Self-Assembled Triple (H+/O2−/e−) Conducting Nanocomposite of Ba-Co-Ce-Y-O into an Electrolyte for Semiconductor Ionic Fuel Cells
Abstract
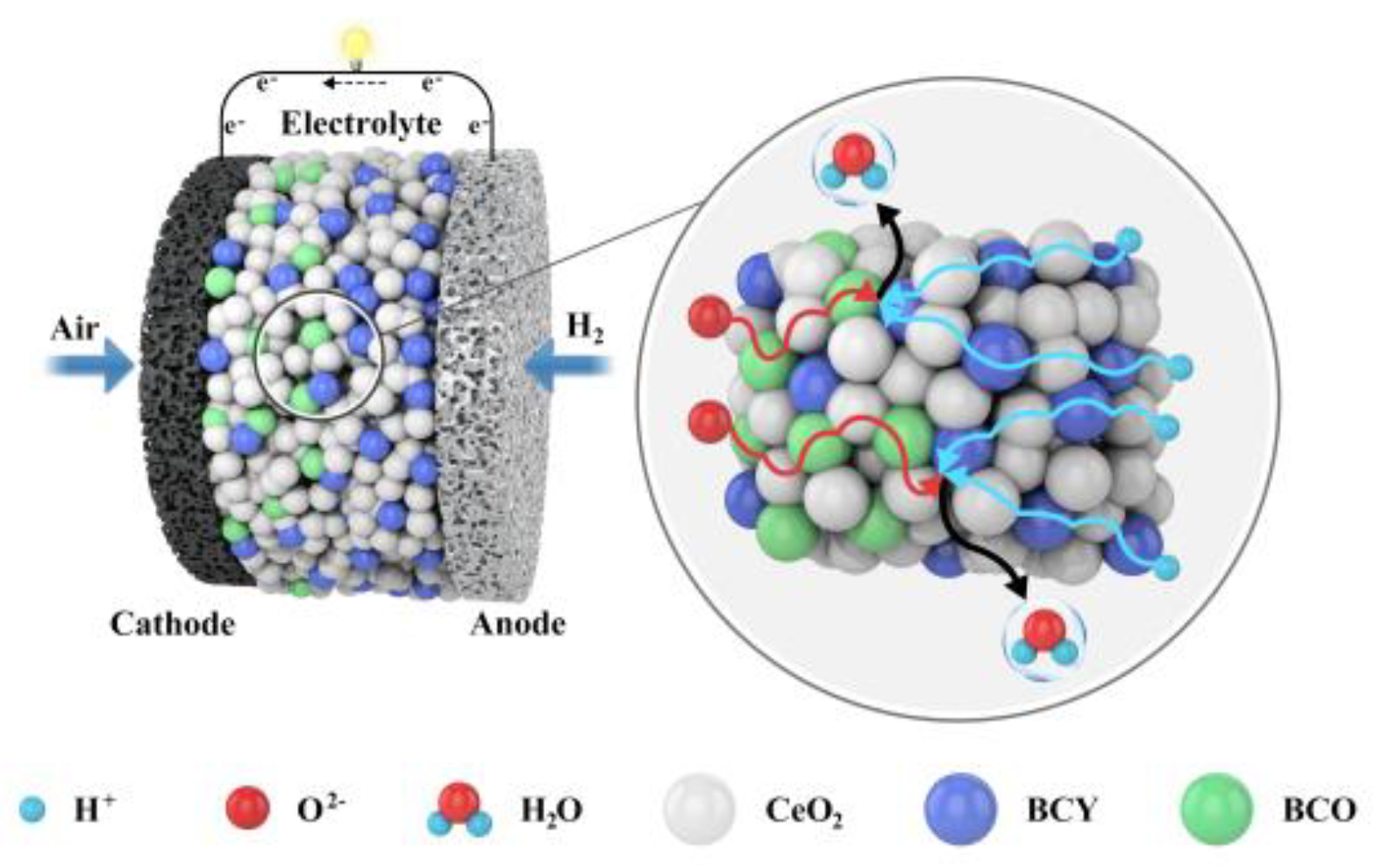
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Fuel Cell Fabrication
2.3. Characterizations and Electrochemical Measurements
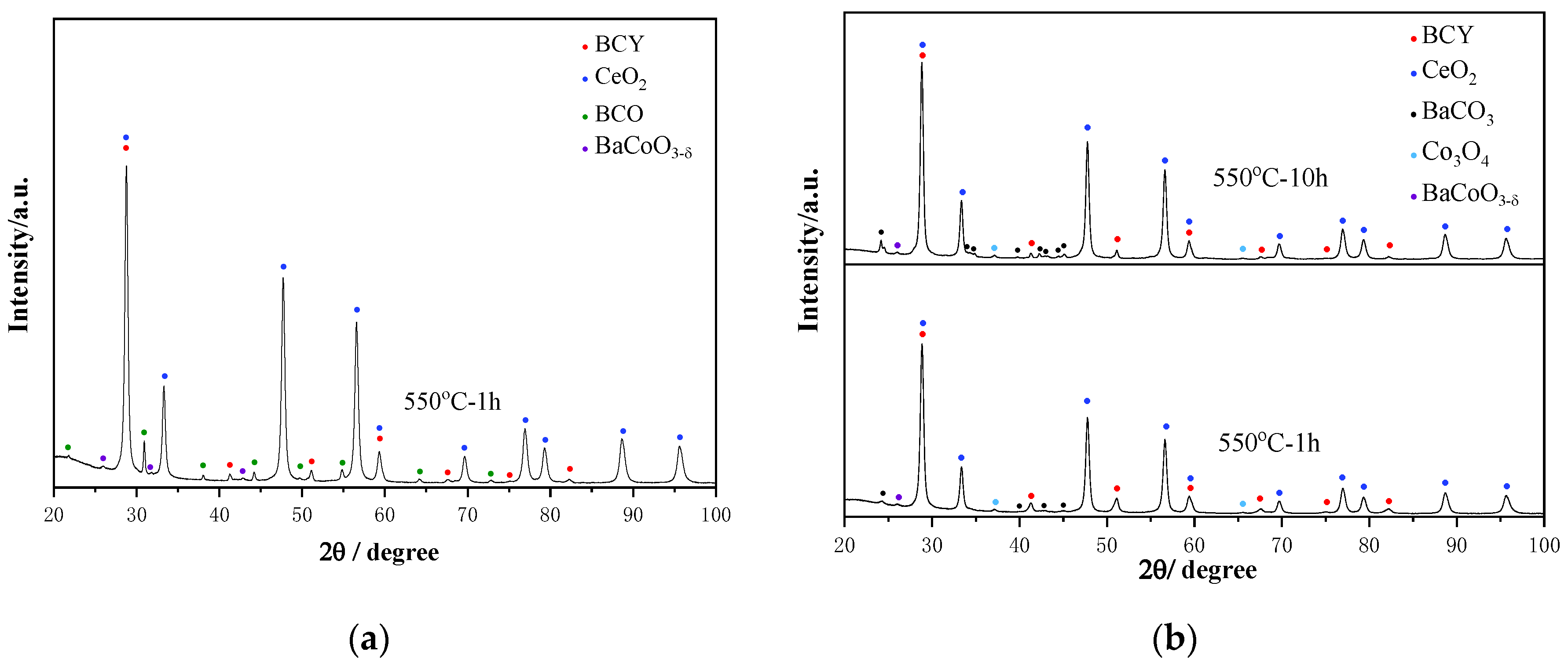
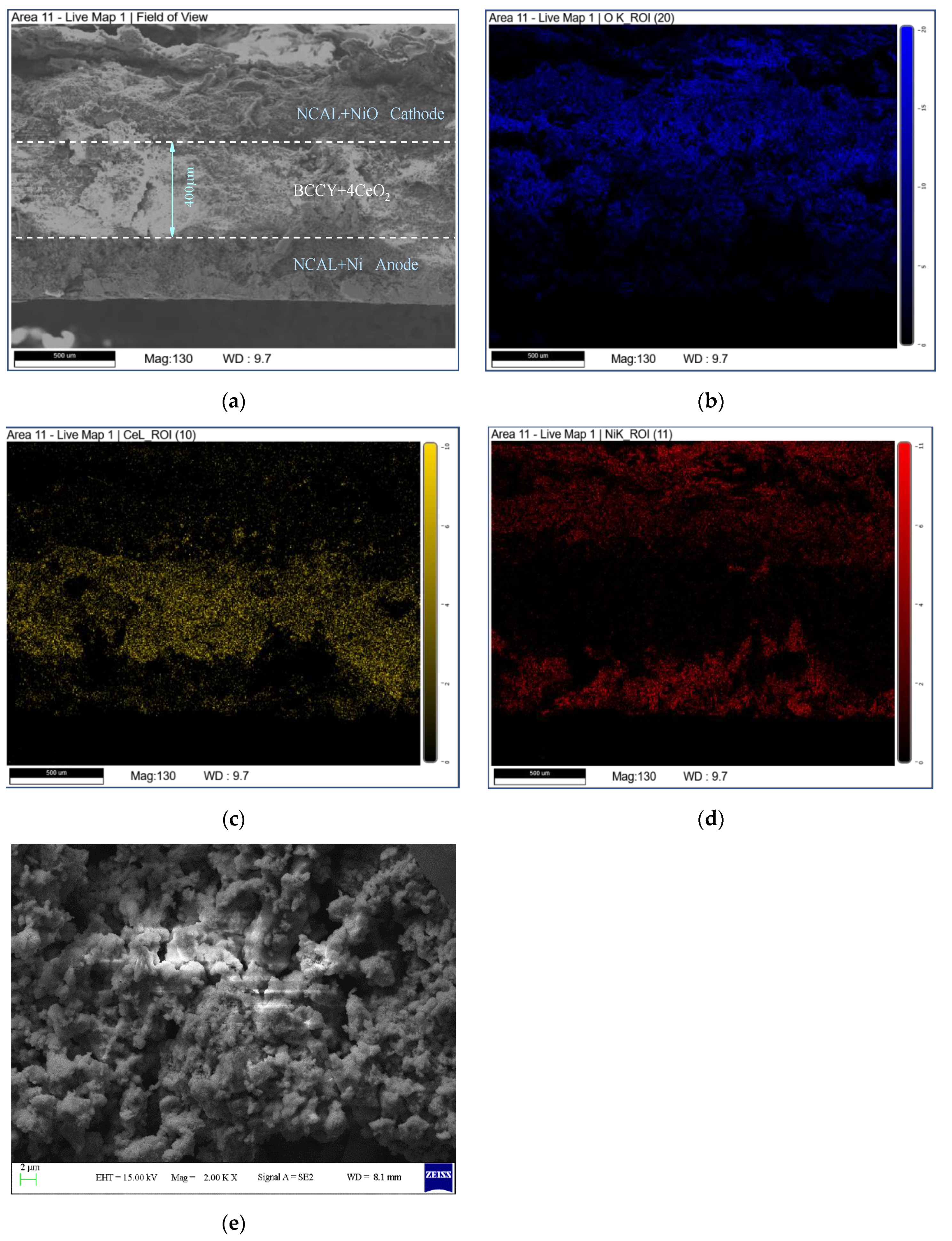
3. Results
3.1. Material Characterizations
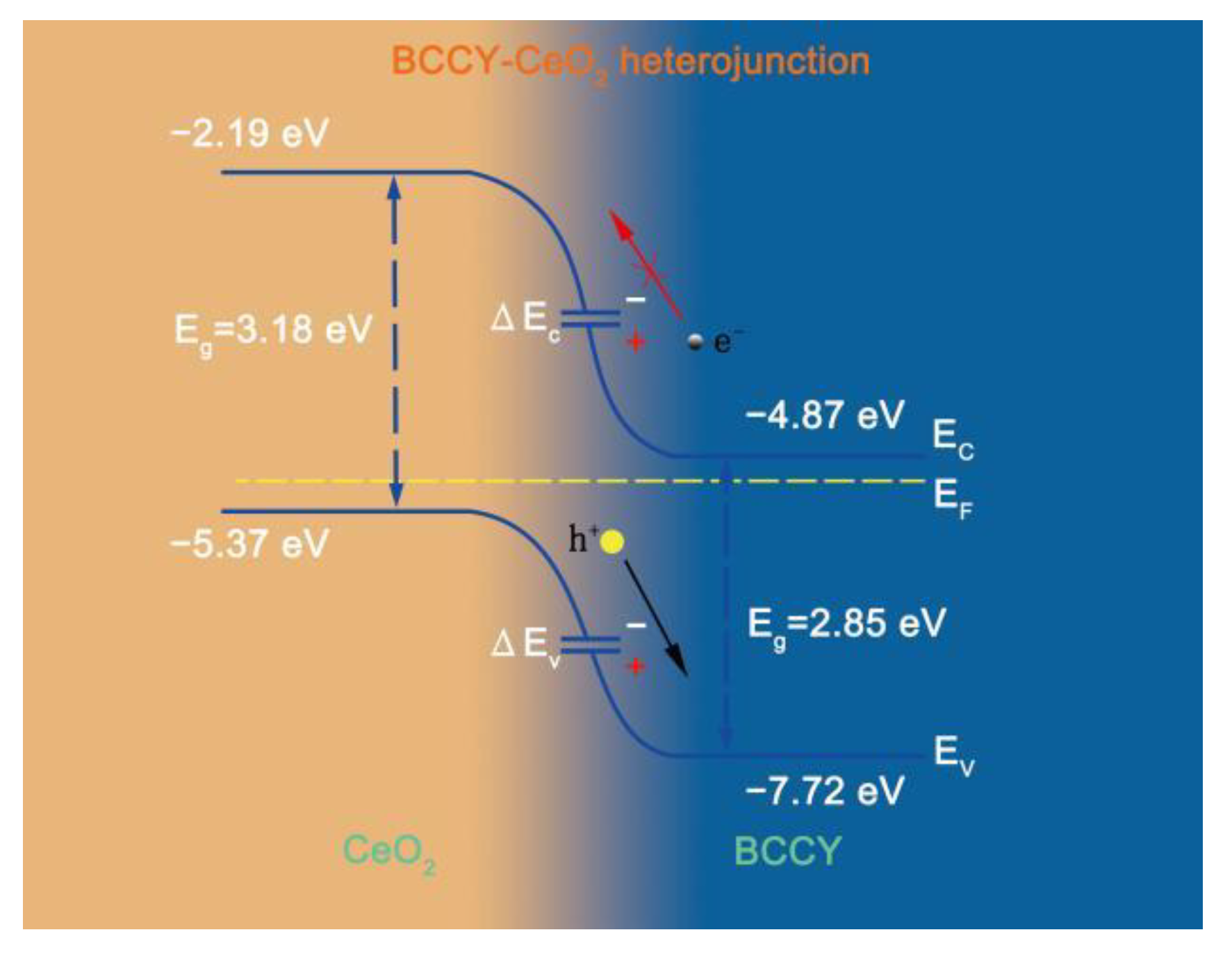
3.2. Electrochemical Performance and Electrical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Qian, Y.; Zhang, Y.; Hao, C.; Dan, D.; Zhuge, W. A review of heat transfer and thermal management methods for temperature gradient reduction in solid oxide fuel cell (SOFC) stacks. Appl. Energy 2020, 280, 115899. [Google Scholar] [CrossRef]
- Malik, V.; Srivastava, S.; Bhatnagar, M.K.; Vishnoi, M. Comparative study and analysis between Solid Oxide Fuel Cells (SOFC) and Proton Exchange Membrane (PEM) fuel cell—A review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Inac, S.; Unverdi, S.Q.; Midilli, A. Global warming, environmental and sustainability aspects of a geothermal energy based biodigester integrated SOFC system. Int. J. Hydrog. Energy 2020, 45, 35039–35052. [Google Scholar] [CrossRef]
- Tu, B.; Wen, H.; Yin, Y.; Zhang, F.; Su, X.; Cui, D.; Cheng, M. Thermodynamic analysis and experimental study of electrode reactions and open circuit voltages for methane-fuelled SOFC. Int. J. Hydrog. Energy 2020, 45, 34069–34079. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W. Processing and characterization of ultra-thin yttria-stabilized zirconia (YSZ) electrolytic films for SOFC. Solid State Ion. 2006, 177, 351–357. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today Off. 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Wachsman, E.; Ishihara, T.; Kilner, J. Low-temperature solid oxide fuel cells. MRS Bull. 2014, 39, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Qin, H.; Fan, L. Single-component and three-component fuel cells. J. Power Sources 2011, 196, 6362–6365. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Q.; Muhammad, A.; Zhu, B. Electrochemical study of lithiated transition metal oxide composite for single layer fuel cell. J. Power Sources 2015, 286, 388–393. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, Y.; Li, L.; Shi, Q.; Shi, J.; Yun, S.; Akbar, M.; Wang, B.; Kim, J.-S.; Zhu, B. Proton shuttles in CeO2/CeO2−δ core-shell structure. ACS Energy Lett. 2019, 11, 2601–2607. [Google Scholar] [CrossRef]
- Mushtaq, N.; Xia, C.; Dong, W.; Wang, B.; Raza, R.; Ali, A.; Afzal, M.; Zhu, B. Tuning the Energy Band Structure at Interfaces of the SrFe0.75Ti0.25O3-δ-Sm0.25Ce0.75O2-δ Heterostructure for Fast Ionic Transport. ACS Appl. Mater. Interfaces 2019, 11, 38737–38745. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Wang, B.; Tang, Y. Layered LiCoO2-LiFeO2 Heterostructure Composite for Semiconductor-Based Fuel Cells. Nanomaterials 2021, 11, 1224. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Q.; Zhu, Z.; Liu, X.; Afzal, M.; He, Y.; Zhu, B. Effects of composition on the electrochemical property and cell performance of single layer fuel cell. J. Power Sources 2015, 275, 476–482. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Akbar, M.; Jin, B.; Tu, Z.; Mushtaq, N.; Wang, B.; Qu, X.; Xia, C.; Huang, Y. A bulk-heterostructure nanocomposite electrolyte of Ce0.8Sm0.2O2-δ-SrTiO3 for low-temperature solid oxide fuel cells. Nano-Micro Lett. 2021, 13, 46. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Singh, M.; Hu, E.; Jiang, Z.; Raza, R.; Wang, F.; Wang, J.; Zhu, B. Superionic conductivity in ceria-based heterostructure composites for low-temperature solid oxide fuel cells. Nano-Micro Lett. 2020, 12, 178. [Google Scholar] [CrossRef]
- Dong, W.; Tong, Y.; Zhu, B.; Xiao, H.; Wei, L.; Huang, C.; Wang, B.; Wang, X.; Kim, J.; Wang, H. Semiconductor TiO2 thin film as an electrolyte for fuel cells. J. Mater. Chem. A 2019, 7, 16728. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Sengodan, S.P.; Kwon, G.; Ding, D.; Shin, J.Y.; Liu, M.; Kim, G.T. Triple-Conducting Layered Perovskites as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. ChemSusChem 2014, 7, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cui, J.; Zou, M.; Mu, S.; Huang, H.; Meng, Y.; He, K.; Brinkman, K.S.; Tong, J. Novel twin-perovskite nanocomposite of Ba-Ce-Fe-Co-O as a promising triple conducting cathode material for protonic ceramic fuel cells. J. Power Sources 2020, 450, 227609. [Google Scholar] [CrossRef]
- Le, L.; Hernandez, C.H.; Rodriguez, M.H.; Zhu, L.; Duan, C.; Ding, H.; O’Hayre, R.P.; Sullivan, N.P. Proton-conducting ceramic fuel cells: Scale up and stack integration. J. Power Sources 2021, 482, 228868. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Chen, Y.; Tan, S.; Shao, Z.; Chen, D. In situ formation of a 3D core-shell and triple-conducting oxygen reduction reaction electrode for proton-conducting SOFCs. J. Power Sources 2018, 385, 76–83. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Stefan, N.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Kiho, B.; Sewook, L.; Dong, Y.; Hyun, J.K.; Hunhyeong, L.; Dongwook, S.; Ji-Won, S.; Joonk, H.S. High-Performance Protonic Ceramic Fuel Cells with Thin-Film Yttrium-Doped Barium Cerate–Zirconate Electrolytes on Compositionally Gradient Anodes. ACS Appl. Mater. Interfaces 2016, 8, 9097–9103. [Google Scholar]
- Cheng, S.; Wang, Y.; Zhuang, L.; Xue, J.; Wei, Y.; Feldhoff, A.; Caro, J.; Wang, H. A dual-phase ceramic membrane with extremely high H2 Permeation flux prepared by autoseparation of a ceramic precursor. Angew. Chem. Int. Ed. 2016, 55, 10895–10898. [Google Scholar] [CrossRef]
- Dong, F.; Ni, M.; He, W.; Chen, Y.; Yang, G.; Chen, D.; Shao, Z. An efficient electrocatalyst as cathode material for solid oxide fuel cells: BaFe0.95Sn0.05O2-δ. J. Power Sources 2016, 326, 459–465. [Google Scholar] [CrossRef]
- Rao, Y.; Zhong, S.; He, F.; Wang, Z.; Peng, R.; Lu, Y. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int. J. Hydrog. Energy 2012, 37, 12522–12527. [Google Scholar] [CrossRef]
- Saqib, M.; Lee, J.I.; Shin, J.S.; Park, K.; Kim, Y.D.; Kim, K.B.; Park, J.Y. Modification of oxygen-ionic transport barrier of BaCo0.4Zr0.1Fe0.4Y0.1O3 steam (air) electrode by impregnating samarium-doped ceria nanoparticles for proton-conducting reversible solid oxide cells. J. Electrochem. Soc. 2019, 166, F746–F754. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Yang, L.; Guan, R.; Jiang, J.; Jin, F.; He, T. Ba0.95La0.05Fe0.8Zn0.2O3-δ cobalt-free perovskite as a triple-conducting cathode for proton-conducting solid oxide fuel cells. Ceram. Int. 2020, 46, 18216–18223. [Google Scholar] [CrossRef]
- Qian, B.; Chen, Y.; Shao, Z. BaCo0.6Fe0.3Sn0.1O3-δ perovskite as a new superior oxygen reduction electrode for intermediate-to low temperature solid oxide fuel cells. J. Mater. Chem. A 2014, 2, 15078–15086. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, W.; Zhou, C.; Zhong, Y.; Yang, G.; Zhou, W.; Liu, M.; Shao, Z. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 2019, 3, 2842–2853. [Google Scholar] [CrossRef]
- Yoshihiro, Y.; Raul, H.; Sossina, M.H. Cation non-stoichiometry in yttrium-doped barium zirconate: Phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar]
- Satapathy, A.; Sinha, E. A comparative proton conductivity study on Yb-doped BaZrO3 perovskite at intermediate temperatures under wet N2 environment. J. Alloy. Compd. 2019, 772, 675–682. [Google Scholar] [CrossRef]
- Mao, X.; Wang, W.; Ma, G. A novel cobalt-free double-perovskite NdBaFe1.9Nb0.1O5+δ cathode material for proton-conducting IT-SOFC. Ceram. Int. 2015, 41, 10276–10280. [Google Scholar] [CrossRef]
- Xie, D.; Guo, W.; Guo, R.; Liu, Z.; Sun, D.; Meng, L.; Wang, B. Synthesis and electrochemical properties of BaFe1-xCuxO3-δ perovskite oxide for IT-SOFC cathode. Fuel Cells 2016, 16, 829–838. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X.; Zhang, W.; Xia, C.; Liu, Y.; Yuan, M.; Zhu, B.; Ji, Y. Novel high ionic conductivity electrolyte membrane based on semiconductor La0.65Sr0.3Ce0.05Cr0.5Fe0.5O3-δ for low-temperature solid oxide fuel cells. J. Power Sources 2019, 421, 33–40. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, B.; Wang, Y.; Raza, R.; Tan, W.; Kim, J.; Aken, P.A.; Lund, P. Charge separation and transport in La0.6Sr0.4Co0.2Fe0.8O3-δ and ion-doping ceria heterostructure material for new generation fuel cell. Nano Energy 2017, 37, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Carvajal, J. FULLPROF. A Rietveld refinement and pattern matching analysis program, laboratoire leon Bril-louin (CEA-CNRS), France. 2000. Available online: https://cdifx.univ-rennes1.fr/fps/fp_rennes.pdf (accessed on 7 September 2021).
- Kim, J.; Jun, A.; Shin, J.; Kim, G. Effect of Fe doping on layered GdBa0.5Sr0.5Co2O5+δ perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Am. Ceram. Soc. 2014, 97, 651–656. [Google Scholar] [CrossRef]
- Frontera, P.; Modafferi, V.; Frusteri, F.; Bonura, G.; Bottari, M.; Siracusano, S.; Antonucci, P.L. Catalytic features of Ni/Ba-Ce0.9-Y0.1 catalyst to produce hydrogen for PCFCs by methane reforming. Int. J. Hydrog. Energy 2010, 35, 11661–11668. [Google Scholar] [CrossRef]
- Joo, J.H.; Choi, G.M. Open-circuit voltage of ceria-based thin film SOFC supported on nano-porous alumina. Solid State Ion. 2007, 178, 1602–1607. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Liu, M. Impedance spectroscopy study of an SDC-based SOFC with high open circuit voltage. Electrochim. Acta 2015, 177, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Mi, Y.; Wang, B.; Lin, B.; Chen, G.; Zhu, B. Shaping triple-conducting semiconductor BaCo0.4Fe0.4Zr0.1Y0.1O3-δ into an electrolyte for low-temperature solid oxide fuel cells. Nat. Commun. 2019, 10, 1707. [Google Scholar] [CrossRef]
- Harumi, Y.; Xiong, Y.; Haruo, K.; Katsuhiko, Y. Thermodynamic reconsiderations on electronic properties of pure- and doped-ceria. ECS Trans. 2010, 28, 165–172. [Google Scholar]
- Yin, L.; Li, C.; Dong, S.; Tang, R. Heteroatomic interface engineering in MOF-derived carbon heterostructures with built-in electric-field effects for high performance Al-ion Batteries. Energy Environ. Sci. 2018, 11, 3201–3211. [Google Scholar]
- Li, Z.; Zhu, Z.; Chueh, C.-C.; Jo, S.B.; Luo, J.; Jang, S.-H.; Jen, A.K.Y. Rational design of dipolar chromophore as an efficient dopant-free hole-transporting material for perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 11833–11839. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, D.; Zeng, Q.; Jiang, Y.; Lin, B. Highly promoted performance of triple-conducting cathode for YSZ-based SOFC via fluorine anion doping. Ceram. Int. 2020, 46, 23964–23971. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, H.; Li, W.; Ma, Z.; Ou, X.; Fan, Y.; Guo, Y.; Wang, X.; Ling, N.Y. Numerical study on the electron-blocking effect and optimized operation parameters of ceria-SOFCs with the pure Sm doping CeO2 electrolyte. Int. J. Hydrog. Energy 2021, 13, 13318–13329. [Google Scholar] [CrossRef]
- Ma, G.; Shimura, T.; Iwahara, H. Simultaneous doping with La3+ and Y3+ for Ba2+- and Ce4+- sites in BaCeO3 and the ionic conduction. Solid State Ion. 1999, 120, 51–60. [Google Scholar] [CrossRef]
- He, W.; Wu, X.; Yang, G.; Shi, H.; Dong, F.; Ni, M. BaCo0.7Fe0.22Y0.08O3-δ as an active oxygen reduction electrocatalyst for low-temperature solid oxide fuel cells below 600 °C. ACS Energy Lett. 2017, 2, 301–305. [Google Scholar] [CrossRef]
- Fan, L.; Ma, Y.; Wang, X.; Singh, M.; Zhu, B. Understanding the electrochemical mechanism of the core-shell ceria-LiZnO nanocomposite in a low temperature solid oxide fuel cell. J. Mater. Chem. A 2014, 2, 5399–5407. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Li, D.; Qi, F.; Yu, L.; Xia, C. Effects of P-N and N-N heterostructures and band alignment on the performance of low-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 9790–9798. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef]
- Xu, D.; Li, K.; Zhou, Y.; Gao, Y.; Yan, D.; Xu, S. The effect of NiO addition on the grain boundary behavior and electrochemical performance of Gd-doped ceria solid electrolyte under different sintering conditions. J. Eur. Ceram. Soc. 2017, 37, 419–425. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, D.P.; Raza, R.; Ma, Y.; Fan, L.; Afzal, M.; Patakangas, J.; He, Y.; Zhao, Y.; Tan, W.; et al. Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv. Energy Mater. 2015, 5, 1401895. [Google Scholar] [CrossRef]
- Hong, T.; Brinkman, K.S.; Xia, C. Barium carbonate nanoparticles as synergistic catalysts for the oxygen reduction reaction on La0.6Sr0.4Co0.2Fe0.8O3¢d solid-oxide fuel cell cathodes. ChemElectroChem 2016, 3, 805–813. [Google Scholar] [CrossRef]
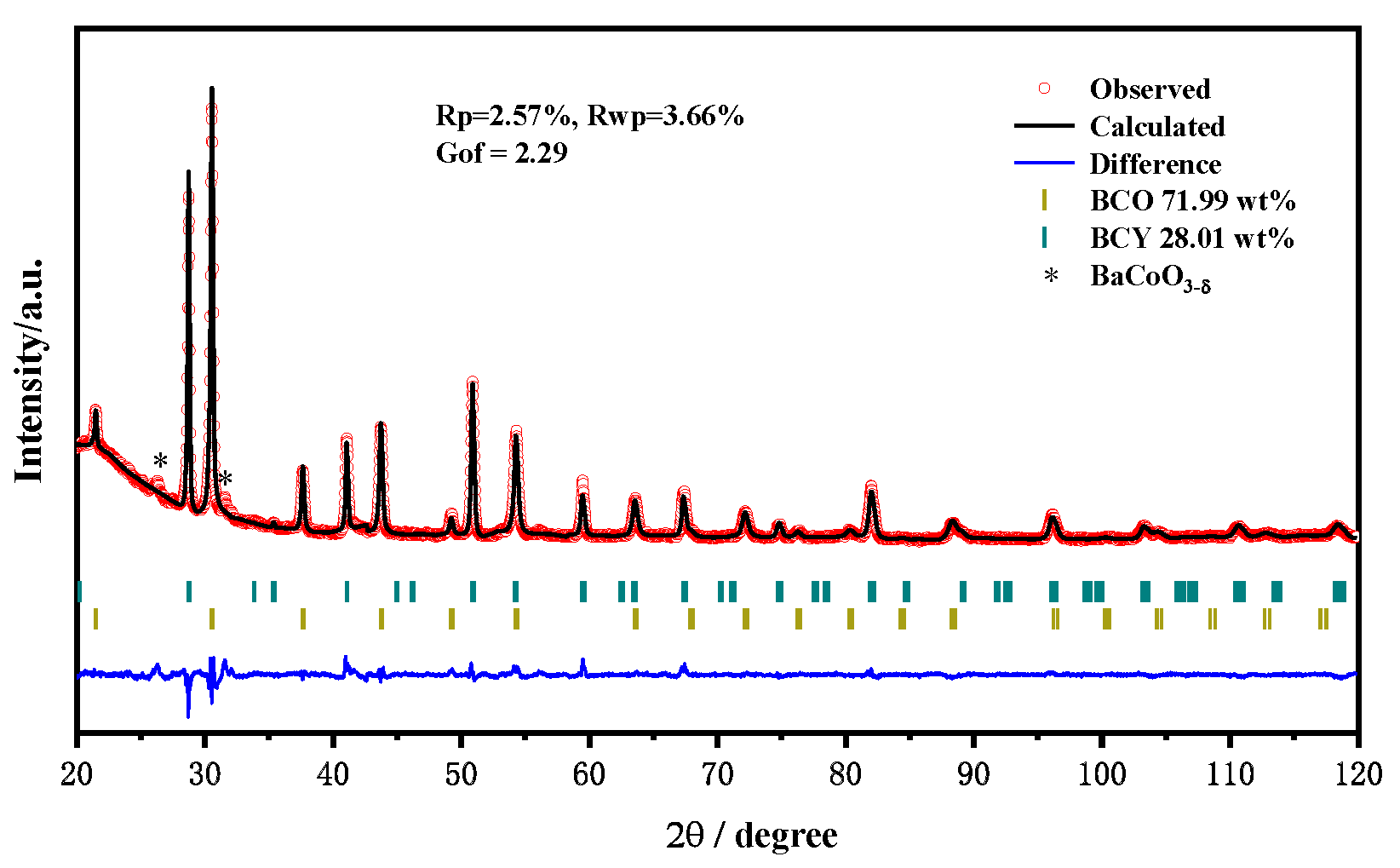




| Phase | a (Å) | b (Å) | c (Å) | Volume (Å3) |
|---|---|---|---|---|
| BaCe0.78Y0.22O3-δ | 6.21239 (14) | 6.21239 (14) | 15.2452 (7) | 509.543 (3) |
| BaCo0.9Ce0.01Y0.09O3-δ | 4.13906 (4) | 4.13906 (4) | 4.13906 (4) | 70.9096 (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yan, A.; Xu, S.; Zhou, Y.; Yang, S.; Zhang, R.; Yang, X.; Lu, Y. Self-Assembled Triple (H+/O2−/e−) Conducting Nanocomposite of Ba-Co-Ce-Y-O into an Electrolyte for Semiconductor Ionic Fuel Cells. Nanomaterials 2021, 11, 2365. https://doi.org/10.3390/nano11092365
Xu D, Yan A, Xu S, Zhou Y, Yang S, Zhang R, Yang X, Lu Y. Self-Assembled Triple (H+/O2−/e−) Conducting Nanocomposite of Ba-Co-Ce-Y-O into an Electrolyte for Semiconductor Ionic Fuel Cells. Nanomaterials. 2021; 11(9):2365. https://doi.org/10.3390/nano11092365
Chicago/Turabian StyleXu, Dan, An Yan, Shifeng Xu, Yongjun Zhou, Shu Yang, Rongyu Zhang, Xu Yang, and Yuzheng Lu. 2021. "Self-Assembled Triple (H+/O2−/e−) Conducting Nanocomposite of Ba-Co-Ce-Y-O into an Electrolyte for Semiconductor Ionic Fuel Cells" Nanomaterials 11, no. 9: 2365. https://doi.org/10.3390/nano11092365





