3D-Printable Nanocellulose-Based Functional Materials: Fundamentals and Applications
Abstract
:1. Introduction
2. Nanocellulose: Preparation, Treatment, Functionality, and 3D Printability
2.1. Preparation
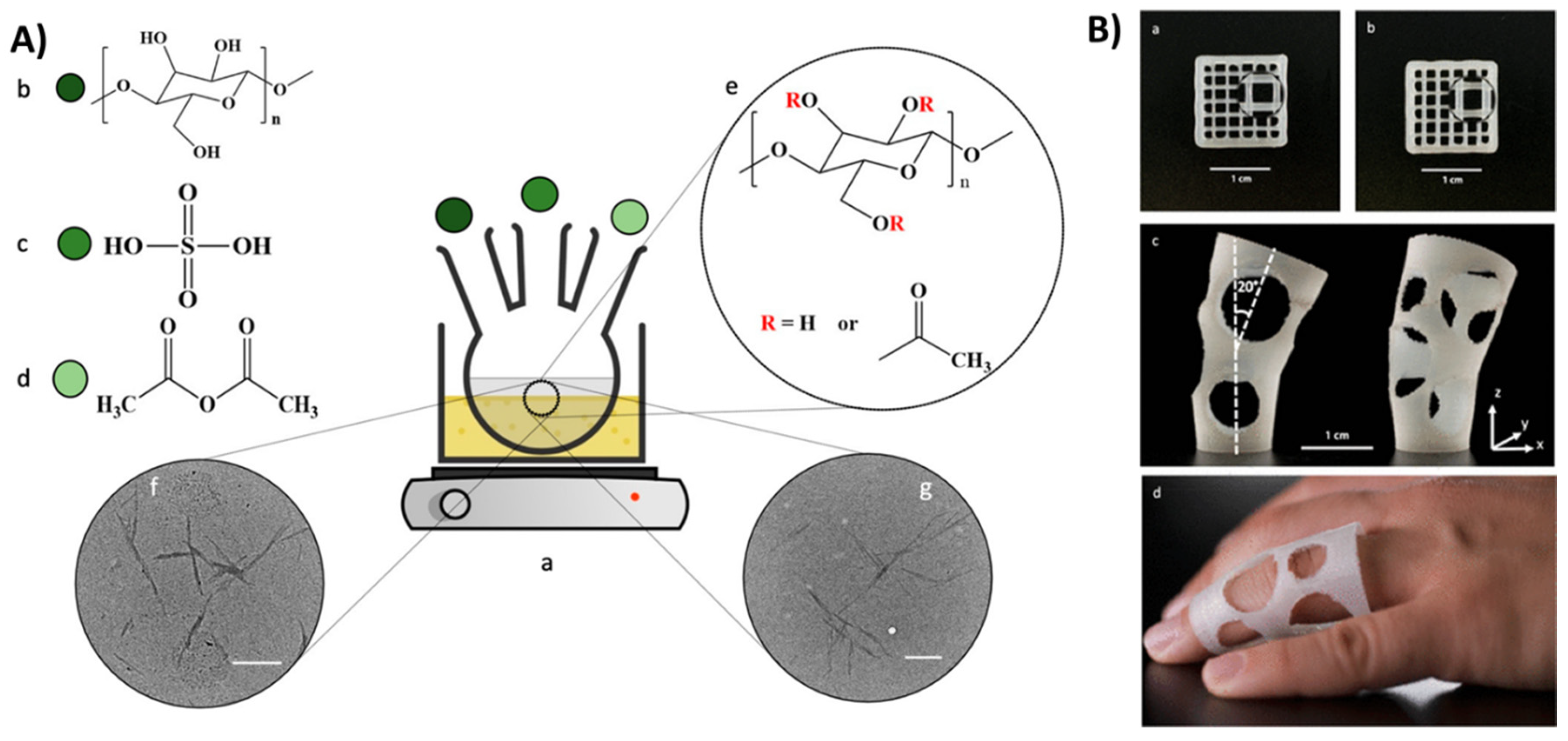
2.2. Functional Nanocellulose-Based Composite Nanostructures
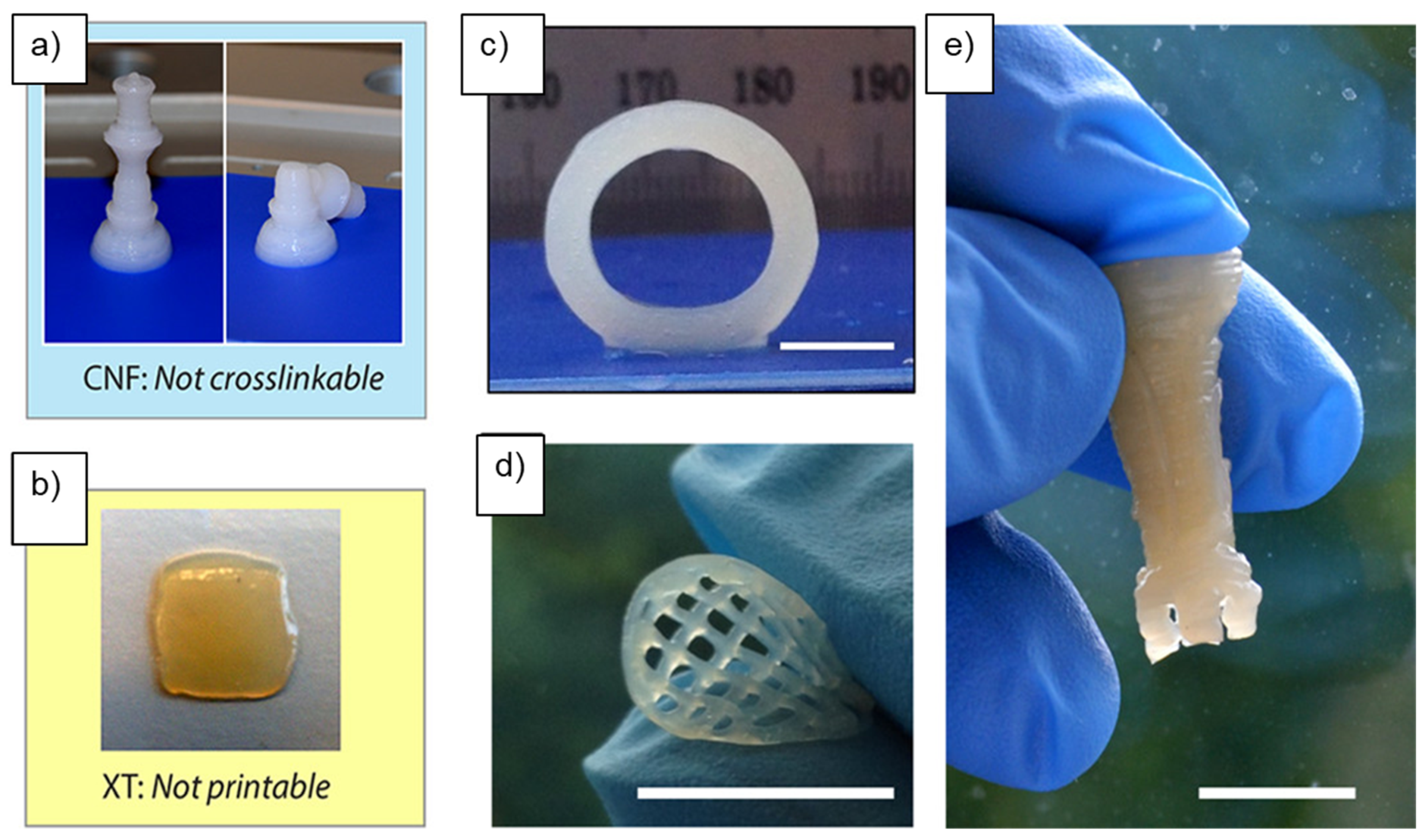
2.3. Three-Dimensional Printability of Nanocellulose Composites
3. Applications of 3D-Printed Nanocellulose-Based Materials
3.1. Environmental Applications
3.2. Food and Packaging Applications
3.3. Energy and Electrochemical Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Kirk, K.A.; Othman, A.; Andreescu, S. Nanomaterial-functionalized Cellulose: Design, Characterization and Analytical Applications. Anal. Sci. 2018, 34, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Aitomaki, Y.; Oksman, K. Reinforcing efficiency of nanocellulose in polymers. React. Funct. Polym. 2014, 85, 151–156. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomaki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.J.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Thunberg, J.; Kalogeropoulos, T.; Kuzmenko, V.; Hagg, D.; Johannesson, S.; Westman, G.; Gatenholm, P. In situ synthesis of conductive polypyrrole on electrospun cellulose nanofibers: Scaffold for neural tissue engineering. Cellulose 2015, 22, 1459–1467. [Google Scholar] [CrossRef]
- Goffin, A.L.; Raquez, J.M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. Poly(epsilon-caprolactone) based nanocomposites reinforced by surface-grafted cellulose nanowhiskers via extrusion processing: Morphology, rheology, and thermo-mechanical properties. Polymer 2011, 52, 1532–1538. [Google Scholar] [CrossRef]
- Gebhardt, A. Understanding Additive Manufacturing; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2011. [Google Scholar]
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D Printed Hydrogel-Based Sensors for Quantifying UV Exposure. ACS Appl. Mater. Interfaces 2020, 12, 43911–43920. [Google Scholar] [CrossRef]
- Othman, A.; Norton, L.; Finny, A.S.; Andreescu, S. Easy-to-use and inexpensive sensors for assessing the quality and traceability of cosmetic antioxidants. Talanta 2020, 208, 120473. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.F.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindstrom, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ujang, F.A.; Janudin, N.; Razak, M.A.I.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; et al. Nanocellulose: The next super versatile material for the military. Mater. Adv. 2021, 2, 1485–1506. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Lee, S.Y.; Wei, T.; Li, J.; Fan, Z.J. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petru, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci.-Nano 2014, 1, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wang, L.; Jin, L.; Wang, J.P.; Jin, D.C. Evolutionary Analysis of Cellulose Gene Family in Grasses. Commun. Comput. Inf. Sci. 2011, 244, 178. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Atiqah, A.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Nurazzi, N.M.; Atikah, M.S.N.; Ansari, M.N.M.; et al. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2020, 41, 459–467. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Ilyas, R.A. Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies. J. Mater. Res. Technol. 2020, 9, 6759–6776. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.A.; Trica, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Ee, L.Y.; Li, S.F.Y. Recent advances in 3D printing of nanocellulose: Structure, preparation, and application prospects. Nanoscale Adv. 2021, 3, 1167–1208. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Rehman, N.; Sharif, A.; Ahmed, E.; Farooqi, Z.H.; Din, M.I. Environmentally benign extraction of cellulose from dunchi fiber for nanocellulose fabrication. Int. J. Biol. Macromol. 2020, 153, 72–78. [Google Scholar] [CrossRef]
- Zhi, S.L.; Liu, Y.L.; Yu, X.Y.; Wang, X.Y.; Lu, X.B. Enzymatic Hydrolysis of Cellulose after Pretreated by Ionic Liquids: Focus on One-pot Process. Energy Procedia 2012, 14, 1741–1747. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Sultan, S.; Abdelhamid, H.N.; Zou, X.D.; Mathew, A.P. CelloMOF: Nanocellulose Enabled 3D Printing of Metal-Organic Frameworks. Adv. Funct. Mater. 2019, 29, 1805372. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Jimenez, A.; Gopakumar, D.A.; Puglia, D.; Thomas, S.; Kenny, J.M.; Chiralt, A.; Torre, L. Revalorization of sunflower stalks as novel sources of cellulose nanofibrils and nanocrystals and their effect on wheat gluten bionanocomposite properties. Carbohydr. Polym. 2016, 149, 357–368. [Google Scholar] [CrossRef]
- Pohanka, M. Photography by Cameras Integrated in Smartphones as a Tool for Analytical Chemistry Represented by an Butyrylcholinesterase Activity Assay. Sensors 2015, 15, 13752–13762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Yuan, J.Y. Poly(1-Vinyl-1,2,4-triazolium) Poly(Ionic Liquid)s: Synthesis and the Unique Behavior in Loading Metal Ions. Macromol. Rapid Commun. 2016, 37, 1124–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhao, Q.; Yuan, J. Porous polyelectrolytes: Charge pores for more functionalities. Angew. Chem. 2017. [Google Scholar] [CrossRef]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Delaney, J.L.; Hogan, C.F.; Tian, J.; Shen, W. Electrogenerated Chemiluminescence Detection in Paper-Based Microfluidic Sensors. Anal. Chem. 2011, 83, 1300–1306. [Google Scholar] [CrossRef]
- Gong, J.; Lin, H.J.; Dunlop, J.W.C.; Yuan, J.Y. Hierarchically Arranged Helical Fiber Actuators Derived from Commercial Cloth. Adv. Mater. 2017, 29, 1605103. [Google Scholar] [CrossRef]
- Lin, H.J.; Gong, J.; Eder, M.; Schuetz, R.; Peng, H.S.; Dunlop, J.W.C.; Yuan, J.Y. Programmable Actuation of Porous Poly(Ionic Liquid) Membranes by Aligned Carbon Nanotubes. Adv. Mater. Interfaces 2017, 4, 1600768. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, S. Material design of nanocellulose/polymer composites via Pickering emulsion templating. Polym. J. 2021, 53, 103–109. [Google Scholar] [CrossRef]
- Mingwei, T.; Lijun, Q.; Xiansheng, Z.; Kun, Z.; Shifeng, Z.; Xiaoqing, G.; Guangting, H.; Xiaoning, T.; Yaning, S. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef]
- Lasrado, D.; Ahankari, S.; Kar, K. Nanocellulose-based polymer composites for energy applications-A review. J. Appl. Polym. Sci. 2020, 137, 48959. [Google Scholar] [CrossRef]
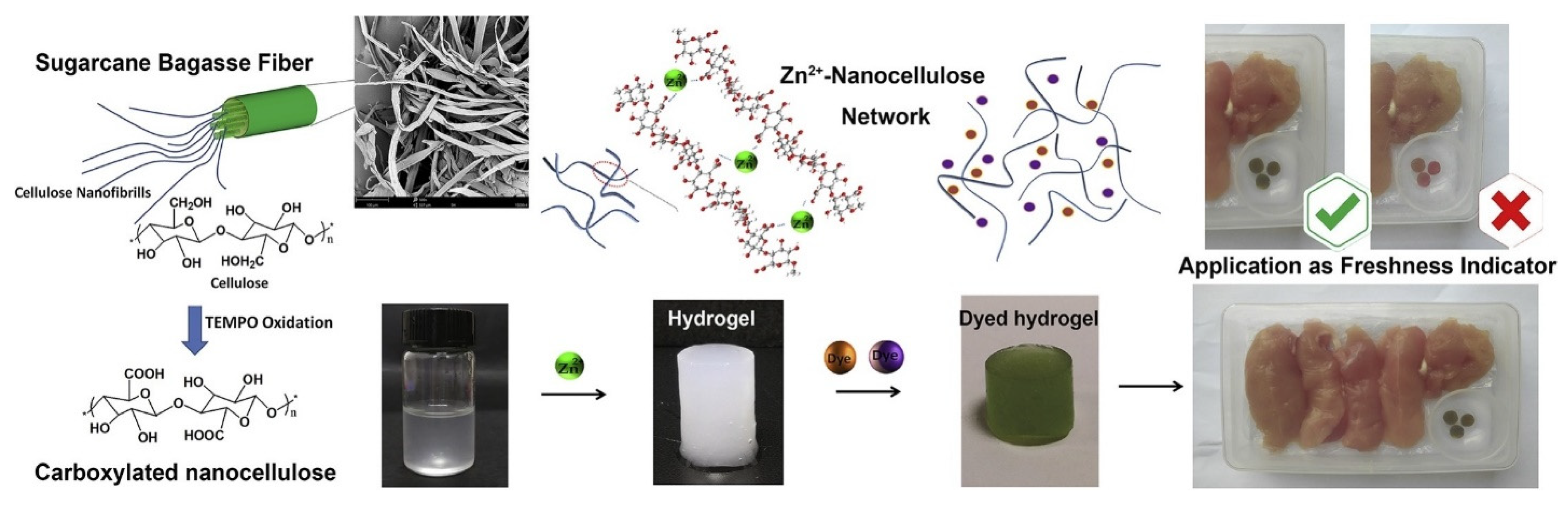
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.X.; Wu, M.; Wang, S.F. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Huang, F.; Lin, S.; Zhang, H.; Cao, S.; Chen, L.; He, Z.; Lutes, R.; Yang, J.; et al. Preparation and Characterization of Cellulose-Based Nanofiltration Membranes by Interfacial Polymerization with Piperazine and Trimesoyl Chloride. ACS Sustain. Chem. Eng. 2018, 6, 13168–13176. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K.; Saito, T.; Isogai, A. Fabrication of ultrathin nanocellulose shells on tough microparticles via an emulsion-templated colloidal assembly: Towards versatile carrier materials. Nanoscale 2019, 11, 15004–15009. [Google Scholar] [CrossRef] [Green Version]
- Elder, B.; Neupane, R.; Tokita, E.; Ghosh, U.; Hales, S.; Kong, Y.L. Nanomaterial Patterning in 3D Printing. Adv. Mater. 2020, 32, 1907142. [Google Scholar] [CrossRef] [PubMed]
- Klar, V.; Pere, J.; Turpeinen, T.; Karki, P.; Orelma, H.; Kuosmanen, P. Shape fidelity and structure of 3D printed high consistency nanocellulose. Sci. Rep.-UK 2019, 9, 3822. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic Inks Based on Cellulose Nanofibrils and Cross-Linkable Xylans for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fang, J.W.; Tang, S.W.; Wu, Z.G.; Wang, X.Y. 3D-Printed Nanocellulose-Based Cushioning-Antibacterial Dual-Function Food Packaging Aerogel. Molecules 2021, 26, 3543. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Hartemann, P.; Hoet, P.; Proykova, A.; Fernandes, T.; Baun, A.; De Jong, W.; Filser, J.; Hensten, A.; Kneuer, C.; Maillard, J.Y.; et al. Nanosilver: Safety, health and environmental effects and role in antimicrobial resistance. Mater. Today 2015, 18, 122–123. [Google Scholar] [CrossRef]
- Fourmann, O.; Hausmann, M.K.; Neels, A.; Schubert, M.; Nystrom, G.; Zimmermann, T.; Siqueira, G. 3D printing of shape-morphing and antibacterial anisotropic nanocellulose hydrogels. Carbohydr. Polym. 2021, 259. [Google Scholar] [CrossRef]
- Giubilini, A.; Siqueira, G.; Clemens, F.J.; Sciancalepore, C.; Messori, M.; Nystrom, G.; Bondioli, F. 3D-Printing Nanocellulose-Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Biodegradable Composites by Fused Deposition Modeling. ACS Sustain. Chem. Eng. 2020, 8, 10292–10302. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Syrovy, T.; Maronova, S.; Kubersky, P.; Ehman, N.V.; Vallejos, M.E.; Pretl, S.; Felissia, F.E.; Area, M.C.; Chinga-Carrasco, G. Wide range humidity sensors printed on biocomposite films of cellulose nanofibril and poly(ethylene glycol). J. Appl. Polym. Sci. 2019, 136, 47920. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, Z.; Nordli, H.R.; Pukstad, B.; Gethin, D.T.; Chinga-Carrasco, G. Translucent and ductile nanocellulose-PEG bionanocomposites-A novel substrate with potential to be functionalized by printing for wound dressing applications. Ind. Crop. Prod. 2016, 93, 193–202. [Google Scholar] [CrossRef]
- Sun, F.Z.; Nordli, H.R.; Pukstad, B.; Gamstedt, E.K.; Chinga-Carrasco, G. Mechanical characteristics of nanocellulose-PEG bionanocomposite wound dressings in wet conditions. J. Mech. Behav. Biomed. 2017, 69, 377–384. [Google Scholar] [CrossRef]
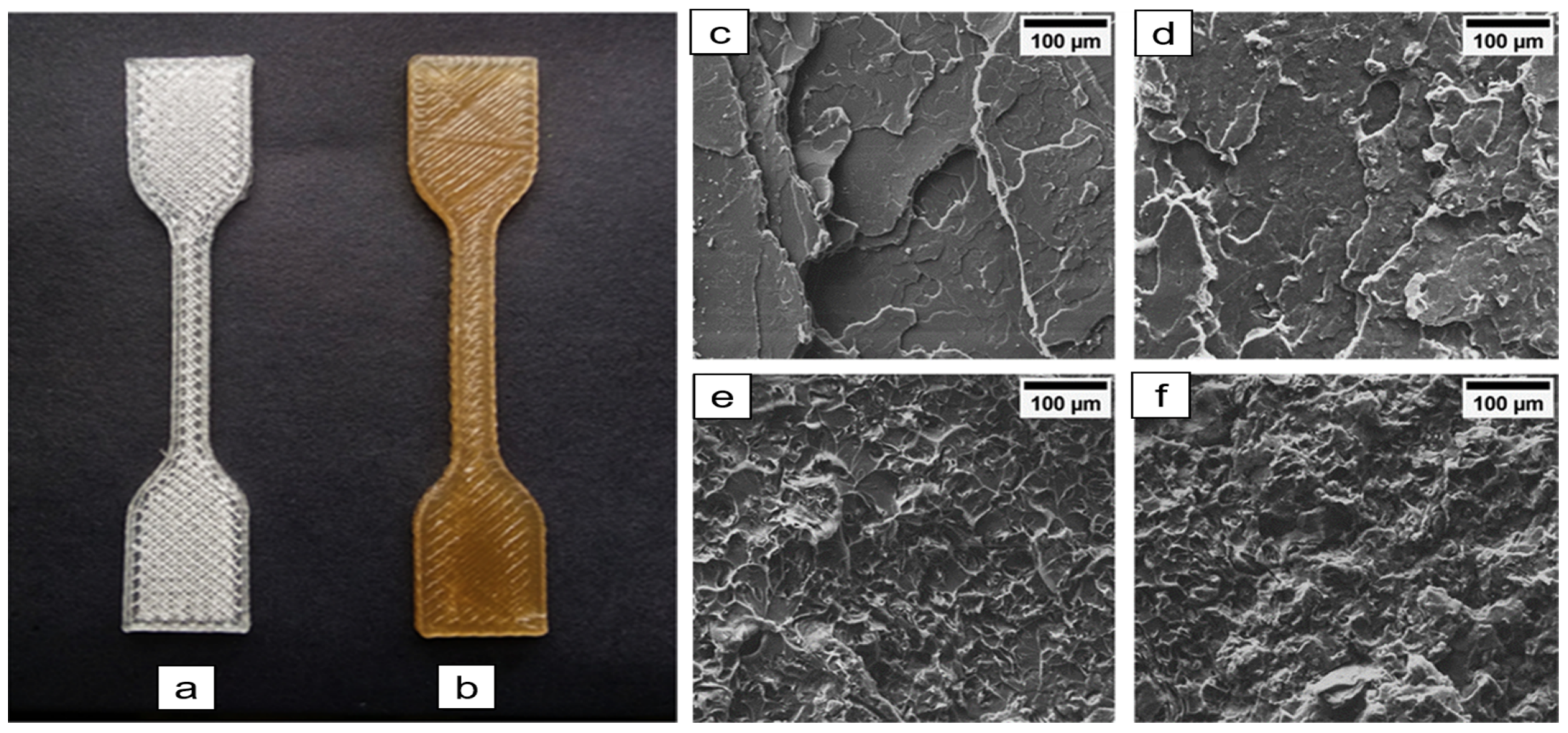
- Ambone, T.; Torris, A.; Shanmuganathan, K. Enhancing the mechanical properties of 3D printed polylactic acid using nanocellulose. Polym. Eng. Sci. 2020, 60, 1842–1855. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Gao, Q.; Lei, M.; Zhou, K.M.; Liu, X.L.; Wang, S.F. Nanocellulose in Food Packaging-A Short Review. J. Biobased Mater. Bioenergy 2020, 14, 431–443. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef] [PubMed]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsa-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Zhou, R.; Su, S.Q.; Li, Y.F. Effects of cushioning materials on the firmness of Huanghua pears (Pyrus pyrifolia Nakai cv. Huanghua) during distribution and storage. Packag. Technol. Sci. 2008, 21, 1–11. [Google Scholar] [CrossRef]
- Jarimopas, B.; Singh, S.P.; Sayasoonthorn, S.; Singh, J. Comparison of package cushioning materials to protect post-harvest impact damage to apples. Packag. Technol. Sci. 2007, 20, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.H.; Chen, J.H.; Chen, F.; Zhu, C.Q.; Wu, D.; Wang, J.; Chen, K.S. Effects of cushioning materials and temperature on quality damage of ripe peaches according to the vibration test. Food Packag. Shelf 2020, 25. [Google Scholar] [CrossRef]
- Karagiannidis, P.G.; Hodge, S.A.; Lombardi, L.; Tomarchio, F.; Decorde, N.; Milana, S.; Goykhman, I.; Su, Y.; Mesite, S.V.; Johnstone, D.N.; et al. Microfluidization of Graphite and Formulation of Graphene-Based Conductive Inks. ACS Nano 2017, 11, 2742–2755. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Dominguez, J.M.; Baigorri, A.; Alvarez-Sanchez, M.A.; Colom, E.; Villacampa, B.; Anson-Casaos, A.; Garcia-Bordeje, E.; Benito, A.M.; Maser, W.K. Waterborne Graphene- and Nanocellulose-Based Inks for Functional Conductive Films and 3D Structures. Nanomaterials 2021, 11, 1435. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, J.M.; Anson-Casaos, A.; Grasa, L.; Abenia, L.; Salvador, A.; Colom, E.; Mesonero, J.E.; Garcia-Bordeje, J.E.; Benito, A.M.; Maser, W.K. Unique Properties and Behavior of Nonmercerized Type-II Cellulose Nanocrystals as Carbon Nanotube Biocompatible Dispersants. Biomacromolecules 2019, 20, 3147–3160. [Google Scholar] [CrossRef]





| Nanocellulose Treatment | Type and Source | Formulation | Printing Method | Area of Application | Reference |
|---|---|---|---|---|---|
| Hydrothermal and soda pretreatment, PEG plasticizer, interdigitated electrodes, relative humidity sensing | CNF from bagasse (sugarcane residue) | FS_T3.8_P40; CNF film from bagasse treated with NaOH and 3.8 molar, 40% PEG, IDE screen printing | Screen printing via flatbed screen printer (Everbright S-200HF) | Environmental (relative humidity sensing) | [56] |
|
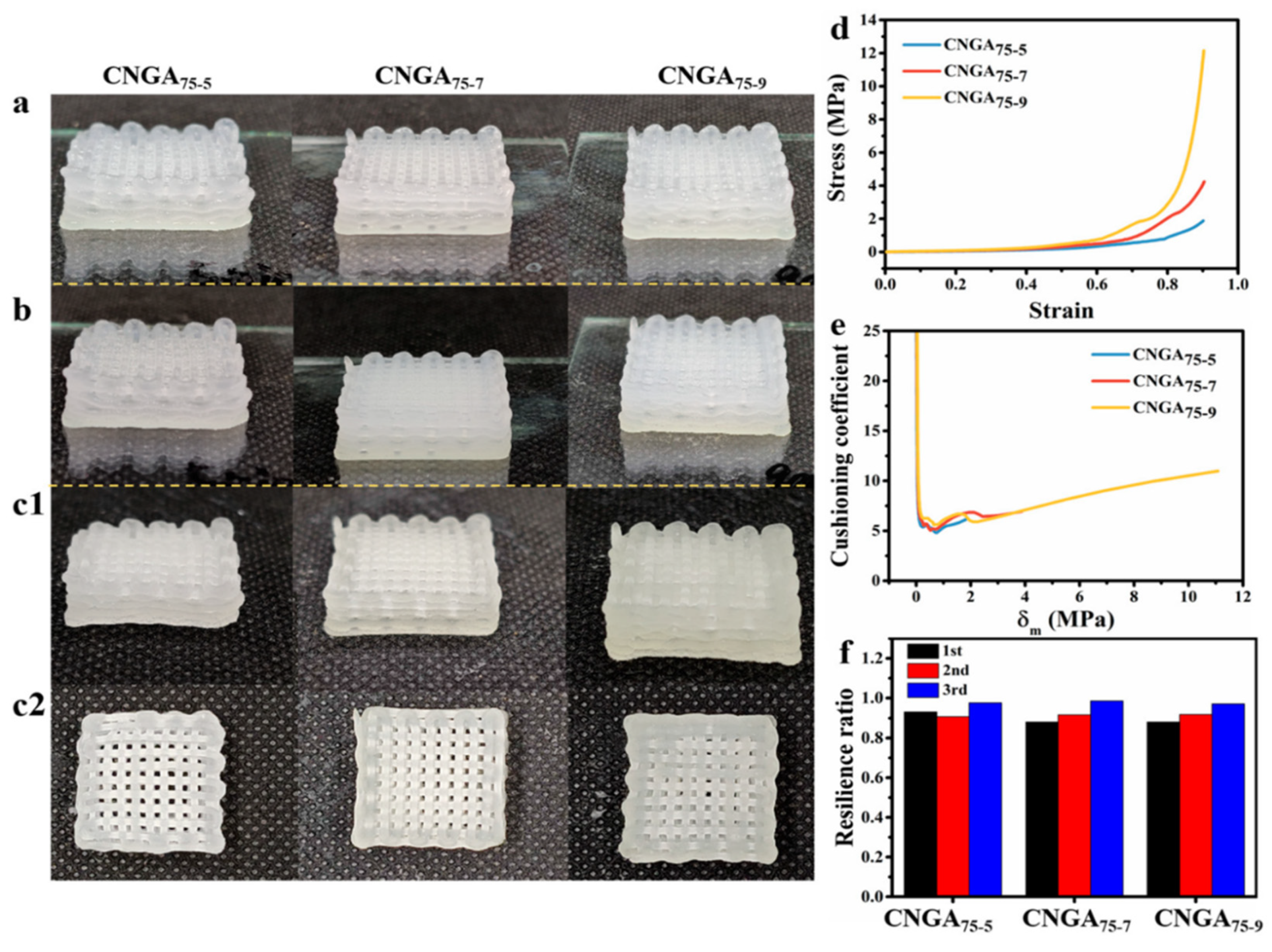
Carboxymethyl nanocellulose, glycerin, acrylamide, chitosan/AgNPs, cushioning, and antibacterial composite | CMC, (commercially sourced) | (CNGA/C–AgNPs); cushioning 3D-printed structure from 75 vol% glycerine, 1.2 g of N-(2-hydroxyethyl) acrylamide, and functionalized with chitosan–silver nanoparticle (Cts/AgNPS) | Coaxial printing technology via Y&D7300N 3D printer for matrix printing while LSP04-1A syringe pump extrudes the core solution (Cts/AgNPs) concurrently | Food packaging with cushioning and antimicrobial property | [50] |
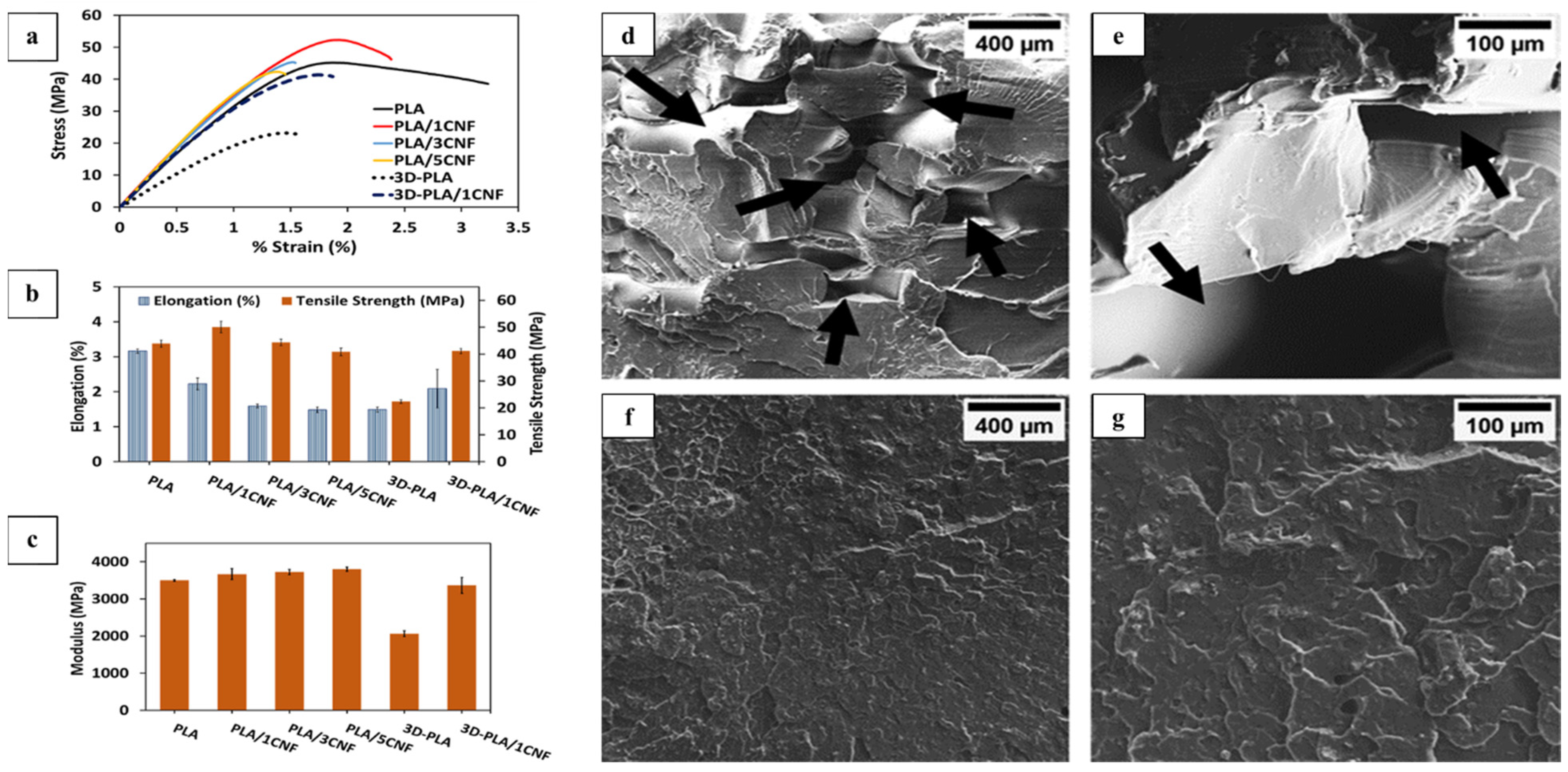
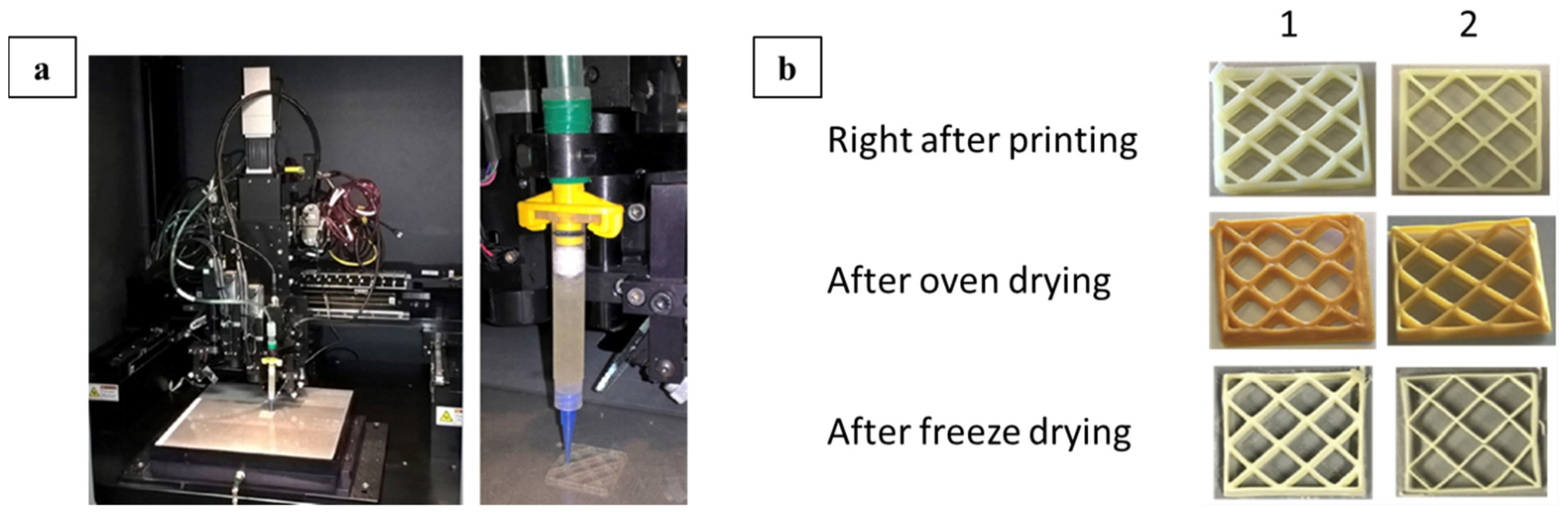
| Fused filament fabrication (FFF)-3D printing. Sisal nanocellulose, polylatic acid composite | CNF from raw sisal fibers |
(3D-PLA/1CNF); polylactic acid with varying CNF concentrations (1/3/5%) | Compression molding and 3D printing using FFF Desktop 3D printer (Fracktal Works Julia V2) | Diverse | [59] |
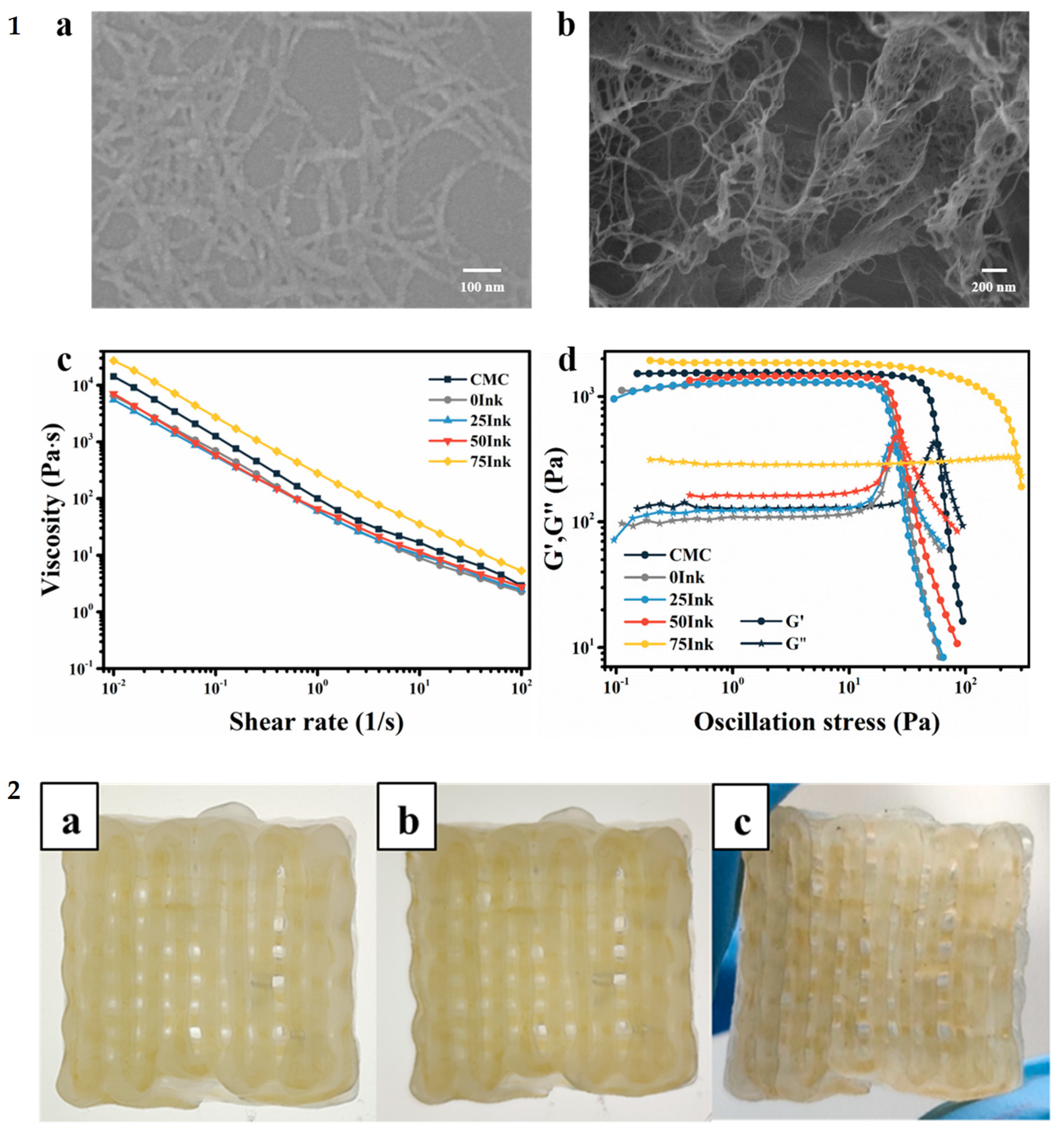
| Hydrothermal treatment, hydrogel–aerogel unidirectional freezing conversion | CNC from cotton-linters-derived microcrystalline cellulose (MCC) powder. | NH4OH for basic medium, carbon nanotube, and CNC for reinforcement and higher viscosity, reduced Graphene oxide (GO) for conductivity | Spray and rod coating | Metal-free electrodes (electrochemical) | [70] |
| Plant pulp, nutritional pastes, drying condition | CNF from birch kraft pulp | 10% cold swelling starch + 15% SMP, 60% SSMP, 30% rye bran, 35% OPC or 45% FBPC | Extrusion-based printer | Food printing | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finny, A.S.; Popoola, O.; Andreescu, S. 3D-Printable Nanocellulose-Based Functional Materials: Fundamentals and Applications. Nanomaterials 2021, 11, 2358. https://doi.org/10.3390/nano11092358
Finny AS, Popoola O, Andreescu S. 3D-Printable Nanocellulose-Based Functional Materials: Fundamentals and Applications. Nanomaterials. 2021; 11(9):2358. https://doi.org/10.3390/nano11092358
Chicago/Turabian StyleFinny, Abraham Samuel, Oluwatosin Popoola, and Silvana Andreescu. 2021. "3D-Printable Nanocellulose-Based Functional Materials: Fundamentals and Applications" Nanomaterials 11, no. 9: 2358. https://doi.org/10.3390/nano11092358
APA StyleFinny, A. S., Popoola, O., & Andreescu, S. (2021). 3D-Printable Nanocellulose-Based Functional Materials: Fundamentals and Applications. Nanomaterials, 11(9), 2358. https://doi.org/10.3390/nano11092358







