Solid-State Synthesis
3. Modified Self-Propagating High-Temperature Synthesis
4. Solution-Derived Carbothermal Synthesis
5. Plasma-Enhanced Exothermic Reaction
6. Perspective Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nayebi, B.; Parvin, N.; Mohandesi, J.A.; Asl, M.S. Densification and toughening mechanisms in spark plasma sintered ZrB2-based composites with zirconium and graphite additives. Ceram. Int. 2020, 46, 13685–13694. [Google Scholar] [CrossRef]
- Medve, D.; Balko, J.; Sedlák, R.; Kovalčíková, A.; Shepa, I.; Naughton-Duszová, A. Wear resistance of ZrB2 based ceramic composites. Int. J. Refract. Met. Hard Mater. 2019, 81, 214–224. [Google Scholar] [CrossRef]
- Monteverde, F.; Grohsmeyer, R.J.; Stanfield, A.D.; Hilmas, G.E.; Fahrenholtz, W.G. Densification behavior of ZrB2-MoSi2 ceramics: The formation and evolution of coreshell solid solution structures. J. Alloys Compd. 2019, 779, 950–961. [Google Scholar] [CrossRef]
- Nekahi, S.; Vaferi, K.; Vajdi, M.; Moghanlou, F.S.; Asl, M.S.; Shokouhimehr, M. A numerical approach to the heat transfer and thermal stress in a gas turbine stator blade made of HfB2. Ceram. Int. 2019, 45, 24060–24069. [Google Scholar] [CrossRef]
- Shi, A.H.; Yang, X.; Fang, C.Q.; Chen, L.; Weng, Y.Q.; Liu, H.Z.; Huang, Q.Z. Effect of CNTs addition on microstructure, ablation property and mechanism of ZrC-SiC coating for C/C-ZrC-SiC composites. Vacuum 2020, 172, 109099. [Google Scholar] [CrossRef]
- Zavjalov, A.P.; Nikiforov, P.A.; Kosyanov, D.Y.; Zakharenko, A.M.; Trukhin, V.O.; Talskikh, K.Y.; Shichalin, O.O.; Papynov, E.K. Phase Formation and Densification Peculiarities of Hf–C–N Solid Solution Ceramics during Reactive Spark Plasma Sintering. Adv. Eng. Mater. 2020, 22. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Simonenko, E.P.; Zavjalov, A.P.; Belov, A.A.; Portnyagin, A.S.; Gerasimenko, A.V.; Drankov, A.N. Spark Plasma Sintering-Reactive Synthesis of SiC and SiC–HfB2 Ceramics Based on Natural Renewable Raw Materials. Russ. J. Inorg. Chem. 2021, 66, 629–637. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, X.-H.; Han, J.-C.; Luo, X.-G.; Du, S.-Y. Effect of Various Additives on the Oxidation Behavior of ZrB2-Based Ultra-High-Temperature Ceramics at 1800 °C. J. Am. Ceram. Soc. 2010, 93, 345–349. [Google Scholar] [CrossRef]
- Orooji, Y.; Alizadeh, A.; Ghasali, E.; Derakhshandeh, M.R.; Alizadeh, M.M. Co-reinforcing of mullite-TiN-CNT composites with ZrB2 and TiB2 compounds. Ceram. Int. 2019, 45, 20844–20854. [Google Scholar] [CrossRef]
- Asl, M.S.; Ahmadi, Z.; Namini, A.S.; Babapoor, A.; Motallebzadeh, A. Spark plasma sintering of TiC-SiCw ceramics. Ceram. Int. 2019, 45, 19808–19821. [Google Scholar]
- Nekahi, S.; Vajdi, M.; Moghanlou, F.S.; Vaferi, K.; Motallebzadeh, A.; Ozen, M. TiB2-SiC-based ceramics as alternative efficient micro heat exchangers. Ceram. Int. 2019, 45, 19060–19067. [Google Scholar] [CrossRef]
- Asl, M.S.; Delbari, S.A.; Shayesteh, F.; Ahmadi, Z.; Motallebzadeh, A. Reactive spark plasma sintering of TiB2-SiC-TiN novel composite. Int. J. Refract. Met. Hard Mater. 2019, 81, 119–126. [Google Scholar]
- Delbari, S.A.; Nayebi, B.; Ghasali, E.; Shokouhimehr, M.; Asl, M.S. Spark plasma sintering of TiN ceramics codoped with SiC and CNT. Ceram. Int. 2018, 45, 3207–3216. [Google Scholar] [CrossRef]
- Mahaseni, Z.H.; Germi, M.D.; Ahmadi, Z.; Asl, M.S. Microstructural investigation of spark plasma sintered TiB2 ceramics with Si3N4 addition. Ceram. Int. 2018, 44, 13367–13372. [Google Scholar] [CrossRef]
- Zhang, X.H.; Hu, P.; Han, J.C.; Du, S.Y. Study on Thermal Shock Resistance and Oxidation Resistance of Ultra-High Temperature Ceramics. Mater. China 2011, 30, 27–31. [Google Scholar]
- Zhang, X.; Hu, P.; DU, S.; Han, J.; Meng, S. Research progress on ultra-high temperature ceramic composites. Chin. Sci. Bull. 2015, 60, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Kesava, B.; Reddy, J.J.; Srinivas, V.; Kumari, S.; Prasad, V.B. Microstructure, mechanical properties and oxidation behavior of short carbon fiber reinforced ZrB2-20v/oSiC-2v/oB4C composite. Mater. Sci. Eng. A 2018, 719, 206–226. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Gordeev, A.N.; Vasilevskii, S.A.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Avramenko, V.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Behavior of HfB2-SiC (10, 15, and 20 vol %) ceramic materials in high-enthalpy air flows. Russ. J. Inorg. Chem. 2016, 61, 1203–1218. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, N.; Gordeev, A.; Kolesnikov, A.F.; Papynov, E.; Shichalin, O.; Avramenko, V.A.; Kuznetsov, N. Behavior of a sample of the ceramic material HfB2–SiC (45 vol %) in the flow of dissociated air and the analysis of the emission spectrum of the boundary layer above its surface. Russ. J. Inorg. Chem. 2015, 60, 1360–1373. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, E.P.; Gordeev, A.N.; Simonenko, N.P.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Av-ramenko, V.A.; Kuznetsov, N.T. HfB2@SiC (45 vol%) Ceramic Material: Manufacture and Behavior under Long@Term Ex-posure to Dissociated Air Jet Flow. Russ. J. Inorg. Chem. 2014, 59, 1298–1311. [Google Scholar] [CrossRef]
- Vajdi, M.; Moghanlou, F.S.; Niari, E.R.; Asl, M.S.; Shokouhimehr, M. Heat transfer and pressure drop in a ZrB2 microchannel heat sink: A numerical approach. Ceram. Int. 2019, 46, 1730–1735. [Google Scholar] [CrossRef]
- Sakkaki, M.; Moghanlou, F.S.; Vajdi, M.; Pishgar, F.; Shokouhimehr, M.; Asl, M.S. The effect of thermal contact resistance on the temperature distribution in a WC made cutting tool. Ceram. Int. 2019, 45, 22196–22202. [Google Scholar] [CrossRef]
- Vajdi, M.; Moghanlou, F.S.; Ahmadi, Z.; Motallebzadeh, A.; Asl, M.S. Thermal diffusivity and microstructure of spark plasma sintered TiB2SiC Ti composite. Ceram. Int. 2019, 45, 8333–8344. [Google Scholar] [CrossRef]
- Balak, Z. Shrinkage, hardness and fracture toughness of ternary ZrB2-SiC-HfB2 composite with different amount of HfB2. Mater. Chem. Phys. 2019, 235, 121706. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Zhang, X.H.; Hu, P.; Han, W.B.; Hong, C.Q. Effect of graphite flake on microstructure as well as mechanical properties and thermal shock resistance of ZrB2–SiC matrix ultrahigh temperature ceramics. J. Alloys Compd. 2009, 484, 390–394. [Google Scholar] [CrossRef]
- Lu, S.C. Handbook of Powder Technology; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Guo, J.K. Research on advanced structural ceramics. J. Inorg. Mater. 1999, 14, 193–202. [Google Scholar]
- Guo, J.K. Research and Prospect of advanced ceramics in China. Chin. J. Mater. Res. 1997, 11, 594–600. [Google Scholar]
- Huang, Y.; Zhang, L.M.; Wang, C.A.; Yang, J.L.; Xie, Z.P. Review on research progress of advanced structural ceramics. J. Chin. Ceram. Soc. 2005, 5, 91–100. [Google Scholar]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Sevastyanov, V.G.; Kuznetsov, N.T. Behavior of HfB2–30 vol% SiC UHTC obtained by sol–gel approach in the supersonic airflow. J. Sol-Gel Sci. Technol. 2019, 92, 386–397. [Google Scholar] [CrossRef]
- Khanra, A.; Pathak, L.; Godkhindi, M. Double SHS of ZrB2 powder. J. Mater. Process. Technol. 2008, 202, 386–390. [Google Scholar] [CrossRef]
- Krishnarao, R.; Alam, Z.; Das, D.K.; Prasad, V.B. Synthesis of ZrB2–SiC composite powder in air furnace. Ceram. Int. 2014, 40, 15647–15653. [Google Scholar] [CrossRef]
- Setoudeh, N.; Welham, N. Formation of zirconium diboride (ZrB2) by room temperature mechanochemical reaction between ZrO2, B2O3 and Mg. J. Alloys Compd. 2006, 420, 225–228. [Google Scholar] [CrossRef]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Sonber, J.K.; Murthy, T.S.R.C.; Subramanian, C.; Kumar, S.; Fotedar, R.K.; Suri, A.K. Investigations on synthesis of ZrB2 and composites with HfB2, and TiSi2. Int. J. Refract. Met. Hard Mater. 2011, 29, 21–30. [Google Scholar] [CrossRef]
- Fahrenholtz, W.; Hilmas, G.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Mishra, S.; Das, S.; Pathak, L. Defect structures in zirconium diboride powder prepared by self-propagating high-temperature synthesis. Mater. Sci. Eng. A 2004, 364, 249–255. [Google Scholar] [CrossRef]
- Sacks, M.D.; Wang, C.-A.; Yang, Z.; Jain, A. Carbothermal reduction synthesis of nanocrystalline zirconium carbide and hafnium carbide powders using solution-derived precursors. J. Mater. Sci. 2004, 39, 6057–6066. [Google Scholar] [CrossRef]
- Nishiyama, K.; Nakamur, T.; Utsumi, S.; Sakai, H.; Abe, M. Preparation of ultrafine boride powders by metallothermic reduction method. J. Phys. Conf. Ser. 2009, 176, 012043. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Z.P.; Hou, Y.D. Reaction mechanism of 0.80Pb(Mg1/3Nb2/3)O3-0.20PbTiO3 ceramics prepared by semichemical method. J. Inorg. Mater. 2002, 17, 737–744. [Google Scholar]
- Aruna, S.T.; Rajam, K.S. Mixture of fuels approach for the solution combustion synthesis of Al2O3-ZrO2 nanoeomposite. Mater. Res. Bull. 2004, 39, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, K.; Mukasyan, A.; Varma, A. Aqueous Combustion Synthesis of Strontium-Doped Lanthanum Chromite Ceramics. J. Am. Ceram. Soc. 2003, 86, 1149–1154. [Google Scholar] [CrossRef]
- Kozhukharov, V.; Brashkova, N.; Machkova, M.; Carda, J.; Ivanova, M. Ultrasonic Spray Pyrolysis for Powder Synthesis. Solid State Phenom. 2003, 90, 553–558. [Google Scholar] [CrossRef]
- Nimmo, W.; Hind, D.; Ali, N.J.; Hampartsoumian, E.; Milne, S.J. The production of ultrafine zirconium oxide powders by spray pyrolysis. J. Mater. Sci. 2002, 37, 3381–3387. [Google Scholar] [CrossRef]
- Radev, D.D.; Klissurski, D. Mechanochemieal synthesis and SHS of diborides of titanium and zirconium. J. Mater. Synth. Proc. 2001, 9, 131–136. [Google Scholar] [CrossRef]
- Camurlu, H.E.; Filippo, M. Preparation of nano-size ZrB2 powder by self-propagating high-temperature synthesis. J. Eur. Ceram. Soc. 2009, 29, 1501. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, H.; Fu, Z.Y. Composition of ZrB2 ceramic powder via Zr-B system by self-propagating high-temperaturesy nthesis. J. Chin. Ceram. Soc. 2004, 32, 1016. [Google Scholar]
- Makarenko, G.N.; Krushinskaya, L.A.; Timofeeva, I.I.; Matsera, V.E.; Vasil’Kovskaya, M.A.; Uvarova, I.V. Formation of Diborides of Groups IV–VI Transition Metals During Mechanochemical Synthesis. Powder Met. Met. Ceram. 2015, 53, 514–521. [Google Scholar] [CrossRef]
- Radev, D.D. Properties of titanium and zirconium diborides obtained by conventional and nonconventional synthesis methods. Metall 1996, 9, 561–564. [Google Scholar]
- Jin, Y.X.; Zhang, E.L. Self Propagating Synthesis Technology and In Situ Composites; Harbin Institute of Technology Press: Harbin, China, 2002. [Google Scholar]
- Merzhanov, A.G. SHS on the pathway to industrialization. Int. J. SHS 2001, 10, 237–257. [Google Scholar]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2016, 62, 203–239. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion Synthesis of Advanced Materials: Principles and Applications. Korean J. Chem. Eng. 1998, 24, 79–226. [Google Scholar]
- Varma, A.; Lebrat, J.-P. Combustion synthesis of advanced materials. Chem. Eng. Sci. 1992, 47, 2179–2194. [Google Scholar] [CrossRef]
- Guo, W.M. Preparation and Properties of ZrB2 Based Ultra-High Temperature Ceramics; Fudan University: Shanghai, China, 2013. [Google Scholar]
- Zhang, P.L. Synthesis of TiB2 and ZrB2 Ceramics by Magnesium Thermite Reaction Self-Propagating High Temperature Synthesis and Its Macrokinetics; Lanzhou University of Technology: Lanzhou, China, 2008. [Google Scholar]
- Ye, D.L.; Hu, J.H. Practical Handbook of Inorganic Thermodynamic Data; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Campos, K.S.; Silva, G.F.B.L.; Nunes, E.H.M.; Vasconcelos, W.; Silva, G.F.B.L. Preparation of Zirconium, Titanium, and Magnesium Diborides by Metallothermic Reduction. Refract. Ind. Ceram. 2014, 54, 407–412. [Google Scholar] [CrossRef]
- Zhang, W. Effect of Raw Materials on Self-Propagating High Temperature Synthesis of ZrB2 Powder; Xi’an University of Architecture and Technology: Xi’an, China, 2017. [Google Scholar]
- Zhang, W.; Xiao, G.Q.; Ding, D.H. Effect of borax on self-propagating high temperature synthesis of ZrB2 powder. Chin. Mater. Rev. B 2017, 31, 125–128. [Google Scholar]
- Zhang, T.M. Preparation of High Purity Zirconium Diboride Powder by Self-Propagating Magnesium Thermal Reduction; Harbin Institute of Technology Press: Harbin, China, 2006. [Google Scholar]
- Khanra, A.K.; Pathak, L.C.; Mishra, S.K.; Godkhindi, M.M. Self-propagating-high-temperature synthesis (SHS) of ultrafine ZrB2 powder. J. Mater. Sci. Lett. 2003, 22, 1189–1191. [Google Scholar] [CrossRef]
- Olevsky, E.A.; Dudina, D.V. Field-Assisted Sintering Science and Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Bai, L.; Wang, Y.; Chen, H.; Yuan, F. Large-scale production of well-dispersed submicro ZrB2and ZrC powders. Cryst. Res. Technol. 2016, 51, 428–432. [Google Scholar] [CrossRef]
- Bai, L.Y.; Ni, S.L.; Jin, H.C.; He, J.P.; Ouyang, Y.G.; Yuan, F.L. ZrB2 powders with low oxygen content: Synthesis and characterization. Int. J. Appl. Ceram. Technol. 2018, 15, 508–513. [Google Scholar] [CrossRef]
- Lv, G.B. Preparation of zirconium boride powder by carbon reduction. Chin. J. Ceram. 2019, 2, 25–30. [Google Scholar]
- Li, M.; Ke, C.; Zhang, J. Synthesis of ZrB2 powders by molten-salt participating silicothermic reduction. J. Alloys Compd. 2020, 834, 155062. [Google Scholar] [CrossRef]
- Mukasyan, A.; Rogachev, A.S.; Aruna, S.T. Combustion synthesis in nanostructured reactive systems. Adv. Powder Technol. 2015, 26, 954–976. [Google Scholar] [CrossRef] [Green Version]
- Patil, K.C.; Hedge, M.S.; Rattan, T. Chemistry of Nano-Crystalline Oxide Materials: Combustion Synthesis, Properties and Applications; World Scientific: New Jersey, CA, USA, 2008. [Google Scholar]
- Yan, Y.J.; Huang, Z.G.; Dong, S.M.; Jiang, D.L. New route to synthesize ultra-fine zirconium diboride powders using inorganic-organic hybrid precursors. J. Am. Ceram. Soc. 2006, 89, 3585–3588. [Google Scholar] [CrossRef]
- Qiu, W.F.; Ye, L.; Han, W.J.; Zhao, T. Progress in synthesis of ultra-high temperature ceramic precursors. Mater. China 2015, 34, 751–761. [Google Scholar]
- Xu, C.H. Synthesis and Related Research of New Ceramic Precursors; University of Chinese Academy of Sciences: Beijing, China, 2001. [Google Scholar]
- Tsirlina, A.M.; Shcherbakova, G.I. Nano-Structured metal-containing polymer precursors for high temperature non-oxide ceramics and ceramic fibers-synthesis, pyrolysis and properties. J. Eur. Ceram. Soc. 2002, 22, 2577–2585. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.X.; Jiang, Y.B.; Zhao, B.; Li, J.P.; Feng, Z.H. Synthesis of nano ZrB2 powder by wet chemical method. Chin. J. Inorg. Chem. 2011, 27, 1788–1792. [Google Scholar]
- Dollé, M.; Gosset, D.; Bogicevic, C.; Karolak, F.; Simeone, D.; Baldinozzi, G. Synthesis of nanosized zirconium carbide by a sol–gel route. J. Eur. Ceram. Soc. 2007, 27, 2061–2067. [Google Scholar] [CrossRef]
- Li, R.X.; Zhang, Y.; Zhao, B.; Jiang, Y.B.; Li, J.P.; Feng, Z.H. Synthesis of nano ZrB2 powders by carbothermal reduction and sol-gel method. Mater. China 2012, 31, 59–63. [Google Scholar]
- Yang, B.Y.; Li, J.P.; Wang, T.Y.; Li, R.X.; Feng, Z.H.; Cai, H.N. Effect of gel aging on the preparation of ZrB2 powder. Chin. J. Inorg. Mater. 2014, 29, 717–721. [Google Scholar]
- Zhao, Y.; Li, J.P.; Wang, T.Y.; Ki, R.X.; Feng, Z.H.; Cai, H.N. Synthesis of nano ZrB2 powder using xylitol. J. Inorg. Mater. 2016, 31, 597–601. [Google Scholar]
- Yang, B.Y.; Li, J.P.; Zhao, B.; Hu, Y.Z.; Wang, T.Y.; Sun, D.F.; Li, R.X.; Yin, S.; Feng, Z.H.; Tang, Q.; et al. Synthesis of hexagonal-prism-like ZrB2 by a sol–gel route. Powder Technol. 2014, 256, 522–528. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Gao, B.; Wang, J.; Wang, Y.; Chen, S.; Gou, Y. Synthesis and characterization of a novel precursor-derived ZrC/ZrB2ultra-high-temperature ceramic composite. Appl. Organomet. Chem. 2012, 27, 79–84. [Google Scholar] [CrossRef]
- Yuan, F.L.; Jin, H.C.; Hou, G.L.; Bai, L.Y.; Ding, F.; Li, B.Q.; Chen, Y.F. Progress on preparation of special powders using HF thermal plasma. Chin. J. Process Eng. 2018, 18, 1139–1145. [Google Scholar]
- He, J.; Bai, L.; Jin, H.; Jia, Z.; Hou, G.; Yuan, F. Simulation and experimental observation of silicon particles’ vaporization in RF thermal plasma reactor for preparing Si nano-powder. Powder Technol. 2017, 313, 27–35. [Google Scholar] [CrossRef]
- Hou, G.; Cheng, B.; Yao, M.-S.; Cao, Y.; Ding, F.; Hu, P.; Ma, R.; Yuan, F. Well dispersed silicon nanospheres synthesized by RF thermal plasma treatment and their high thermal conductivity and dielectric constant in polymer nanocomposites. RSC Adv. 2015, 5, 9432–9440. [Google Scholar] [CrossRef]
- Hu, P.; Yuan, F.L.; Bai, L.Y. Plasma synthesis of large quantities of zinc oxide nanorods. J. Phys. Chem. C 2007, 111, 194–200. [Google Scholar]
- Zhang, H.; Yao, M.-S.; Bai, L.; Xiang, W.; Jin, H.; Li, J.; Yuan, F. Synthesis of uniform octahedral tungsten trioxide by RF induction thermal plasma and its application in gas sensing. CrystEngComm 2013, 15, 1432–1438. [Google Scholar] [CrossRef]
- Ouyang, Y.; Ding, F.; Bai, L.; Li, X.; Hou, G.; Fan, J.; Yuan, F. Design of network Al2O3 spheres for significantly enhanced thermal conductivity of polymer composites. Compos. Part A Appl. Sci. Manuf. 2019, 128, 105673. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Ding, F.; Bai, L.; Yuan, F. Simultaneously enhance thermal conductive property and mechanical properties of silicon rubber composites by introducing ultrafine Al2O3 nanospheres prepared via thermal plasma. Compos. Sci. Technol. 2020, 190, 108019. [Google Scholar] [CrossRef]
- He, J.; Bai, L.; Jin, H.; Yuan, F. Optimization of tungsten particles spheroidization with different size in thermal plasma reactor based on numerical simulation. Powder Technol. 2016, 302, 288–297. [Google Scholar] [CrossRef]
- Bai, L.; He, J.; Ouyang, Y.; Liu, W.; Liu, H.; Yao, H.; Li, Z.; Song, J.; Wang, Y.; Yuan, F. Modeling and Selection of RF Thermal Plasma Hot-Wall Torch for Large-Scale Production of Nanopowders. Materials 2019, 12, 2141. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.B. Preparation of Nano Microsphere Tungsten Powder by High Frequency Thermal Plasma; University of Chinese Academy of Sciences: Beijing, China, 2012. [Google Scholar]
- Bai, L.; Zhang, H.; Jin, H.; Yuan, F.; Huang, S.; Li, J. Radio-Frequency Atmospheric-Pressure Plasma Synthesis of Ultrafine ZrC Powders. Int. J. Appl. Ceram. Technol. 2012, 10, E274–E281. [Google Scholar] [CrossRef]
- Van Laar, J.; Slabber, J.; Meyer, J.; van der Walt, I.; Puts, G.; Crouse, P. Microwave-plasma synthesis of nano-sized silicon carbide at atmospheric pressure. Ceram. Int. 2014, 41, 4326–4333. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.-M.; Koo, S.-M.; Cho, W.-S.; Hwnag, K.-T.; Kim, J.-H. Synthesis of SiC nano-powder from organic precursors using RF inductively coupled thermal plasma. Ceram. Int. 2012, 38, 1959–1963. [Google Scholar] [CrossRef]
- Szépvölgyi, J.; Mohai, I.; GálI, L. Synthesis of nanosized ceramic powders in a radio frequency thermal plasma reactor. J. Eur. Ceram. Soc. 2008, 28, 895–899. [Google Scholar] [CrossRef]
- Bai, L.Y. Synthesis of ultrafine ZrB2 and ZrC powders by high frequency thermal plasma. Aerosp. Mater. Technol. 2012, 2, 88–90. [Google Scholar]
- Bai, L.; Jin, H.; Lu, C.; Yuan, F.; Huang, S.; Li, J. RF thermal plasma-assisted metallothermic synthesis of ultrafine ZrB2 powders. Ceram. Int. 2015, 41, 7312–7317. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, F.; Fang, Z.; Wang, Q.; Ouyang, Y.; Jin, H.; He, J.; Liu, W.; Wang, Y. RF Thermal Plasma Synthesis of Ultrafine ZrB2-ZrC Composite Powders. Nanomaterials 2020, 10, 2497. [Google Scholar] [CrossRef] [PubMed]
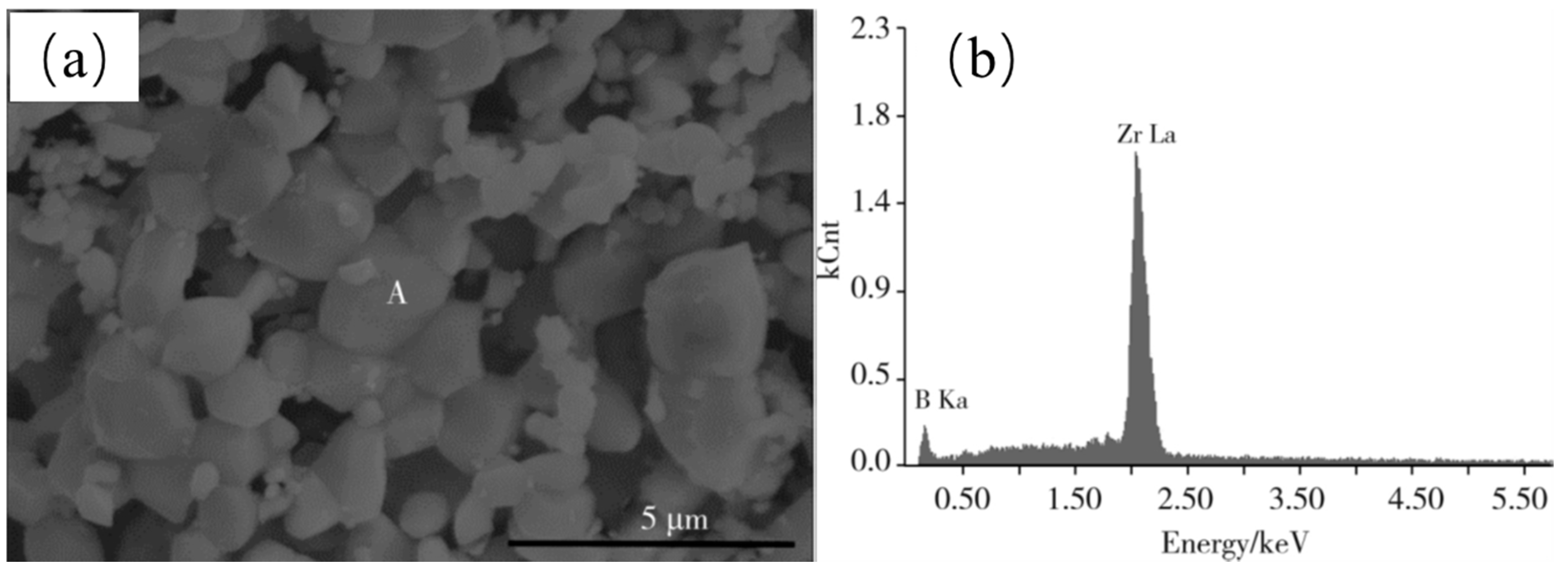


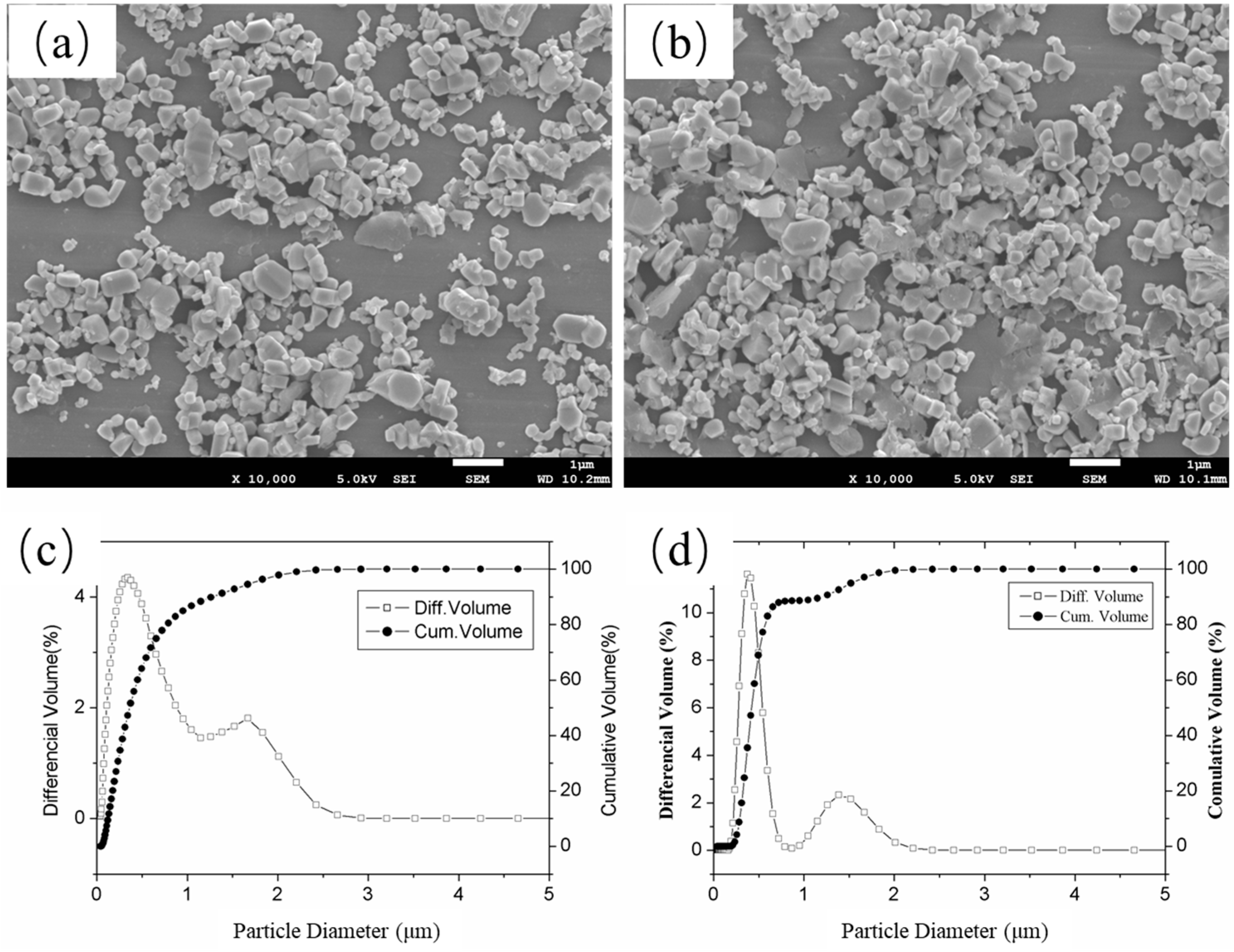

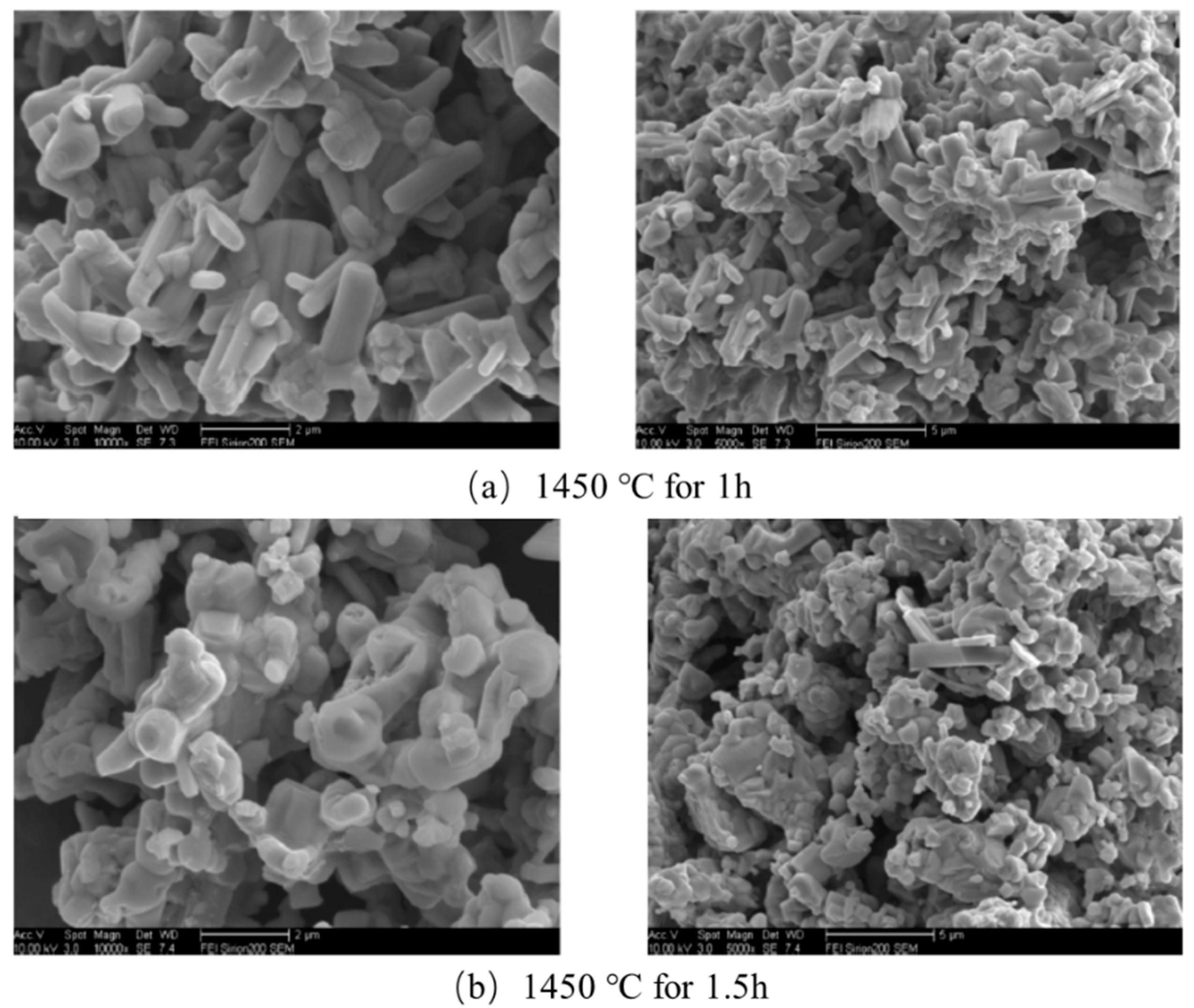
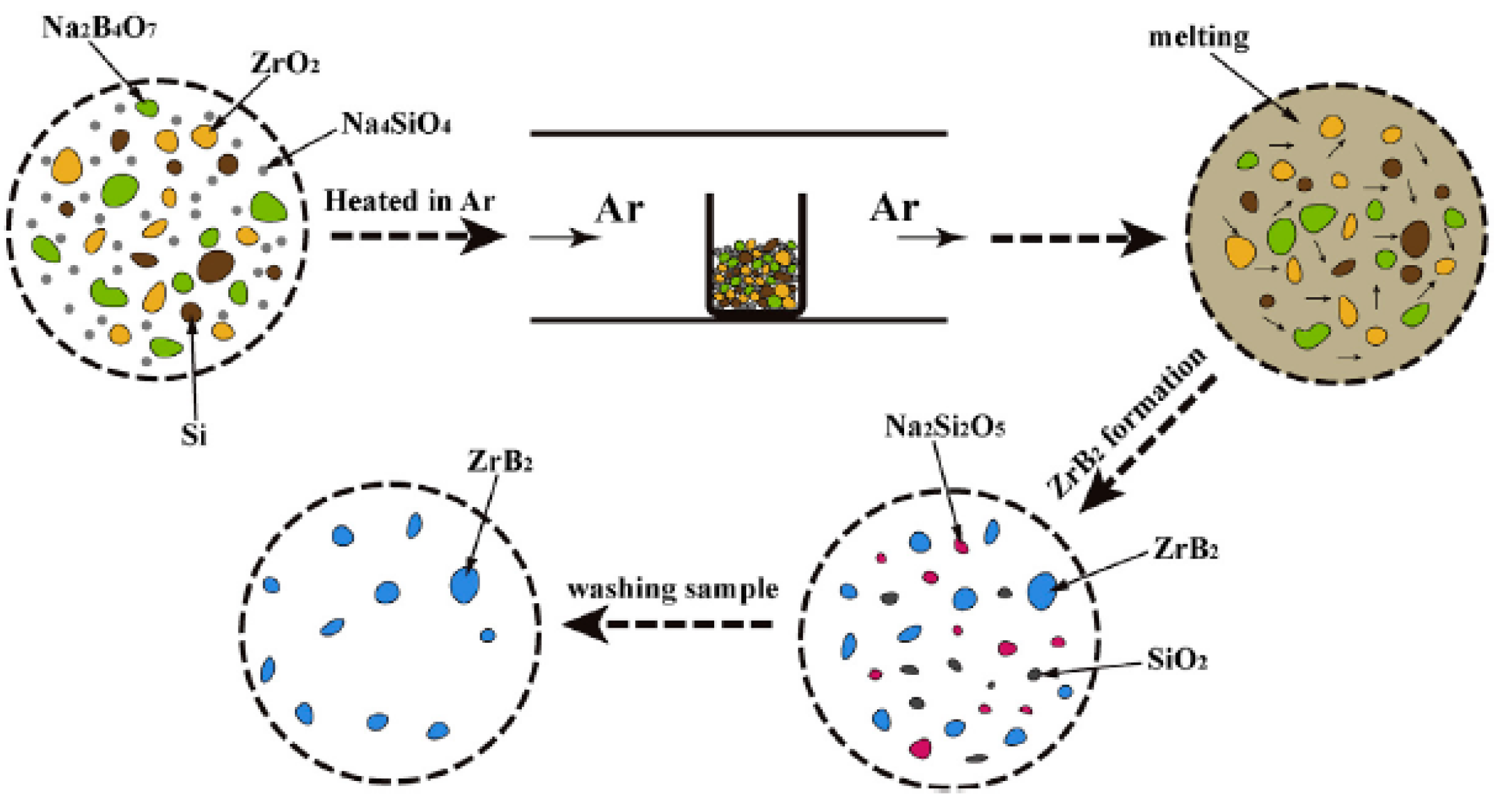

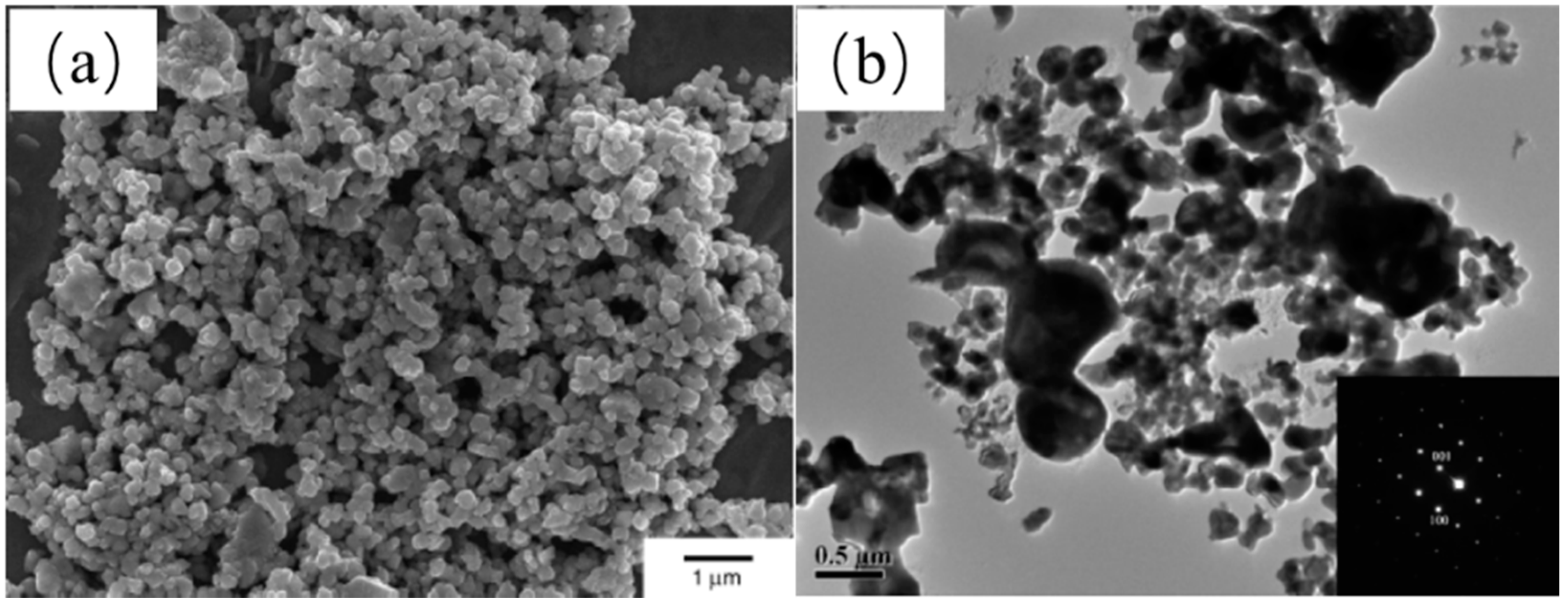

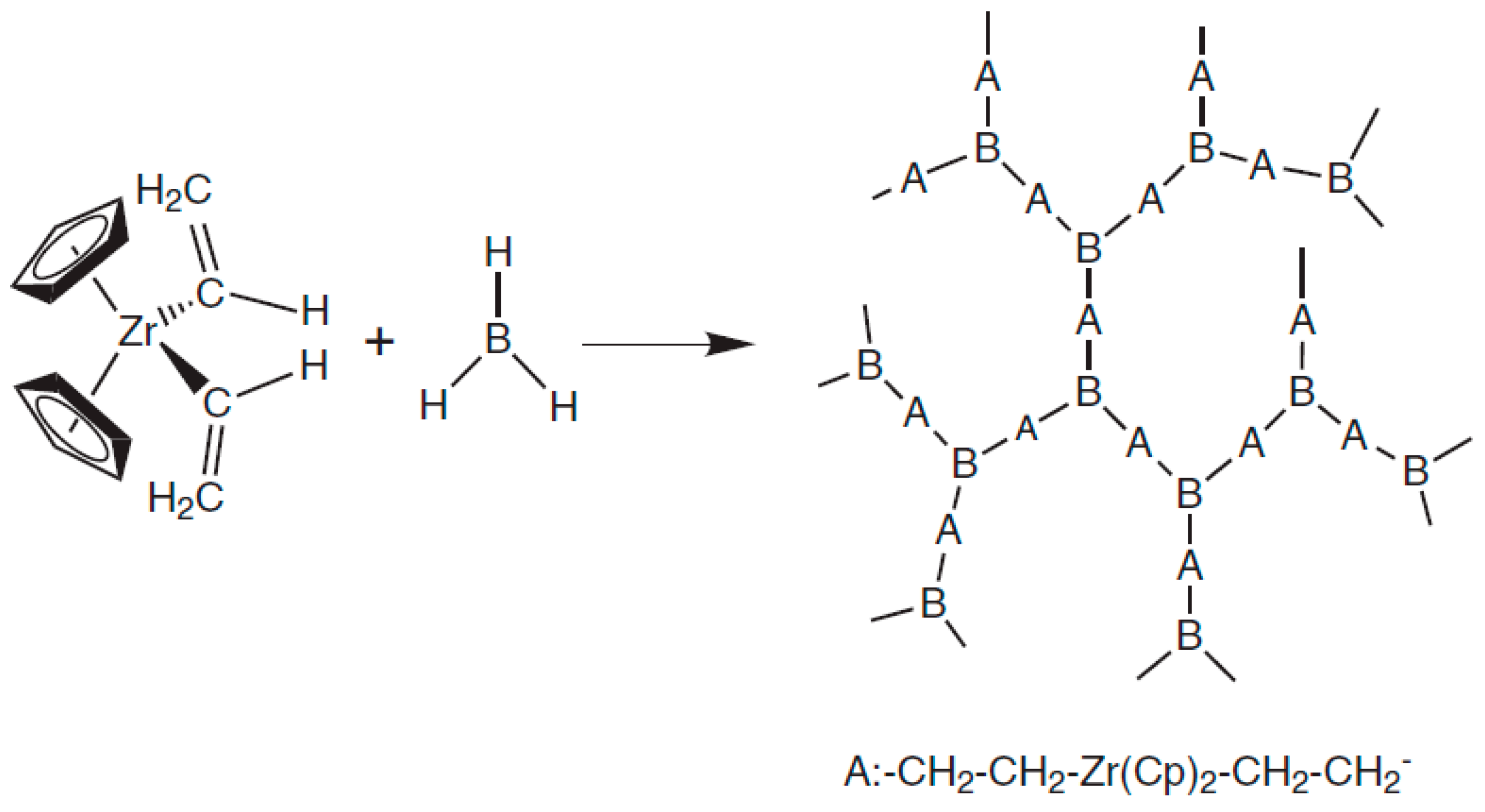

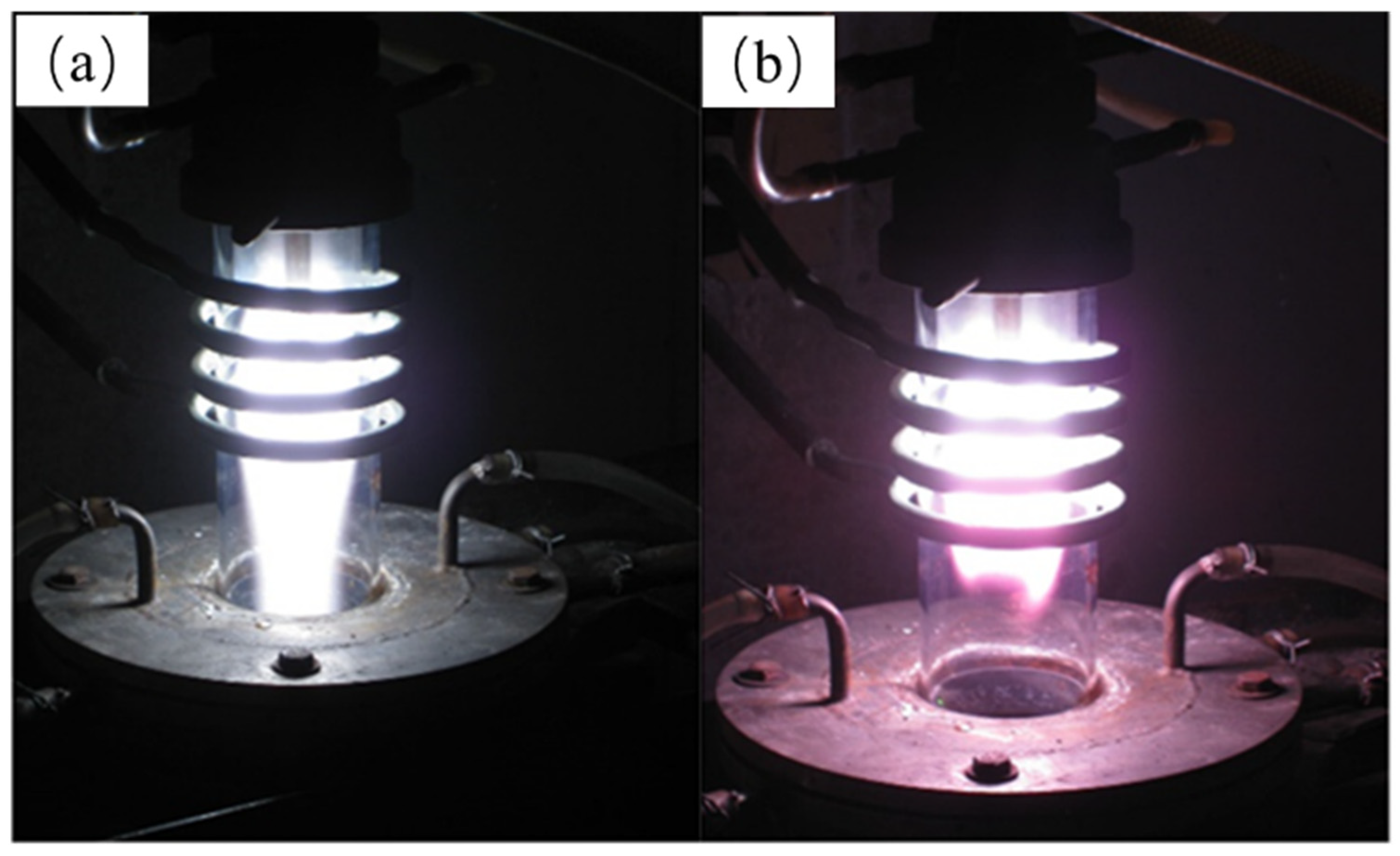
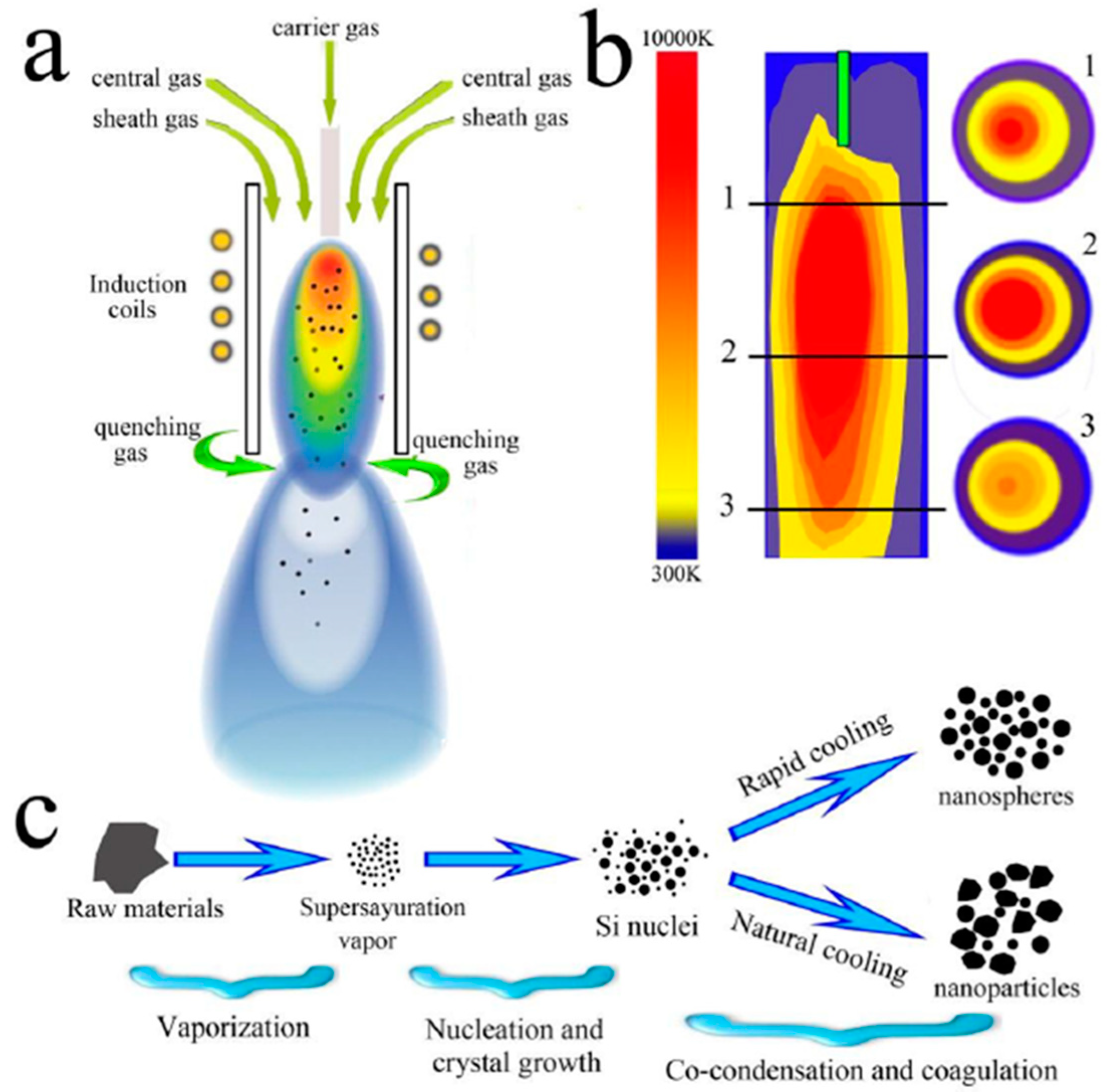

| NaCl (wt.%) | Crystal Size (nm) |
|---|---|
| 0 | 25 |
| 5 | 20 |
| 10 | 18 |
| 15 | 16 |
| 20 | 13 |
| Characteristics of Plasma Process | Properties of ZrB2 Powders |
|---|---|
| High temperature | High temperature |
| Rapid cooling | Nano size |
| No electrode pollution | High purity |
| Controllable atmosphere | Non-oxide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Ouyang, Y.; Yuan, F. Progress in Preparation of ZrB2 Nanopowders Based on Traditional Solid-State Synthesis. Nanomaterials 2021, 11, 2345. https://doi.org/10.3390/nano11092345
Bai L, Ouyang Y, Yuan F. Progress in Preparation of ZrB2 Nanopowders Based on Traditional Solid-State Synthesis. Nanomaterials. 2021; 11(9):2345. https://doi.org/10.3390/nano11092345
Chicago/Turabian StyleBai, Liuyang, Yuge Ouyang, and Fangli Yuan. 2021. "Progress in Preparation of ZrB2 Nanopowders Based on Traditional Solid-State Synthesis" Nanomaterials 11, no. 9: 2345. https://doi.org/10.3390/nano11092345
APA StyleBai, L., Ouyang, Y., & Yuan, F. (2021). Progress in Preparation of ZrB2 Nanopowders Based on Traditional Solid-State Synthesis. Nanomaterials, 11(9), 2345. https://doi.org/10.3390/nano11092345





