Ionic Liquid-Based Materials for Biomedical Applications
Abstract
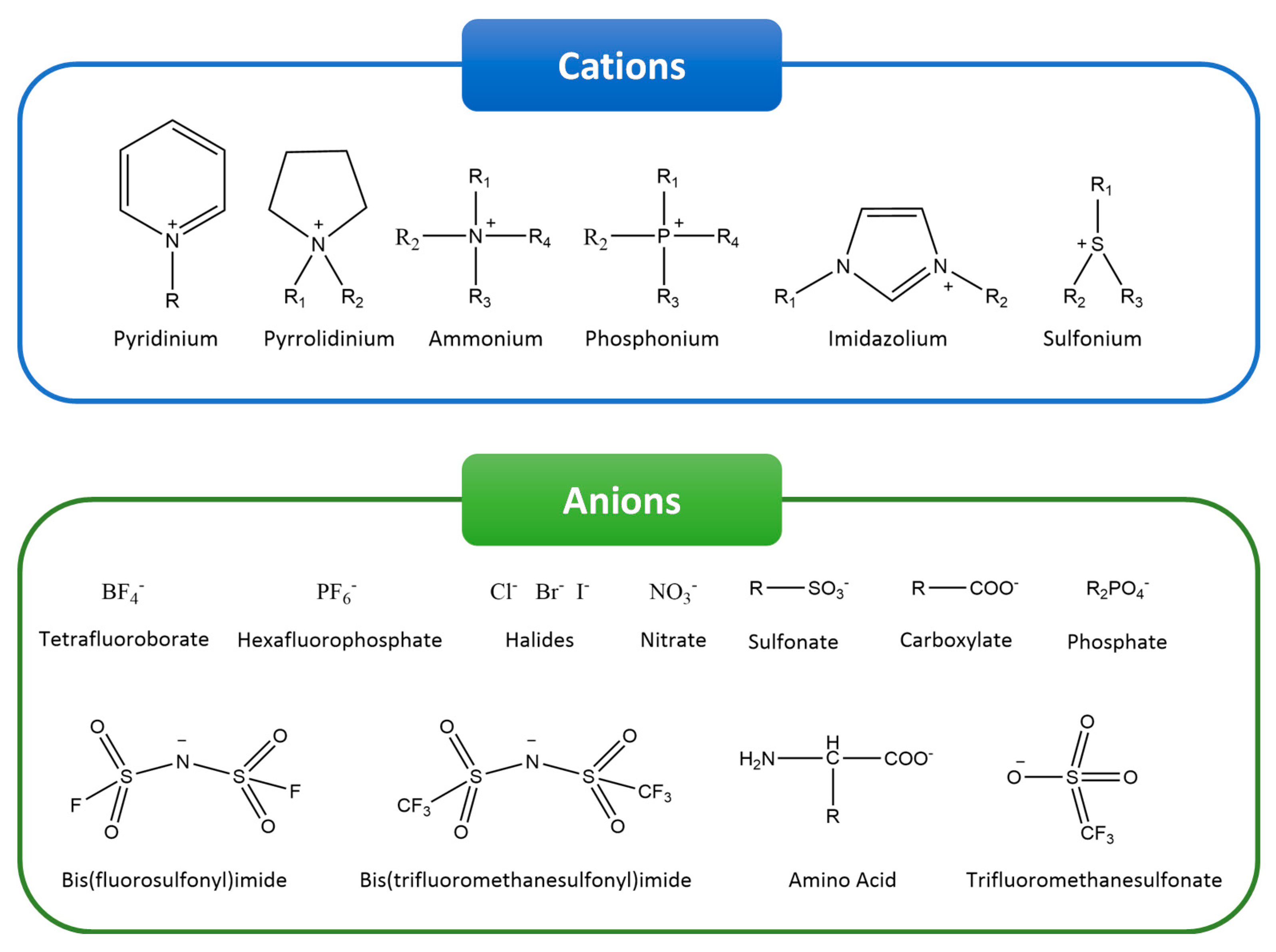
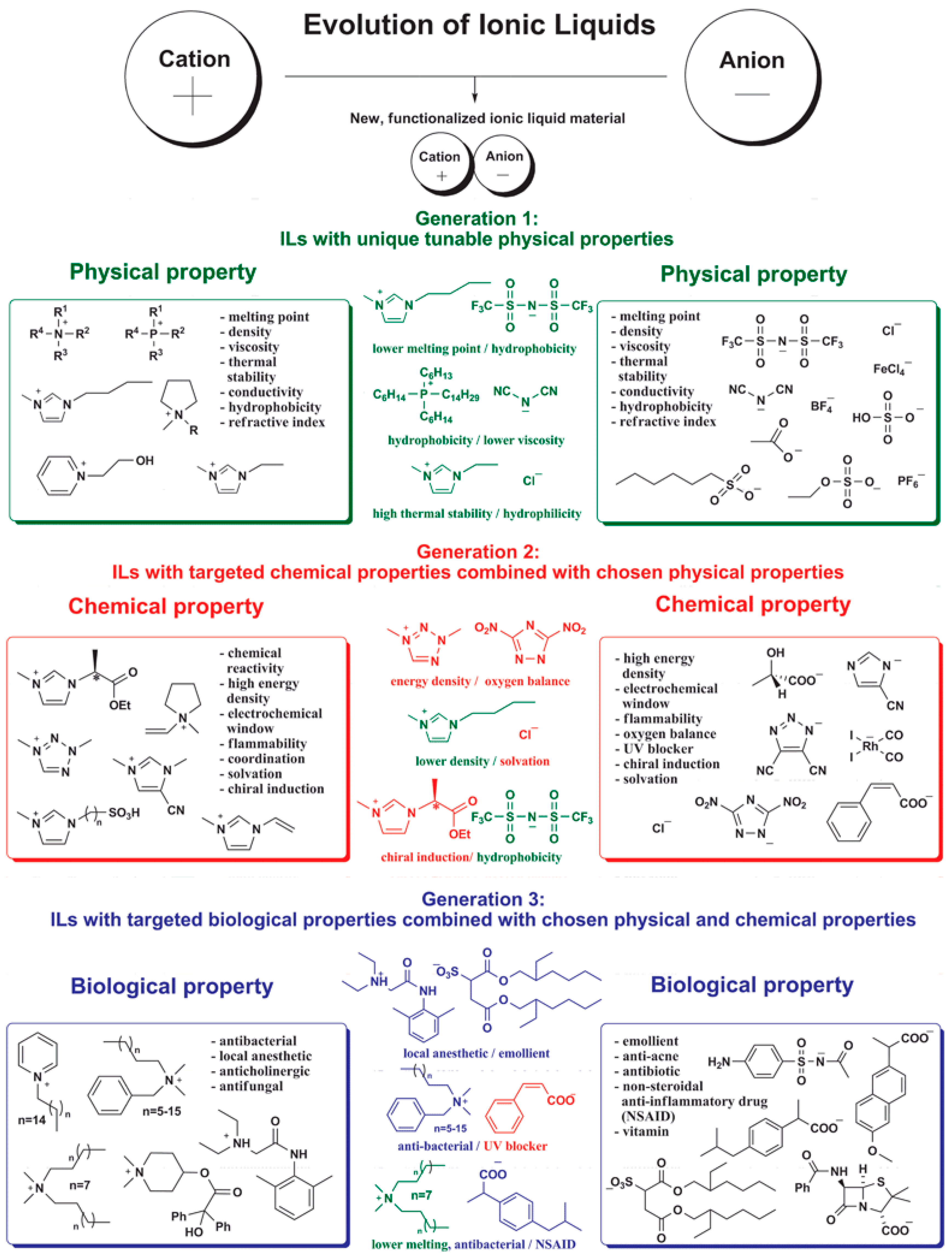
:1. Introduction
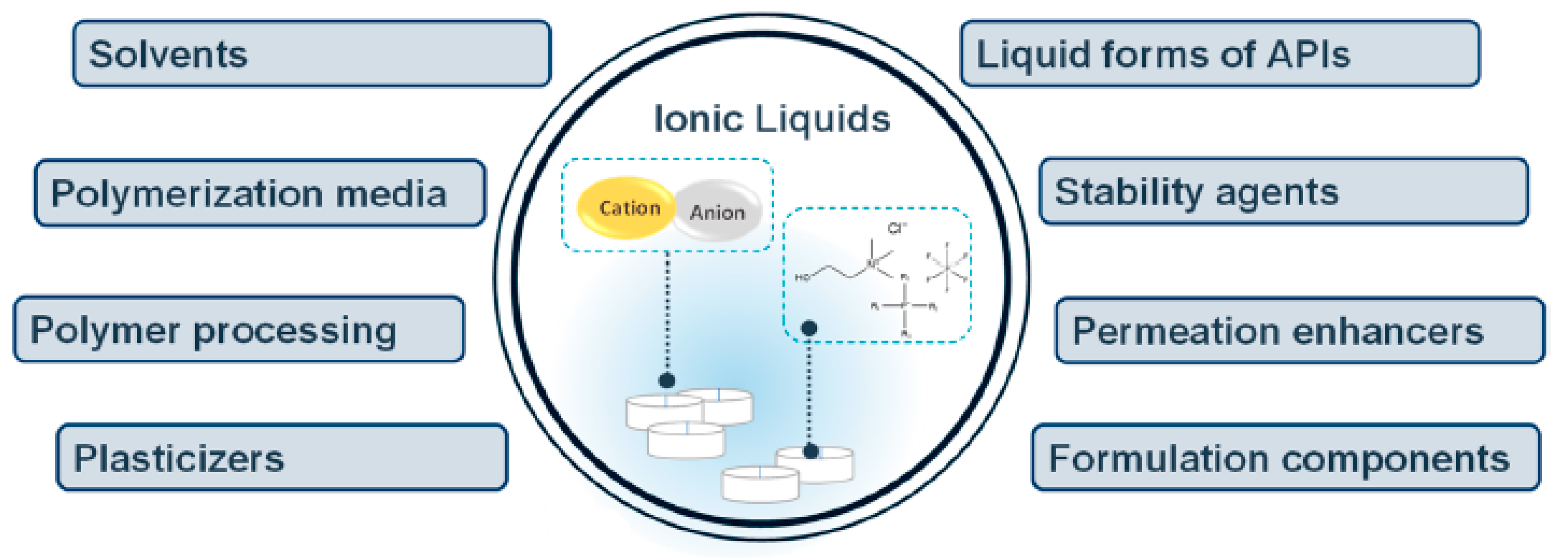
1.1. Ionic Liquids for Biomedical Applications
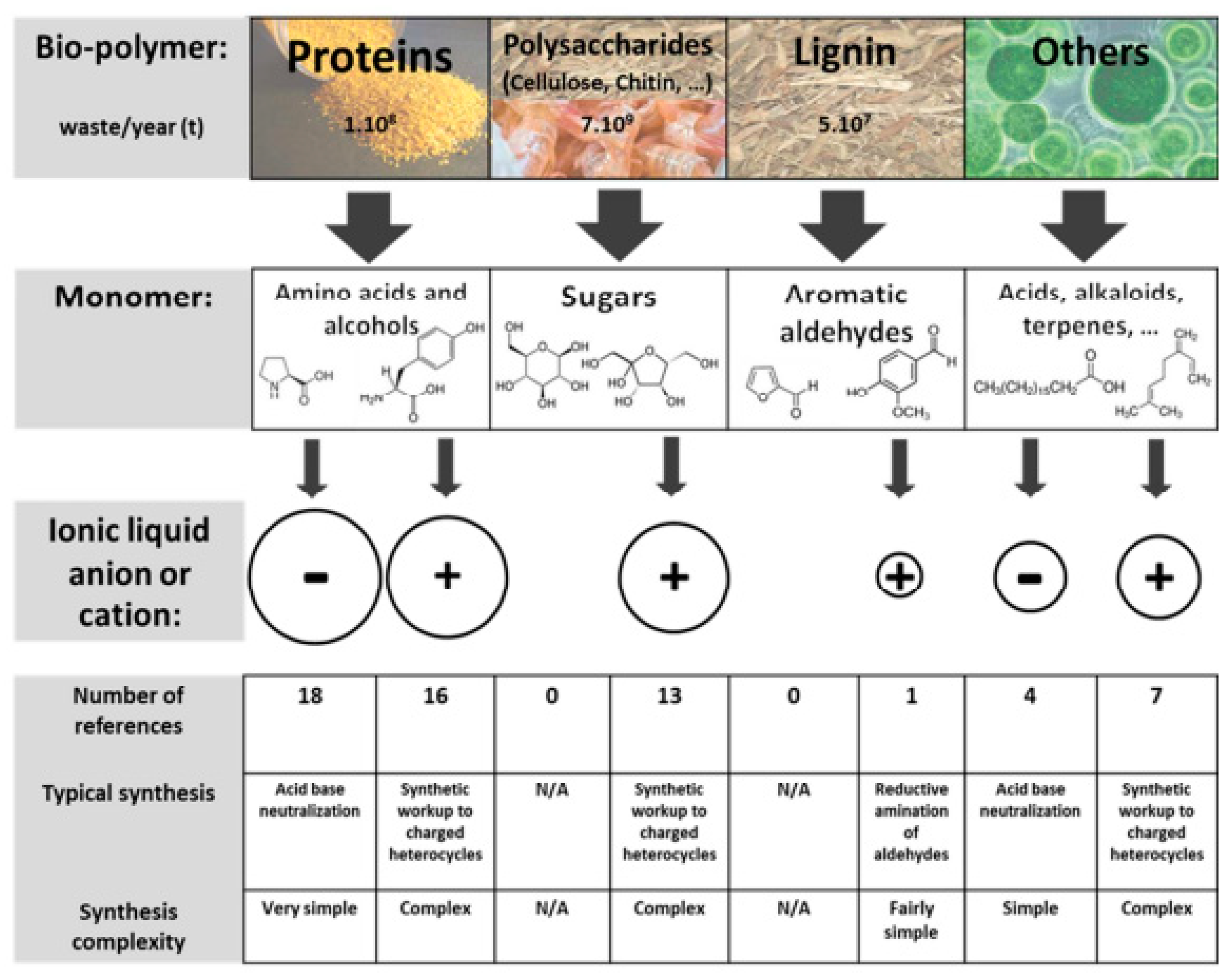
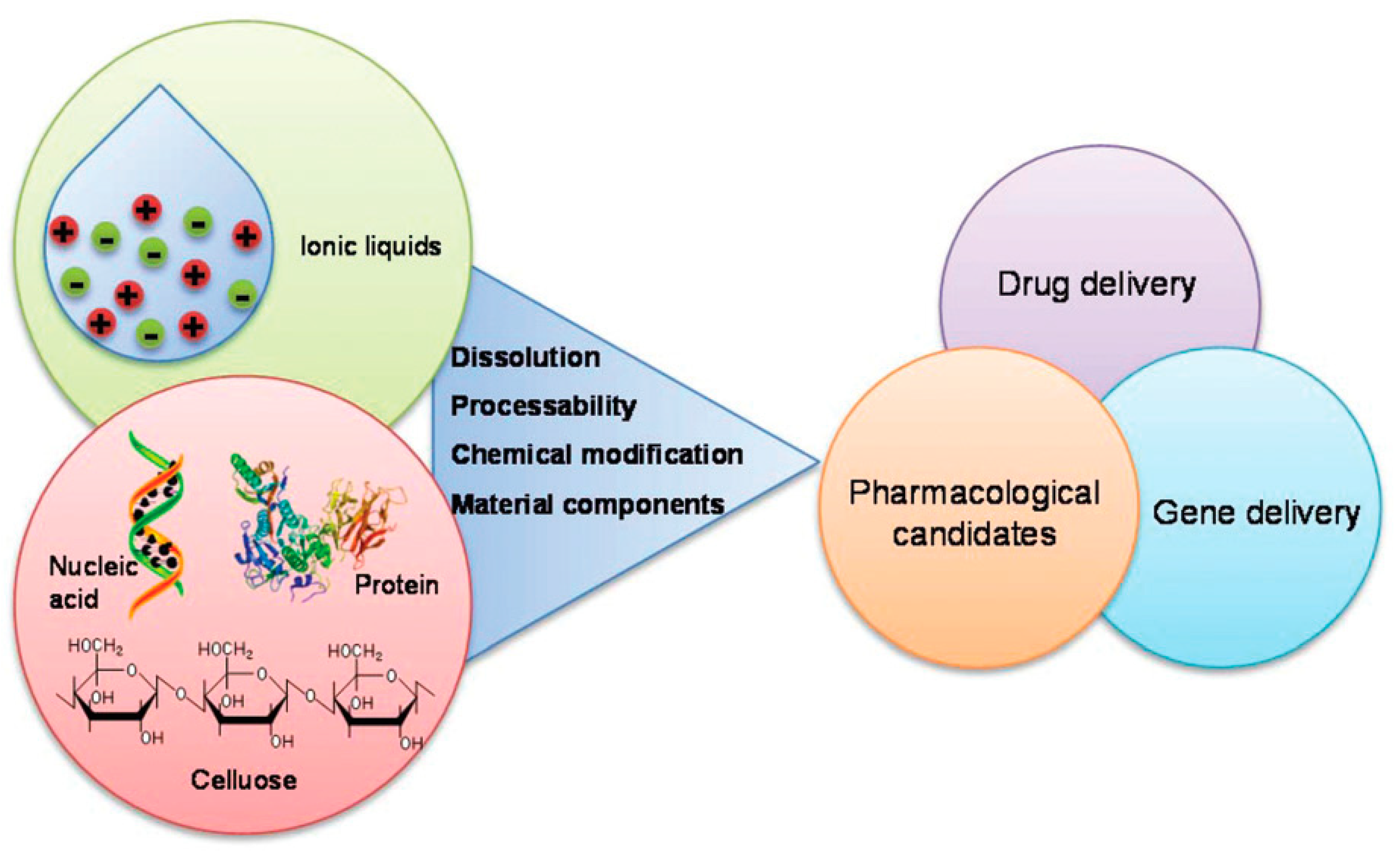
1.2. Ionic Liquids for the Development of Biomaterials
1.2.1. Dissolution
1.2.2. Polymer Regeneration
1.2.3. Preparation of Ionic-Liquid-Based Hybrid Materials
2. Biomedical Applications of Ionic Liquids-Based Materials
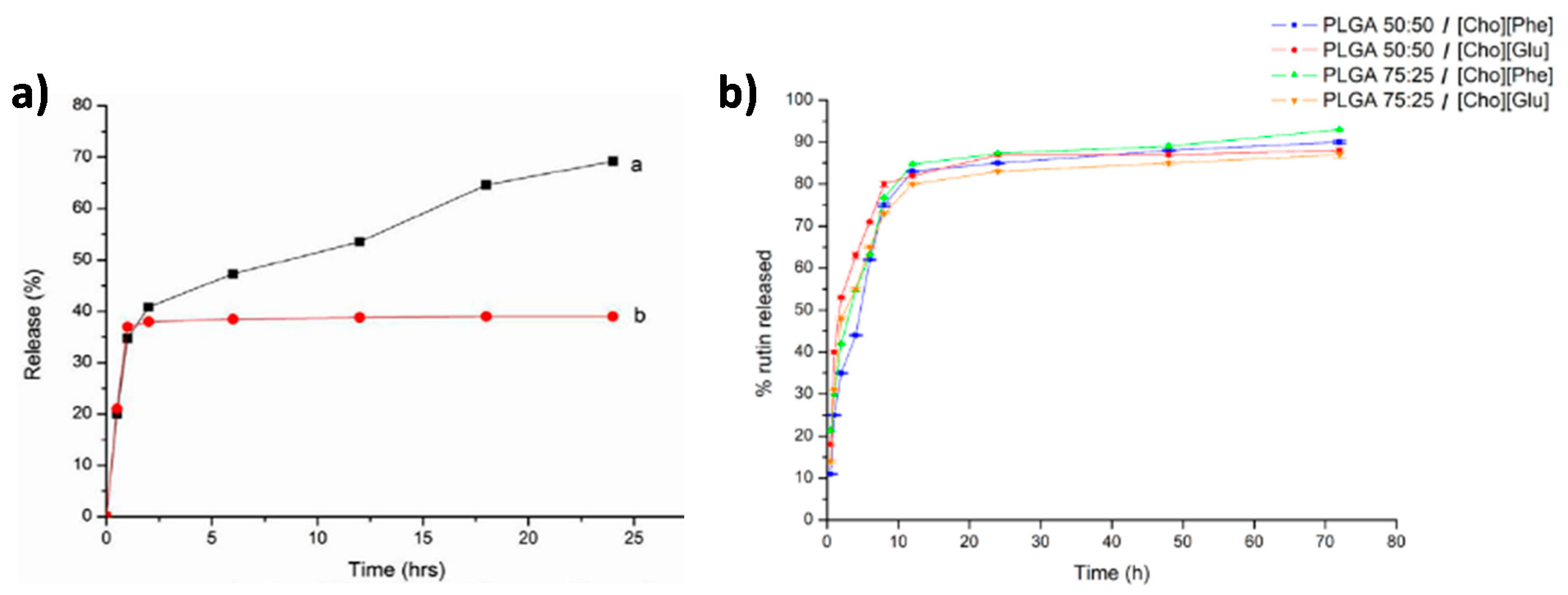
2.1. Drug Delivery
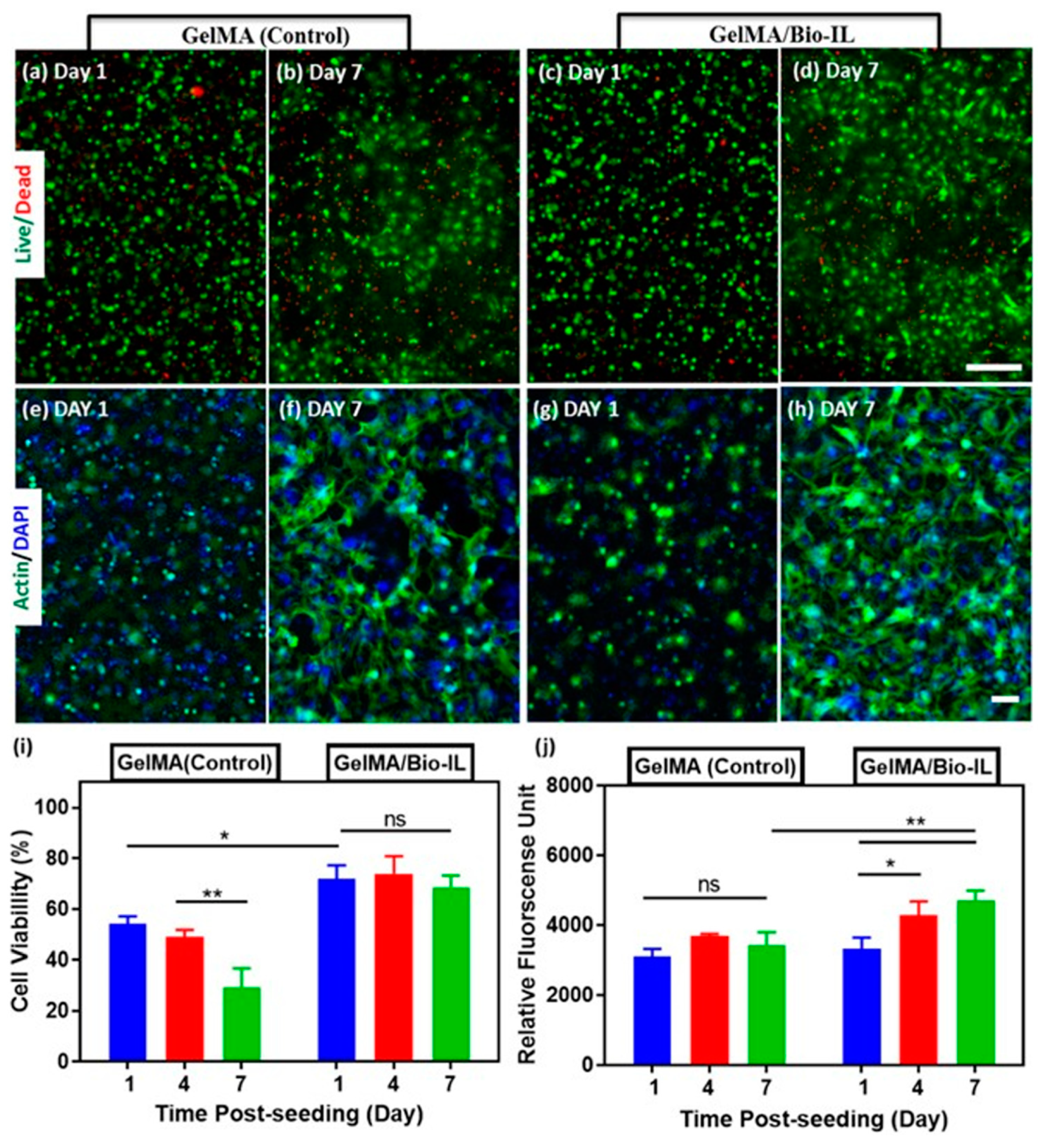
2.2. Tissue Engineering
2.3. Cancer Therapy
2.4. Antimicrobial Agents
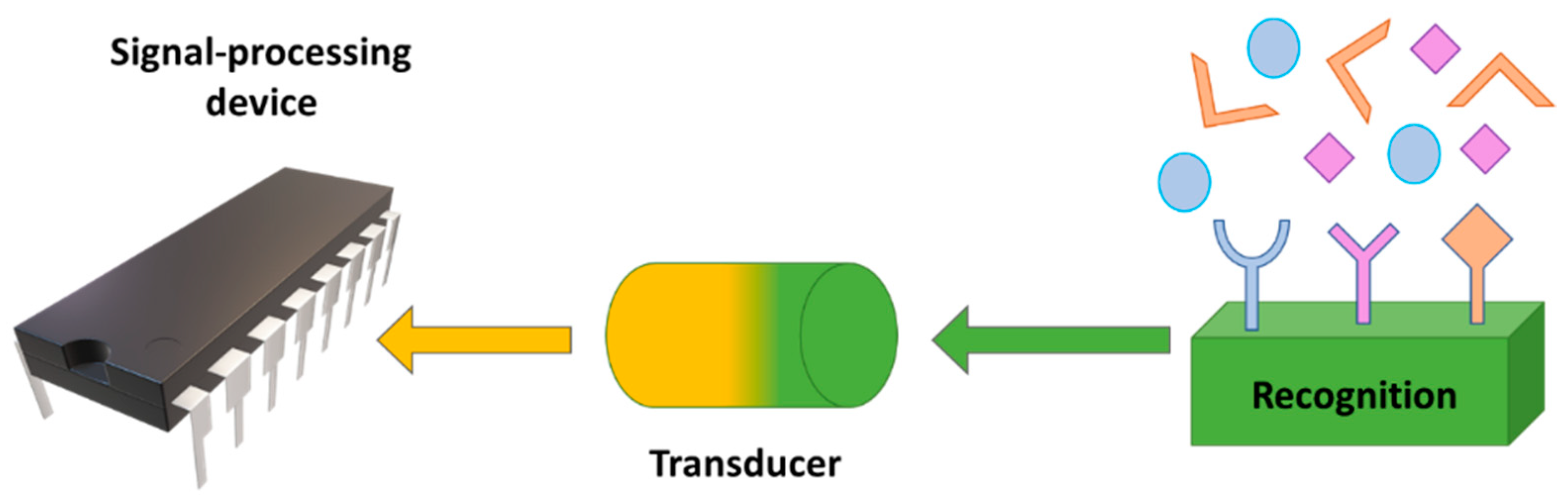
2.5. Biosensors and Biomedical Sensors
3. Main Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ionic Liquid | Name |
| [Amim][Br] | 1-allyl-3-methylimidazolium bromide |
| [Amim][Cl] | 1-n-allyl-3-methylimidazolium chloride |
| [Ch][Hex] | choline hexanoate |
| [Ch][Cit] | choline citrate |
| [Ch][DHP] | 2-hydroxyethyl-trimethylammonium dihydrogen phosphate |
| [Ch][Cl] | choline chloride |
| [Cho][Phe] | 2-hydroxyethyl)-trimethylammonium-L-phenylalaninate |
| [Cho][Glu] | 2-hydroxyethyl)-trimethylammonium-L-glutaminate |
| [Ch][MA] | cholinium malonate |
| [C2OHmim][Cl] | 1-(2-Hydroxyethyl)-3-methyl imidazolium chloride |
| [Chol][HSO4] | choline hydrogen sulphate |
| [Emim][OH] | 1-ethyl-3-methylimidazolium hydroxide |
| [Emim][Ac] | 1-ethyl-3-methylimidazolium acetate |
| [Emim][Cl] | 1-ethyl-3 imidazolium chlorate |
| [Emim][TFSI]/[C2mim][NTf2] | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [Emim][PF6] | 1-ethyl-3-methylimidazolium hexafluorophosphate |
| [Emim] [BF4] | 1-ethyl-3-methylimidazolium tetrafluoroborate |
| [Bmim][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [Bmim][Ac] | 1-butyl-3-methylimidazolium acetate |
| [Bmim][OAc] | 1-butyl-imidazolium acetate |
| [Bmim][Cl] | 1-butyl-3-methylimidazolium chloridev |
| [Bmim][HSO4] | 1-butyl-3-methylimidazolium hydrogen sulphate |
| [Bmim]2[NiCl4] | bis(1-butyl-3-methylimidazolium) tetrachloronickelate |
| [Bmim][TFSI] | 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [MIM][Cl] | 1-methylimidazolium chloride |
| [Hmim][HSO4] | 1-methylimidazolium hydrogen sulphate |
| [P66614][HBO] | trihexyl(tetradecyl) phosphonium 2-(2′-hydroxyphenyl) benzoxazole |
| [TEA][A] | triethanolamine acetate |
| [TEA][SO4H] | triethylammonium hydrogen sulfate |
| [TEA][PO4H] | triethylammonium hydrogen phosphate |
| [VAPim][BF4] | 1-vinyl-3-(3-aminopropyl)-imidazolium tetrafluoroborate |
| [VHim][NTf2] | 1-vinyl-3-hexylimidazolium bis(trifluoromethanesulfonyl)imide |
| [VEIm][DCA] | 1-vinyl-3-ethylimidazolium dicyanamide |
References
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Cruz, C.; Ciach, A. Phase Transitions and Electrochemical Properties of Ionic Liquids and Ionic Liquid—Solvent Mixtures. Molecules 2021, 26, 3668. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2012, 58, 2885–2899. [Google Scholar] [CrossRef]
- Yan, F.; Xia, S.; Wang, Q.; Yang, Z.; Ma, P. Predicting the melting points of ionic liquids by the Quantitative Structure Property Relationship method using a topological index. J. Chem. Thermodyn. 2013, 62, 196–200. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Dong, J.; Zhang, G.; Wang, C. A novel family of green ionic liquids with surface activities. Sci. China Ser. B Chem. 2007, 50, 238–242. [Google Scholar] [CrossRef]
- Soltanmohammadi, F.; Jouyban, A.; Shayanfar, A. New aspects of deep eutectic solvents: Extraction, pharmaceutical applications, as catalyst and gas capture. Chem. Pap. 2021, 75, 439–453. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800, 405–422. [Google Scholar]
- Silva, W.; Zanatta, M.; Ferreira, A.S.; Corvo, M.C.; Cabrita, E.J. Revisiting Ionic Liquid Structure-Property Relationship: A Critical Analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Atilhan, M. Mixed Ionic Liquids: The Case of Pyridinium-Based Fluids. J. Phys. Chem. B 2012, 116, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, K.A.; Sintra, T.E.; Neves, C.M.; Shimizu, K.; Canongia Lopes, J.N.; Gonçalves, F.; Ventura, S.P.; Freire, M.G.; Santos, L.M.; Coutinho, J.A. The effect of the cation alkyl chain branching on mutual solubilities with water and toxicities. Phys. Chem. Chem. Phys. 2014, 16, 19952–19963. [Google Scholar] [CrossRef] [PubMed]
- Dani, U.; Bahadur, A.; Kuperkar, K. Validating interfacial behaviour of surface-active ionic liquids (SAILs) with computational study integrated with biocidal and cytotoxic assessment. Ecotoxicol. Environ. Saf. 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.M.; Gonçalves, F.; Coutinho, J.A.P. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef]
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3- methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Thiele, K.; Juffernholz, T.; Arning, J.; Ranke, J.; Welz-Biermann, U.; Jastorff, B. The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco)toxicological test battery. Green Chem. 2007, 9, 1198–1207. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, Y.; Zhang, Z.D. Toxicity of ionic liquids. Clean Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased Ionic Liquids: Solvents for a Green Processing Industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Wang, X.; Ohlin, C.A.; Lu, Q.; Fei, Z.; Hu, J.; Dyson, P.J. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007, 9, 1191–1197. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Santos de Almeida, T.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef]
- Mikkola, S.-K.; Robciuc, A.; Lokajová, J.; Holding, A.J.; Lämmerhofer, M.; Kilpeläinen, I.; Holopainen, J.M.; King, A.W.T.; Wiedmer, S.K. Impact of Amphiphilic Biomass-Dissolving Ionic Liquids on Biological Cells and Liposomes. Environ. Sci. Technol. 2015, 49, 1870–1878. [Google Scholar] [CrossRef]
- Bachowska, B.; Kazmierczak-Baranska, J.; Cieslak, M.; Nawrot, B.; Szczęsna, D.; Skalik, J.; Bałczewski, P. High Cytotoxic Activity of Phosphonium Salts and Their Complementary Selectivity towards HeLa and K562 Cancer Cells: Identification of Tri-n-butyl-n-hexadecylphosphonium bromide as a Highly Potent Anti-HeLa Phosphonium Salt. ChemistryOpen 2012, 1, 33–38. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Fukumoto, K.; Ohno, H. Design and synthesis of hydrophobic and chiral anions from amino acids as precursor for functional ionic liquids. Chem. Commun. 2006, 3081–3083. [Google Scholar] [CrossRef]
- Tao, G.H.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun. 2005, 3562–3564. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Handy, S.T.; Okello, M.; Dickinson, G. Solvents from biorenewable sources—Ionic liquids based on fructose. Org. Lett. 2003, 5, 2513, Erratum in 2004, 6, 4137. [Google Scholar] [CrossRef]
- Kumar, V.; Pei, C.; Olsen, C.E.; Schäffer, S.J.C.; Parmar, V.S.; Malhotra, S.V. Novel carbohydrate-based chiral ammonium ionic liquids derived from isomannide. Tetrahedron Asymmetry 2008, 19, 664–671. [Google Scholar] [CrossRef]
- Jha, A.K.; Jain, N. Synthesis of glucose-tagged triazolium ionic liquids and their application as solvent and ligand for copper(I) catalyzed amination. Tetrahedron Lett. 2013, 54, 4738–4741. [Google Scholar] [CrossRef]
- Chiappe, C.; Marra, A.; Mele, A. Synthesis and applications of ionic liquids derived from natural sugars. Top. Curr. Chem. 2010, 295, 177–195. [Google Scholar] [CrossRef]
- Poletti, L.; Chiappe, C.; Lay, L.; Pieraccini, D.; Polito, L.; Russo, G. Glucose-derived ionic liquids: Exploring low-cost sources for novel chiral solvents. Green Chem. 2007, 9, 337–341. [Google Scholar] [CrossRef]
- Huang, L.; Zhai, M.; Peng, J.; Xu, L.; Li, J.; Wei, G. Synthesis of room temperature ionic liquids from carboxymethylated chitosan. Carbohydr. Pol. 2008, 71, 690–693. [Google Scholar] [CrossRef]
- Socha, A.M.; Parthasarathi, R.; Shi, J.; Pattathil, S.; Whyte, D.; Bergeron, M.; George, A.; Tran, K.; Stavila, V.; Venkatachalam, S.; et al. Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc. Natl. Acad. Sci. USA 2014, 111, E3587–E3595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadilohar, B.L.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Zeisel, S.H. A brief history of choline. Ann. Nutr. Metab. 2012, 61, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.I.; Gonçalves, A.M.M.; Pereira, J.L.; Figueiredo, B.F.H.T.; Silva, F.A.E.; Coutinho, J.A.P.; Ventura, S.P.M.; Gonçalves, F. Environmental safety of cholinium-based ionic liquids: Assessing structure-ecotoxicity relationships. Green Chem. 2015, 17, 4657–4668. [Google Scholar] [CrossRef]
- Hou, X.D.; Liu, Q.P.; Smith, T.J.; Li, N.; Zong, M.H. Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Dean, P.M.; Turanjanin, J.; Yoshizawa-Fujita, M.; MacFarlane, D.R.; Scott, J.L. Exploring an anti-crystal engineering approach to the preparation of pharmaceutically active ionic liquids. Cryst. Growth Des. 2009, 9, 1137–1145. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araújo, J.M.M.; Rebelo, L.P.N.; Da Ponte, M.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, E. Development of novel ionic liquids based on ampicillin. MedChemComm 2012, 3, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Araújo, J.M.M.; Florindo, C.; Pereiro, A.B.; Vieira, N.S.M.; Matias, A.A.; Duarte, C.M.M.; Rebelo, L.P.N.; Marrucho, I.M. Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv. 2014, 4, 28126–28132. [Google Scholar] [CrossRef]
- Taha, M.; Silva, F.A.E.; Quental, M.V.; Ventura, S.P.M.; Freire, M.G.; Coutinho, J.A.P. Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014, 16, 3149–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Singh, R.M.M. Hydrogen Ion Buffers for Biological Research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Almeida, M.R.; Silva, F.A.E.; Domingues, P.; Ventura, S.P.M.; Coutinho, J.A.P.; Freire, M.G. Novel biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem. Eur. J. 2015, 21, 4781–4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, S.C.; Rocha, A.; Teixeira, R.; Fonseca, L.J.P.; Lourenço, N.M.T. Synthesis of choline sulfonate buffers and their effect on cytochrome c dissolution and oxidation state. RSC Adv. 2014, 4, 15597–15601. [Google Scholar] [CrossRef]
- Taha, M.; Quental, M.V.; Correia, I.; Freire, M.G.; Coutinho, J.A.P. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good’s buffers ionic liquids. Process Biochem. 2015, 50, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic liquids derived from esters of Glycine Betaine: Synthesis and characterization. J. Mol. Liq. 2015, 207, 60–66. [Google Scholar] [CrossRef]
- De Gaetano, Y.; Hubert, J.; Mohamadou, A.; Boudesocque, S.; Plantier-Royon, R.; Renault, J.H.; Dupont, L. Removal of pesticides from wastewater by ion pair Centrifugal Partition Extraction using betaine-derived ionic liquids as extractants. Chem. Eng. J. 2016, 285, 596–604. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Hao, J.W.; Mo, L.P.; Zhang, Z.H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Canongia Lopes, J.N.A.; Pádua, A.A.H. Nanostructural Organization in Ionic Liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef]
- Schlosser, Š.; Marták, J.; Blahušiak, M. Specific phenomena in carboxylic acids extraction by selected types of hydrophobic ionic liquids. Chem. Pap. 2018, 72, 567–584. [Google Scholar] [CrossRef]
- Egorova, K.S.; Posvyatenko, A.V.; Fakhrutdinov, A.N.; Kashin, A.S.; Ananikov, V.P. Assessing possible influence of structuring effects in solution on cytotoxicity of ionic liquid systems. J. Mol. Liq. 2020, 297, 111751. [Google Scholar] [CrossRef]
- Passos, H.; Trindade, M.P.; Vaz, T.S.M.; da Costa, L.P.; Freire, M.G.; Coutinho, J.A.P. The impact of self-aggregation on the extraction of biomolecules in ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2013, 108, 174–180. [Google Scholar] [CrossRef]
- Wang, R.; Leng, W.; Gao, Y.; Yu, L. Microemulsion-like aggregation behaviour of an LCST-type ionic liquid in water. RSC Adv. 2014, 4, 14055–14062. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.; Li, X.; Chen, L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Welton, T. Ionic liquids assisted processing of renewable resources for the fabrication of biodegradable composite materials. Green Chem. 2017, 19, 2051–2075. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Advances in Processing Chitin as a Promising Biomaterial from Ionic Liquids. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 177–198. [Google Scholar]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Comm. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Shirzaei Sani, E.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Oh, K.K.; Lee, S.H. Biopolymer-Based Composite Materials Prepared Using Ionic Liquids. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 133–176. [Google Scholar]
- Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Dissolution and regeneration of silk from silkworm Bombyx mori in ionic liquids and its application to medical biomaterials. Int. J. Biol. Macromol. 2020, 143, 594–601. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Heckel, T.; Konieczna, D.; Wilhelm, R. An Ionic Liquid Solution of Chitosan as Organocatalyst. Catalysts 2013, 3, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Tomé, L.C.; Silva, N.H.C.S.; Soares, H.R.; Coroadinha, A.S.; Sadocco, P.; Marrucho, I.M.; Freire, C.S.R. Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem. 2015, 17, 4291–4299. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Kollau, L.J.B.M.; van den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Huang, H. Modified pineapple peel cellulose hydrogels embedded with sepia ink for effective removal of methylene blue. Carbohydr. Polym. 2016, 148, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Meng, L.; Zhang, X.; Fu, C.; Lu, Q. The ionic liquid-associated synthesis of a cellulose/SWCNT complex and its remarkable biocompatibility. J. Mater. Chem. 2009, 19, 3612–3617. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Oliveira, M.B.; Nayak, S.; Subia, B.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Silk hydrogels from non-mulberry and mulberry silkworm cocoons processed with ionic liquids. Acta Biomater. 2013, 9, 8972–8982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantz, R.; Fox, D.; Marshall, J.; Green Iii, J.M.; Fylstra, P.; De Long, H.; Trulove, P. Dissolution of Biopolymers Using Ionic Liquids. Z. Für Nat. A 2007, 62. [Google Scholar] [CrossRef]
- Kim, M.H.; An, S.; Won, K.; Kim, H.J.; Lee, S.H. Entrapment of enzymes into cellulose–biopolymer composite hydrogel beads using biocompatible ionic liquid. J. Mol. Catal. B Enzym. 2012, 75, 68–72. [Google Scholar] [CrossRef]
- Ghasemi, M.; Tsianou, M.; Alexandridis, P. Assessment of solvents for cellulose dissolution. Bioresour. Technol. 2017, 228, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Barber, P.S.; Griggs, C.S.; Bonner, J.R.; Rogers, R.D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013, 15, 601–607. [Google Scholar] [CrossRef]
- Zadegan, S.; Hossainalipour, M.; Ghassai, H.; Rezaie, H.R.; Naimi-Jamal, M.R. Synthesis of cellulose–nanohydroxyapatite composite in 1-n-butyl-3-methylimidazolium chloride. Ceram. Int. 2010, 36, 2375–2381. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z. Physico-chemical processes in imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Sciarini, L.S.; Rolland-Sabaté, A.; Guilois, S.; Decaen, P.; Leroy, E.; Le Bail, P. Understanding the destructuration of starch in water–ionic liquid mixtures. Green Chem. 2015, 17, 291–299. [Google Scholar] [CrossRef]
- Mundsinger, K.; Müller, A.; Beyer, R.; Hermanutz, F.; Buchmeiser, M.R. Multifilament cellulose/chitin blend yarn spun from ionic liquids. Carbohydr. Polym. 2015, 131, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, K.; Kärkkäinen, J.; Lajunen, M. Dissolution and depolymerization of barley starch in selected ionic liquids. Carbohydr. Polym. 2013, 93, 89–94. [Google Scholar] [CrossRef]
- Komulainen, S.; Verlackt, C.; Pursiainen, J.; Lajunen, M. Oxidation and degradation of native wheat starch by acidic bromate in water at room temperature. Carbohydr. Polym. 2013, 93, 73–80. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and resourcfulization of biopolymers in ionic liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Ahn, Y.; Kang, Y.; Park, B.; Ku, M.K.; Lee, S.H.; Kim, H. Influence of lignin on rheological behaviors and electrospinning of polysaccharide solution. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Elhi, F.; Aid, T.; Koel, M. Ionic liquids as solvents for making composite materials from cellulose. Proc. Est. Acad. Sci. 2016, 65, 255. [Google Scholar] [CrossRef]
- Muginova, S.V.; Myasnikova, D.A.; Kazarian, S.G.; Shekhovtsova, T.N. Evaluation of novel applications of cellulose hydrogel films reconstituted from acetate and chloride of 1-butyl-3-methylimidazolium by comparing their optical, mechanical, and adsorption properties. Mater. Today Commun. 2016, 8, 108–117. [Google Scholar] [CrossRef]
- Johns, M.A.; Bernardes, A.; De Azevêdo, E.R.; Guimarães, F.E.G.; Lowe, J.P.; Gale, E.M.; Polikarpov, I.; Scott, J.L.; Sharma, R.I. On the subtle tuneability of cellulose hydrogels: Implications for binding of biomolecules demonstrated for CBM 1. J. Mater. Chem. B 2017, 5, 3879–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; De Long, H.C.; Mantz, R.A. Dissolution and Regeneration of Bombyx mori Silk Fibroin Using Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Shinohara, Y.; Takizawa, J.; Ren, S.; Sagisaka, K.; Lin, Y.; Hattori, Y.; Hinestroza, J.P. Versatile Molding Process for Tough Cellulose Hydrogel Materials. Sci. Rep. 2015, 5, 16266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendaoud, A.; Kehrbusch, R.; Baranov, A.; Duchemin, B.; Maigret, J.E.; Falourd, X.; Staiger, M.P.; Cathala, B.; Lourdin, D.; Leroy, E. Nanostructured cellulose-xyloglucan blends via ionic liquid/water processing. Carbohydr. Polym. 2017, 168, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.H.; Kim, J.H.; Park, S.; Kim, H.; Won, K.; Lee, S.H. Preparation of artificial wood films with controlled biodegradability. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lee, S.H.; Miyauchi, M.; Dordick, J.S.; Linhardt, R.J. Preparation of Biopolymer-Based Materials Using Ionic Liquids for the Biomedical Application. In Ionic Liquid Applications: Pharmaceuticals, Therapeutics, and Biotechnology. ACS Symp. Ser. Am. Chem. Soc. 2010, 1038, 115–134. [Google Scholar]
- Xie, H.; Li, S.; Zhang, S. Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers. Green Chem. 2005, 7, 606–608. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, Q.; Wu, Y.; Wu, T.; Dai, L. Dissolution and blending of chitosan using 1,3-dimethylimidazolium chloride and 1-H-3-methylimidazolium chloride binary ionic liquid solvent. Carbohydr. Polym. 2011, 83, 233–238. [Google Scholar] [CrossRef]
- Ahn, Y.; Hu, D.-H.; Hong, J.H.; Lee, S.H.; Kim, H.J.; Kim, H. Effect of co-solvent on the spinnability and properties of electrospun cellulose nanofiber. Carbohydr. Polym. 2012, 89, 340–345. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Won, K.; Choi, J.W.; Kim, Y.H.; Kim, H.J.; Yang, Y.-H.; Lee, S.H. Wood mimetic hydrogel beads for enzyme immobilization. Carbohydr. Polym. 2015, 115, 223–229. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous Magnetic Cellulose–Chitosan Composite Microspheres: Preparation, Characterization, and Application for Cu(II) Adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Wu, R.-L.; Wang, X.-L.; Li, F.; Li, H.-Z.; Wang, Y.-Z. Green composite films prepared from cellulose, starch and lignin in room-temperature ionic liquid. Bioresour. Technol. 2009, 100, 2569–2574. [Google Scholar] [CrossRef]
- Chew, S.A.; Hinojosa, V.A.; Arriaga, M.A. 11-Bioresorbable polymer microparticles in the medical and pharmaceutical fields. In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 229–264. [Google Scholar]
- Julio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos de Almeida, T.; Fonte, P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Meira, R.M.; Correia, D.M.; Ribeiro, S.; Costa, P.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S.; Ribeiro, C. Ionic-Liquid-Based Electroactive Polymer Composites for Muscle Tissue Engineering. ACS Appl. Polym. Mater. 2019, 1, 2649–2658. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.M.A.; Cortez, A.R.; Barsan, M.M.; Santos, J.B.; Brett, C.M.A.; de Sousa, H.C. Development of Greener Multi-Responsive Chitosan Biomaterials Doped with Biocompatible Ammonium Ionic Liquids. ACS Sustain. Chem. Eng. 2013, 1, 1480–1492. [Google Scholar] [CrossRef]
- Dias, J.C.; Correia, D.C.; Lopes, A.C.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Esperança, J.M.S.S.; Laza, J.M.; Vilas, J.L.; et al. Development of poly(vinylidene fluoride)/ionic liquid electrospun fibers for tissue engineering applications. J. Mater. Sci. 2016, 51, 4442–4450. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Ionic Liquids in Drug Delivery. Encyclopedia 2021, 1, 324–339. [Google Scholar] [CrossRef]
- King, C.; Shamshina, J.L.; Gurau, G.; Berton, P.; Khan, N.F.A.F.; Rogers, R.D. A platform for more sustainable chitin films from an ionic liquid process. Green Chem. 2017, 19, 117–126. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline chloride-thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef]
- Tran, C.D.; Mututuvari, T.M. Cellulose, chitosan, and keratin composite materials. Controlled drug release. Langmuir 2015, 31, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Udangawa, W.M.R.N.; Pochiraju, A.; Dong, W.; Zheng, Y.; Linhardt, R.J.; Simmons, T.J. Synthesis of Heparin-Immobilized, Magnetically Addressable Cellulose Nanofibers for Biomedical Applications. ACS Biomater. Sci. Eng. 2016, 2, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.G.; Paninho, A.I.; Nunes, A.V.M.; Fonseca, I.M.; Branco, L.C. Biocompatible locust bean gum mesoporous matrices prepared by ionic liquids and a scCO2 sustainable system. RSC Adv. 2015, 5, 107700–107706. [Google Scholar] [CrossRef]
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. API-ammonium ionic liquid–polymer compounds as a potential tool for delivery systems. J. Mol. Liq. 2017, 248, 972–980. [Google Scholar] [CrossRef]
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. Polymer—Ionic liquid—Pharmaceutical conjugates as drug delivery systems. J. Mol. Struct. 2019, 1180, 573–584. [Google Scholar] [CrossRef]
- Mahkam, M.; Abbaszad Rafi, A.; Mohammadzadeh Gheshlaghi, L. Preparation of novel pH-sensitive nanocomposites based on ionic-liquid modified montmorillonite for colon specific drug delivery system. Polym. Compos. 2016, 37, 182–187. [Google Scholar] [CrossRef]
- Hua, D.; Jiang, J.; Kuang, L.; Jiang, J.; Zheng, W.; Liang, H. Smart Chitosan-Based Stimuli-Responsive Nanocarriers for the Controlled Delivery of Hydrophobic Pharmaceuticals. Macromolecules 2011, 44, 1298–1302. [Google Scholar] [CrossRef]
- Júlio, A.; Costa Lima, S.A.; Reis, S.; Santos de Almeida, T.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Delivery Sci. Technol. 2020, 56, 100915. [Google Scholar] [CrossRef]
- Gupta, M.K.; Khokhar, S.K.; Phillips, D.M.; Sowards, L.A.; Drummy, L.F.; Kadakia, M.P.; Naik, R.R. Patterned silk films cast from ionic liquid solubilized fibroin as scaffolds for cell growth. Langmuir 2007, 23, 1315–1319. [Google Scholar] [CrossRef]
- Yang, Y.J.; Ganbat, D.; Aramwit, P.; Bucciarelli, A.; Chen, J.; Migliaresi, C.; Motta, A. Processing keratin from camel hair and cashmere with ionic liquids. Express Polym. Lett. 2019, 13, 97–108. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Dan, W.; Dan, N.; Gu, Z. Evaluation of 1-ethyl-3-methylimidazolium acetate based ionic liquid systems as a suitable solvent for collagen. J. Appl. Polym. Sci. 2013, 130, 2245–2256. [Google Scholar] [CrossRef]
- Iqbal, B.; Sarfaraz, Z.; Muhammad, N.; Ahmad, P.; Iqbal, J.; Khan, Z.U.H.; Gonfa, G.; Iqbal, F.; Jamal, A.; Rahim, A. Ionic liquid as a potential solvent for preparation of collagen-alginate-hydroxyapatite beads as bone filler. J. Biomater. Sci. Polym. Ed. 2018, 29, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Silva, S.S.; Gomes, J.M.; Oliveira, I.M.; Canadas, R.F.; Maia, F.R.; Radhouani, H.; Reis, R.L.; Oliveira, J.M. Ionic Liquid-Mediated Processing of SAIB-Chitin Scaffolds. ACS Sustain. Chem. Eng. 2020, 8, 3986–3994. [Google Scholar] [CrossRef]
- Singh, N.; Chen, J.; Koziol, K.K.; Hallam, K.R.; Janas, D.; Patil, A.J.; Strachan, A.; Hanley, J.G.; Rahatekar, S.S. Chitin and carbon nanotube composites as biocompatible scaffolds for neuron growth. Nanoscale 2016, 8, 8288–8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soheilmoghaddam, M.; Sharifzadeh, G.; Pour, R.H.; Wahit, M.U.; Whye, W.T.; Lee, X.Y. Regenerated cellulose/β-cyclodextrin scaffold prepared using ionic liquid. Mater. Lett. 2014, 135, 210–213. [Google Scholar] [CrossRef]
- Shin, E.J.; Singh, D.; Choi, S.M.; Zo, S.M.; Lee, Y.H.; Han, S.S. Functionalizing cellulose scaffold prepared by ionic liquid with bovine serum albumin for biomedical application. Fibers Polym. 2013, 14, 1965–1969. [Google Scholar] [CrossRef]
- Silva, S.S.; Santos, T.C.; Cerqueira, M.T.; Marques, A.P.; Reys, L.L.; Silva, T.H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. The use of ionic liquids in the processing of chitosan/silk hydrogels for biomedical applications. Green Chem. 2012, 14, 1463–1470. [Google Scholar] [CrossRef] [Green Version]
- Claus, J.; Brietzke, A.; Lehnert, C.; Oschatz, S.; Grabow, N.; Kragl, U. Swelling characteristics and biocompatibility of ionic liquid based hydrogels for biomedical applications. PLoS ONE 2020, 15, e0231421. [Google Scholar] [CrossRef] [PubMed]
- Jastram, A.; Claus, J.; Janmey, P.A.; Kragl, U. Rheological properties of hydrogels based on ionic liquids. Polym. Test. 2021, 93, 106943. [Google Scholar] [CrossRef]
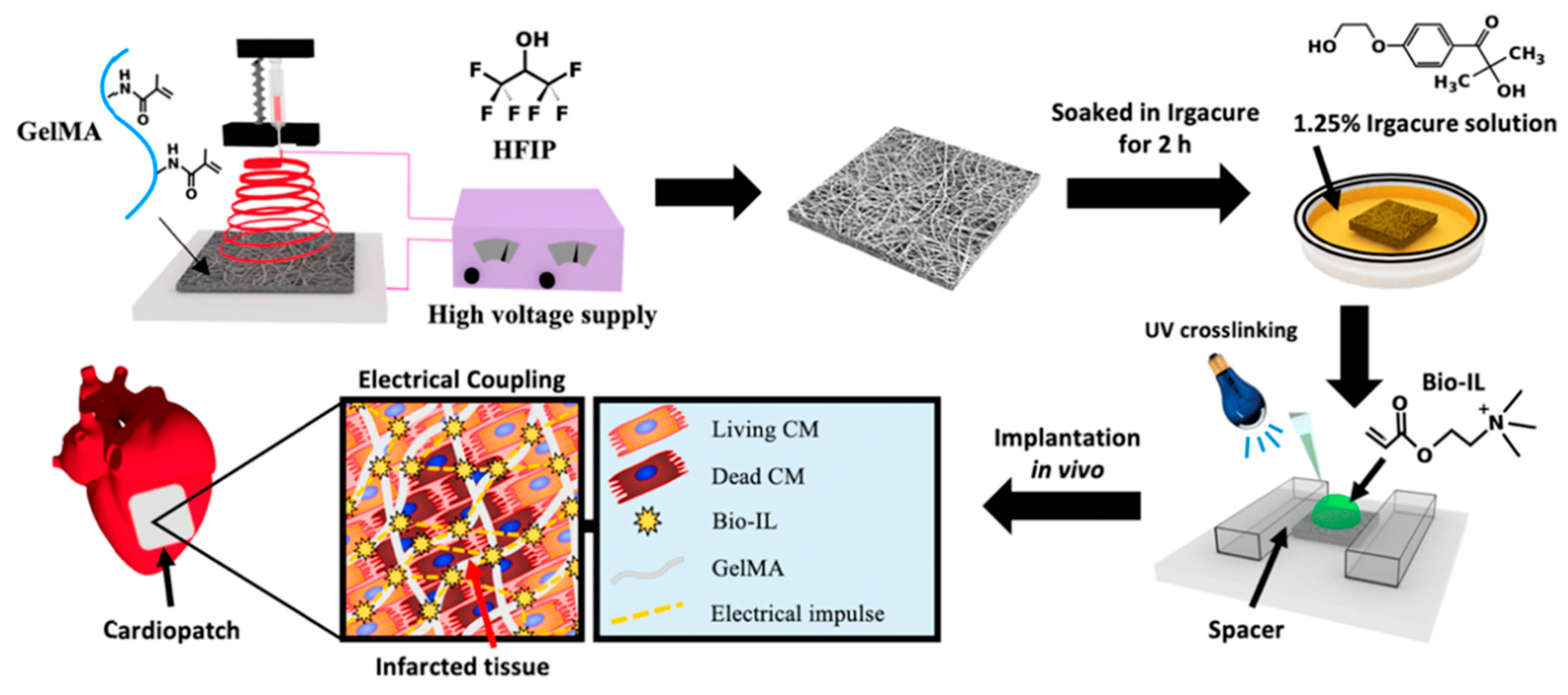
- Walker, B.W.; Lara, R.P.; Yu, C.H.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials 2019, 207, 89–101. [Google Scholar] [CrossRef] [Green Version]
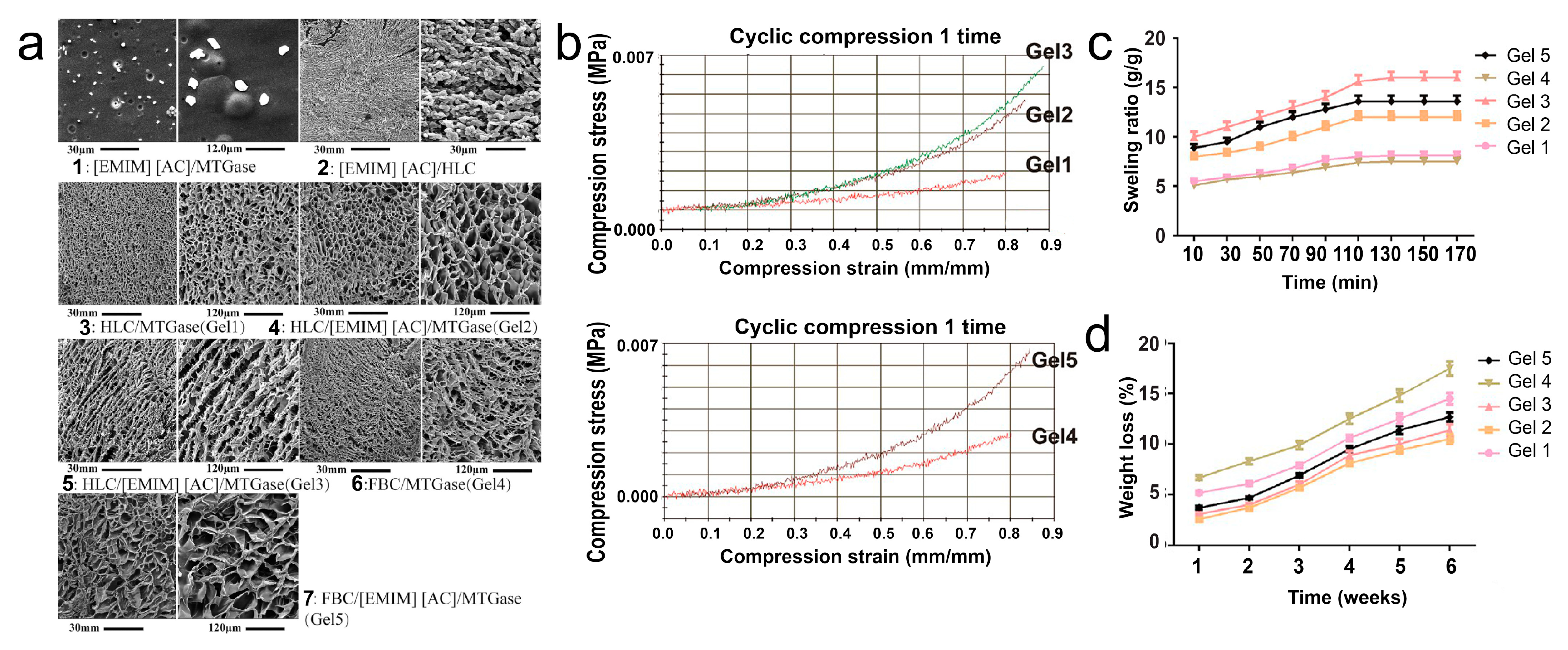
- Li, X.; Fan, D. Smart Collagen Hydrogels Based on 1-Ethyl-3-methylimidazolium Acetate and Microbial Transglutaminase for Potential Applications in Tissue Engineering and Cancer Therapy. ACS Biomater. Sci. Eng. 2019, 5, 3523–3536. [Google Scholar] [CrossRef]
- Liu, P.; Jin, K.; Wong, W.; Wang, Y.; Liang, T.; He, M.; Li, H.; Lu, C.; Tang, X.; Zong, Y.; et al. Ionic liquid functionalized non-releasing antibacterial hydrogel dressing coupled with electrical stimulation for the promotion of diabetic wound healing. Chem. Eng. J. 2021, 415, 129025. [Google Scholar] [CrossRef]
- Dias, A.R.; Costa-Rodrigues, J.; Fernandes, M.H.; Ferraz, R.; Prudêncio, C. The Anticancer Potential of Ionic Liquids. Chem. Med. Chem. 2017, 12, 11–18. [Google Scholar] [CrossRef]
- Dias, A.R.; Costa-Rodrigues, J.; Teixeira, C.; Prudêncio, C.; Gomes, P.; Ferraz, R. Ionic Liquids for Topical Delivery in Cancer. Curr. Med. Chem. 2019, 26, 7520–7532. [Google Scholar] [CrossRef]
- Ranke, J.; Müller, A.; Bottin-Weber, U.; Stock, F.; Stolte, S.; Arning, J.; Störmann, R.; Jastorff, B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol. Environ. Saf. 2007, 67, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.S.; Bertz, C.; Berdusco, N.; Mecozzi, S. Expanding the structural diversity of hydrophobic ionic liquids: Physicochemical properties and toxicity of Gemini ionic liquids. Green Chem. 2021, 23, 4375–4385. [Google Scholar] [CrossRef]
- Frade, R.F.M.; Matias, A.; Branco, L.C.; Afonso, C.A.M.; Duarte, C.M.M. Effect of ionic liquids on human colon carcinoma HT-29 and CaCo-2 cell lines. Green Chem. 2007, 9, 873–877. [Google Scholar] [CrossRef]
- Yoo, B.; Shah, J.K.; Zhu, Y.; Maginn, E.J. Amphiphilic interactions of ionic liquids with lipid biomembranes: A molecular simulation study. Soft Matter 2014, 10, 8641–8651. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Earle, M.J.; Gîlea, M.A.; Gilmore, B.F.; Gorman, S.P.; Seddon, K.R. Cytotoxicity of 1-alkylquinolinium bromide ionic liquids in murine fibroblast NIH 3T3 cells. Green Chem. 2011, 13, 2794–2800. [Google Scholar] [CrossRef]
- Li, X.-Y.; Jing, C.-Q.; Lei, W.-L.; Li, J.; Wang, J.-J. Apoptosis caused by imidazolium-based ionic liquids in PC12 cells. Ecotoxicol. Environ. Saf. 2012, 83, 102–107. [Google Scholar] [CrossRef]
- Li, X.-Y.; Jing, C.-Q.; Zang, X.-Y.; Yang, S.; Wang, J.-J. Toxic cytological alteration and mitochondrial dysfunction in PC12 cells induced by 1-octyl-3-methylimidazolium chloride. Toxicol. In Vitro 2012, 26, 1087–1092. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Wang, J. Cytotoxicity, oxidative stress, and apoptosis in HepG2 cells induced by ionic liquid 1-methyl-3-octylimidazolium bromide. Ecotoxicol. Environ. Saf. 2015, 120, 342–348. [Google Scholar] [CrossRef]
- Jurgen, A.; Marianne, M. Toxicity of Ionic Liquids towards Mammalian Cell Lines. Curr. Org. Chem. 2011, 15, 1905–1917. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Attri, P.; Kaushik, N.; Choi, E.H. Synthesis and antiproliferative activity of ammonium and imidazolium ionic liquids against T98G brain cancer cells. Molecules 2012, 17, 13727–13739. [Google Scholar] [CrossRef]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenko, A.V.; Khrustalev, V.N.; Ananikov, V.P. Cytotoxic Activity of Salicylic Acid-Containing Drug Models with Ionic and Covalent Binding. ACS Med. Chem. Lett. 2015, 6, 1099–1104. [Google Scholar] [CrossRef]
- Cui, W.; Lu, X.; Cui, K.; Niu, L.; Wei, Y.; Lu, Q. Dual-Responsive Controlled Drug Delivery Based on Ionically Assembled Nanoparticles. Langmuir 2012, 28, 9413–9420. [Google Scholar] [CrossRef]
- Mukesh, C.; Bhatt, J.; Prasad, K. A Polymerizable Bioionic Liquid Based Nanogel: A New Nanocarrier for an Anticancer Drug. Macromol. Chem. Phys. 2014, 215, 1498–1504. [Google Scholar] [CrossRef]
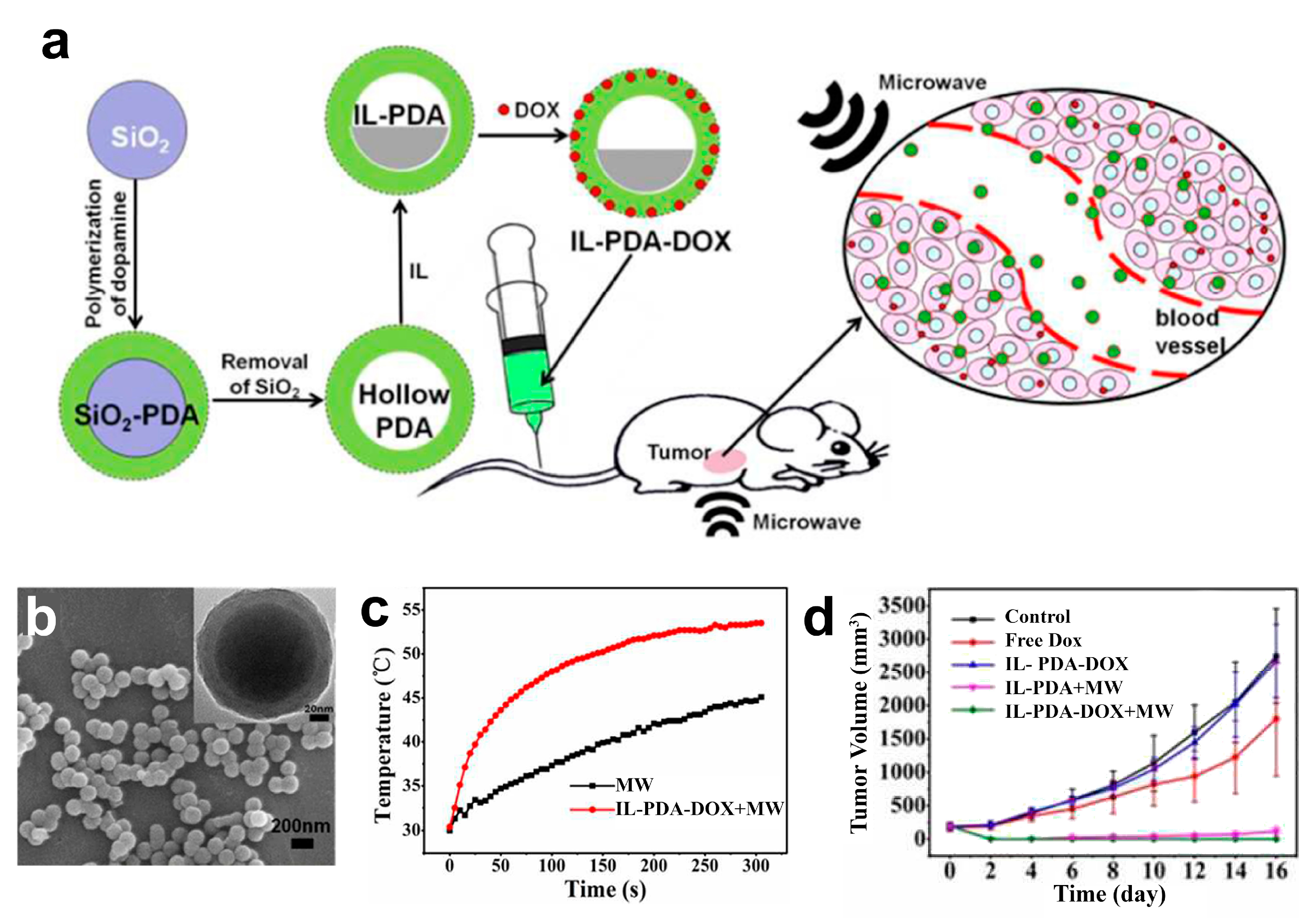
- Tang, W.; Liu, B.; Wang, S.; Liu, T.; Fu, C.; Ren, X.; Tan, L.; Duan, W.; Meng, X. Doxorubicin-loaded ionic liquid–polydopamine nanoparticles for combined chemotherapy and microwave thermal therapy of cancer. RSC Adv. 2016, 6, 32434–32440. [Google Scholar] [CrossRef]
- Tan, L.; Tang, W.; Liu, T.; Ren, X.; Fu, C.; Liu, B.; Ren, J.; Meng, X. Biocompatible Hollow Polydopamine Nanoparticles Loaded Ionic Liquid Enhanced Tumor Microwave Thermal Ablation in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 11237–11245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Surianarayanan, M.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid—In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef]
- Petersen, S.; Kaule, S.; Stein, F.; Minrath, I.; Schmitz, K.-P.; Kragl, U.; Sternberg, K. Novel paclitaxel-coated angioplasty balloon catheter based on cetylpyridinium salicylate: Preparation, characterization and simulated use in an in vitro vessel model. Mater. Sci. Eng. C 2013, 33, 4244–4250. [Google Scholar] [CrossRef]
- Lu, B.; Li, Y.; Wang, Z.; Wang, B.; Pan, X.; Zhao, W.; Ma, X.; Zhang, J. A dual responsive hyaluronic acid graft poly(ionic liquid) block copolymer micelle for an efficient CD44-targeted antitumor drug delivery. New J. Chem. 2019, 43, 12275–12282. [Google Scholar] [CrossRef]
- Stolarska, O.; Rodríguez, H.; Smiglak, M. Eutectic mixtures of pyrrolidinium-based ionic liquids. Fluid Phase Equilibria 2016, 408, 1–9. [Google Scholar] [CrossRef]
- Dickinson, Q.; Bottoms, S.; Hinchman, L.; McIlwain, S.; Li, S.; Myers, C.L.; Boone, C.; Coon, J.J.; Hebert, A.; Sato, T.K.; et al. Mechanism of imidazolium ionic liquids toxicity in Saccharomyces cerevisiae and rational engineering of a tolerant, xylose-fermenting strain. Microb. Cell Fact. 2016, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Collins, A. The Global Risks Report 2019; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; McCann, M.T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 11, 492–497. [Google Scholar] [CrossRef]
- Busetti, A.; Crawford, D.E.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; Laverty, G.; Lowry, A.F.; McLaughlin, M.; Seddon, K.R. Antimicrobial and antibiofilm activities of 1-alkylquinolinium bromide ionic liquids. Green Chem. 2010, 12, 420–425. [Google Scholar] [CrossRef]
- Freemantle, M. An Introduction to Ionic Liquids; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Gilmore, B.F.; Andrews, G.P.; Borberly, G.; Earle, M.J.; Gilea, M.A.; Gorman, S.P.; Lowry, A.F.; McLaughlin, M.; Seddon, K.R. Enhanced antimicrobial activities of 1-alkyl-3-methyl imidazolium ionic liquids based on silver or copper containing anions. New J. Chem. 2013, 37, 873–876. [Google Scholar] [CrossRef]
- Pernak, J.; Kalewska, J.; Ksycińska, H.; Cybulski, J. Synthesis and anti-microbial activities of some pyridinium salts with alkoxymethyl hydrophobic group. Eur. J. Med. Chem. 2001, 36, 899–907. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Memon, S.B.; Caldwell, G.A.; Caldwell, K.A.; Rogers, R.D. Using Caenorhabditis elegans to probe toxicity of 1-alkyl-3-methylimidazolium chloride based ionic liquids. Chem. Commun. 2004, 668–669. [Google Scholar] [CrossRef]
- Pretti, C.; Chiappe, C.; Pieraccini, D.; Gregori, M.; Abramo, F.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids to the zebrafish (Danio rerio). Green Chem. 2006, 8, 238–240. [Google Scholar] [CrossRef]
- Latała, A.; Stepnowski, P.; Nedzi, M.; Mrozik, W. Marine toxicity assessment of imidazolium ionic liquids: Acute effects on the Baltic algae Oocystis submarina and Cyclotella meneghiniana. Aquat. Toxicol. 2005, 73, 91–98. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
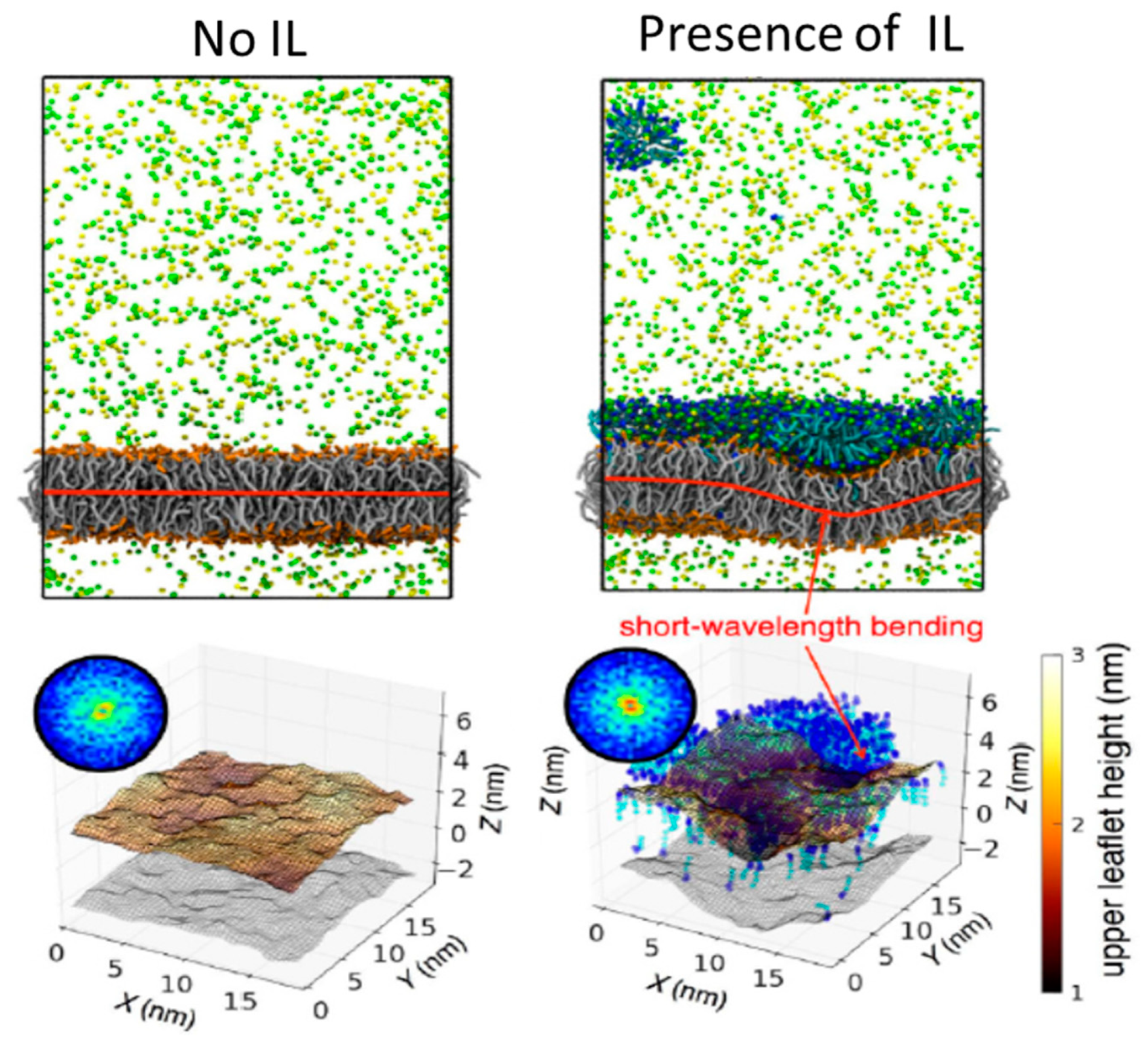
- Bhattacharya, G.; Giri, R.P.; Dubey, A.; Mitra, S.; Priyadarshini, R.; Gupta, A.; Mukhopadhyay, M.K.; Ghosh, S.K. Structural changes in cellular membranes induced by ionic liquids: From model to bacterial membranes. Chem. Phys. Lipids 2018, 215, 1–10. [Google Scholar] [CrossRef]
- Blesic, M.; Marques, M.H.; Plechkova, N.V.; Seddon, K.R.; Rebelo, L.P.N.; Lopes, A. Self-aggregation of ionic liquids: Micelle formation in aqueous solution. Green Chem. 2007, 9, 481–490. [Google Scholar] [CrossRef]
- Jungnickel, C.; Łuczak, J.; Ranke, J.; Fernández, J.F.; Müller, A.; Thöming, J. Micelle formation of imidazolium ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 278–284. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Hou, Y.; Wu, C.; Fan, Y.; Liu, L.; et al. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef]
- Yoo, B.; Jing, B.; Jones, S.E.; Lamberti, G.A.; Zhu, Y.; Shah, J.K.; Maginn, E.J. Molecular mechanisms of ionic liquid cytotoxicity probed by an integrated experimental and computational approach. Sci. Rep. 2016, 6, 19889. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Hartmann, D.O.; Adamová, G.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Unravelling the mechanism of toxicity of alkyltributylphosphonium chlorides in Aspergillus nidulans conidia. New J. Chem. 2012, 36, 56–63. [Google Scholar] [CrossRef]
- Hartmann, D.O.; Silva Pereira, C. A molecular analysis of the toxicity of alkyltributylphosphonium chlorides in Aspergillus nidulans. New J. Chem. 2013, 37, 1569–1577. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Kubicki, M.; Pernak, J. 3-Alkoxymethyl-1-(1R,2S,5R)-(−)-menthoxymethylimidazolium salts-based chiral ionic liquids. Tetrahedron Asymmetry 2010, 21, 2709–2718. [Google Scholar] [CrossRef]
- Gomes, A.; Bessa, L.J.; Correia, P.; Fernandes, I.; Ferraz, R.; Gameiro, P.; Teixeira, C.; Gomes, P. “Clicking” an Ionic Liquid to a Potent Antimicrobial Peptide: On the Route Towards Improved Stability. Int. J. Mol. Sci. 2020, 21, 6174. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial activities of highly bioavailable organic salts and ionic liquids from fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and antimicrobial properties of new chitosan derivatives containing guanidinium groups. Carbohydr. Polym. 2020, 241. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Singh, D.; Kesavan, A.K.; Kang, T.S. Antimicrobial Colloidal Complexes of Lysozyme with Bio-Based Surface Active Ionic Liquids in Aqueous Medium. J. Phys. Chem. B 2020, 124, 3791–3800. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, Q.; Guo, J.; Qin, J.; Mao, H.; Wang, B.; Yan, F. Structure–Antibacterial Activity Relationships of Imidazolium-Type Ionic Liquid Monomers, Poly(ionic liquids) and Poly(ionic liquid) Membranes: Effect of Alkyl Chain Length and Cations. ACS Appl. Mater. Interfaces 2016, 8, 12684–12692. [Google Scholar] [CrossRef]
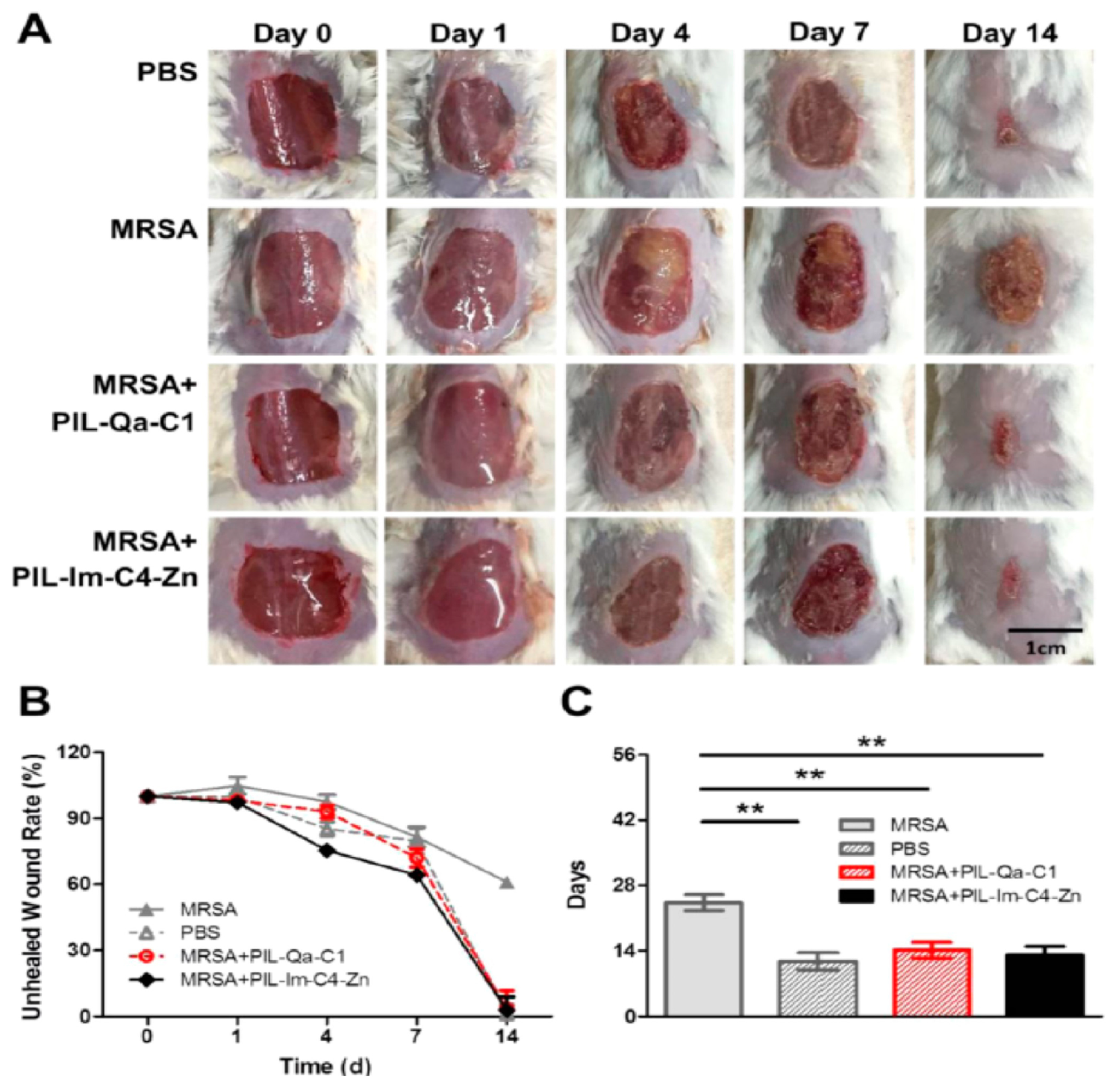
- Xu, Q.; Zheng, Z.; Wang, B.; Mao, H.; Yan, F. Zinc Ion Coordinated Poly(Ionic Liquid) Antimicrobial Membranes for Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 14656–14664. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Nakayama, K.; Kitazume, T. Antibacterial activities of imidazolium, pyrrolidinium and piperidinium salts. Bioorg. Med. Chem. Lett. 2011, 21, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.A.; Voloshina, A.D.; Kulik, N.V.; Zobov, V.V.; Mamedov, V.A. Antimicrobial activity of imidazo[1,5-a]quinoxaline derivatives with pyridinium moiety. Eur. J. Med. Chem. 2013, 66, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Demberelnyamba, D.; Kim, K.-S.; Choi, S.; Park, S.-Y.; Lee, H.; Kim, C.-J.; Yoo, I.-D. Synthesis and antimicrobial properties of imidazolium and pyrrolidinonium salts. Bioorg. Med. Chem. 2004, 12, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative evaluation of antimicrobial activity of different types of ionic liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Gonzalez, J.J.; Comelles, F. Catanionic mixtures of surface-active ionic liquids and N-lauroyl sarcosinate: Surface adsorption, aggregation behavior and microbial toxicity. J. Mol. Liq. 2020, 318, 114040. [Google Scholar] [CrossRef]
- Bromberger, B.; Sommer, J.; Robben, C.; Trautner, C.; Kalb, R.; Rossmanith, P.; Mester, P.J. Evaluation of the antimicrobial activity of pyrithione-based ionic liquids. Sep. Purif. Technol. 2020, 251, 117309. [Google Scholar] [CrossRef]
- Titi, A.; Almutairi, S.M.; Alrefaei, A.F.; Manoharadas, S.; Alqurashy, B.A.; Sahu, P.K.; Hammouti, B.; Touzani, R.; Messali, M.; Ali, I. Novel phenethylimidazolium based ionic liquids: Design, microwave synthesis, in-silico, modeling and biological evaluation studies. J. Mol. Liq. 2020, 315, 113778. [Google Scholar] [CrossRef]
- Brunel, F.; Lautard, C.; Garzino, F.; Raimundo, J.M.; Bolla, J.M.; Camplo, M. Phosphonium-ammonium-based di-cationic ionic liquids as antibacterial over the ESKAPE group. Bioorg. Med. Chem. Lett. 2020, 30, 127389. [Google Scholar] [CrossRef]
- Wylie, M.P.; Bell, S.E.J.; Nockemann, P.; Bell, R.; McCoy, C.P. Phosphonium Ionic Liquid-Infused Poly(vinyl chloride) Surfaces Possessing Potent Antifouling Properties. ACS Omega 2020, 5, 7771–7781. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (−)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Timur, S. Ionic Liquids from Biocompatibility and Electrochemical Aspects toward Applying in Biosensing Devices. Anal. Chem. 2017, 90, 640–648. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Chen, Z.; Watanable, M.; Deng, Y. Beyond solvents and electrolytes: Ionic liquids-based advanced functional materials. Prog. Mater. Sci. 2016, 77, 80–124. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Holbrey, J.D.; Reddy, R.G.; Rogers, R.D. Ionic Liquid Temperature Sensor. U.S. Patent No 6749336, 15 June 2004. [Google Scholar]
- Ji, X.; Banks, C.E.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Electrochemical Ammonia Gas Sensing in Nonaqueous Systems: A Comparison of Propylene Carbonate with Room Temperature Ionic Liquids. Electroanalysis 2007, 19, 2194–2201. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, Y.; Lu, G.; Li, L.; Zhu, X.; Nie, J. A transparent, stretchable, stable, self-adhesive ionogel-based strain sensor for human motion monitoring. J. Mater. Chem. C 2019, 7, 11244–11250. [Google Scholar] [CrossRef]
- Che, S.; Dao, R.; Zhang, W.; Lv, X.; Li, H.; Wang, C. Designing an anion-functionalized fluorescent ionic liquid as an efficient and reversible turn-off sensor for detecting SO2. Chem. Commun. 2017, 53, 3862–3865. [Google Scholar] [CrossRef]
- Che, S.; Guo, J.; Gan, L.; Xiao, Q.; Li, H.; She, Y.; Wang, C. A succinct enhanced luminescence strategy for fluorescent ionic liquids and the application for detecting CO2. Green Energy Environ. 2021, in press. [Google Scholar]
- Chen, X.-W.; Liu, J.-W.; Wang, J.-H. A Highly Fluorescent Hydrophilic Ionic Liquid as a Potential Probe for the Sensing of Biomacromolecules. J. Phys. Chem. B 2011, 115, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.C.; Correia, D.M.; García-Astrain, C.; Pereira, N.; Tariq, M.; Esperança, J.M.S.S.; Lanceros-Méndez, S. Ionic-Liquid-Based Printable Materials for Thermochromic and Thermoresistive Applications. ACS Appl. Mater. Interfaces 2019, 11, 20316–20324. [Google Scholar] [CrossRef]
- Rama, R.; Meenakshi, S.; Pandian, K.; Gopinath, S.C.B. Room Temperature Ionic Liquids-Based Electrochemical Sensors: An Overview on Paracetamol Detection. Crit. Rev. Anal. Chem. 2021, 1–10. [Google Scholar] [CrossRef]
- Santiago-Malagón, S.; Río-Colín, D.; Azizkhani, H.; Aller-Pellitero, M.; Guirado, G.; del Campo, F.J. A self-powered skin-patch electrochromic biosensor. Biosens. Bioelectron. 2021, 175, 112879. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J.; Chen, C.; Yu, H.; Hu, L. A Dynamic Gel with Reversible and Tunable Topological Networks and Performances. Matter 2020, 2, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shen, Y.; Li, J.; Niu, L.; Dong, S.; Ivaska, A. Electrochemical Functionalization of Single-Walled Carbon Nanotubes in Large Quantities at a Room-Temperature Ionic Liquid Supported Three-Dimensional Network Electrode. Langmuir 2005, 21, 4797–4800. [Google Scholar] [CrossRef]
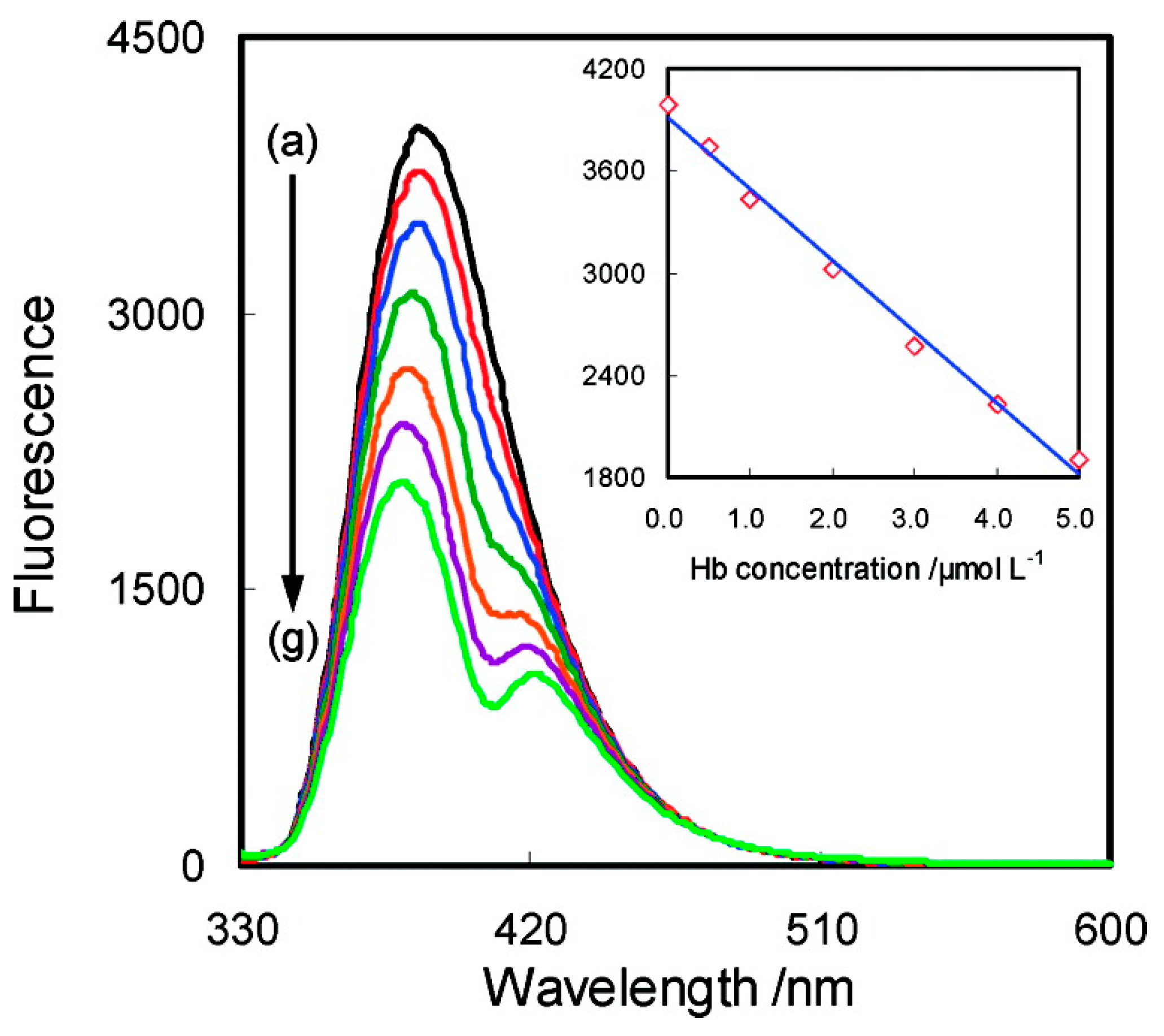
- Lu, X.; Hu, J.; Yao, X.; Wang, Z.; Li, J. Composite System Based on Chitosan and Room-Temperature Ionic Liquid: Direct Electrochemistry and Electrocatalysis of Hemoglobin. Biomacromolecules 2006, 7, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Faruk Emon, M.O.; Vatani, M.; Choi, J.-W. Effect of degree of crosslinking and polymerization of 3D printable polymer/ionic liquid composites on performance of stretchable piezoresistive sensors. Smart Mater. Struct. 2017, 26, 035043. [Google Scholar] [CrossRef]
- Franzoi, A.; Dupont, J.; Spinelli, A.; Vieira, I. Biosensor based on laccase and an ionic liquid for determination of rosmarinic acid in plant extracts. Talanta 2009, 77, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, S.; Zhang, Y.; Lin, Q.; Chen, Y.; Zhu, D.; Sun, L.; Chen, T. Facile Fabrication of a Self-Healing Temperature-Sensitive Sensor Based on Ionogels and Its Application in Detection Human Breath. Nanomaterials 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.J.; Kim, H.; Ok, J.; Shin, Y.J.; Shin, J.H.; Kim, T.H.; Jung, Y.; Kim, T.I. Biocompatible and Biodegradable Organic Transistors Using a Solid-State Electrolyte Incorporated with Choline-Based Ionic Liquid and Polysaccharide. Adv. Funct. Mater. 2020, 30, 1909707. [Google Scholar] [CrossRef]
- Gao, L.; Lin, X.; Zheng, A.; Shuang, E.; Wang, J.; Chen, X. Real-time monitoring of intracellular pH in live cells with fluorescent ionic liquid. Anal. Chim. Acta 2020, 1111, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Salih, F.E.; Oularbi, L.; Halim, E.; Elbasri, M.; Ouarzane, A.; El Rhazi, M. Conducting Polymer/Ionic Liquid Composite Modified Carbon Paste Electrode for the Determination of Carbaryl in Real Samples. Electroanalysis 2018, 30, 1855–1864. [Google Scholar] [CrossRef]
- Ghorbani Zamani, F.; Moulahoum, H.; Ak, M.; Odaci Demirkol, D.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Analyt. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- Javed, F.; Ullah, F.; Zakaria, M.R.; Akil, H.M. An approach to classification and hi-tech applications of room-temperature ionic liquids (RTILs): A review. J. Mol. Liq. 2018, 271, 403–420. [Google Scholar] [CrossRef]
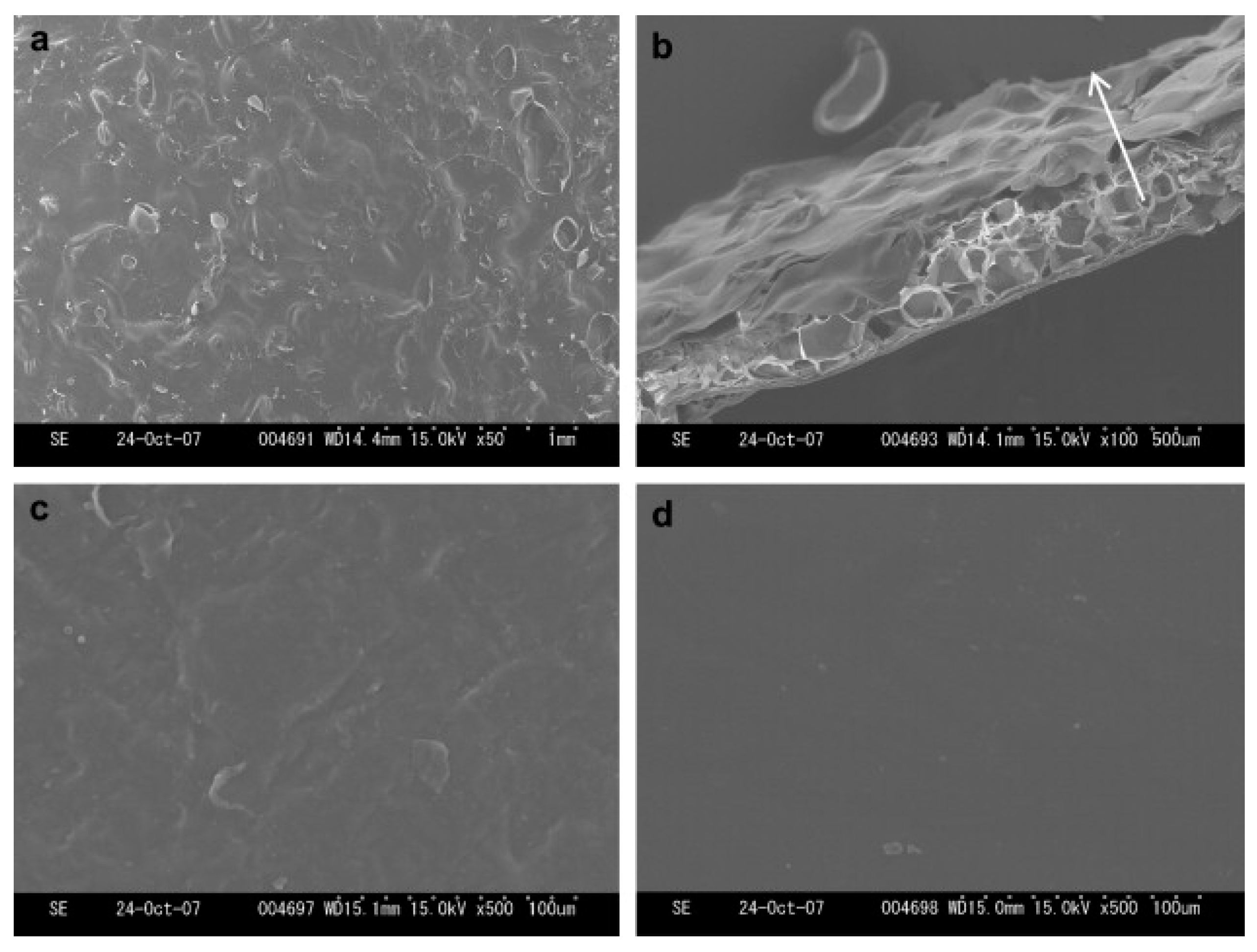
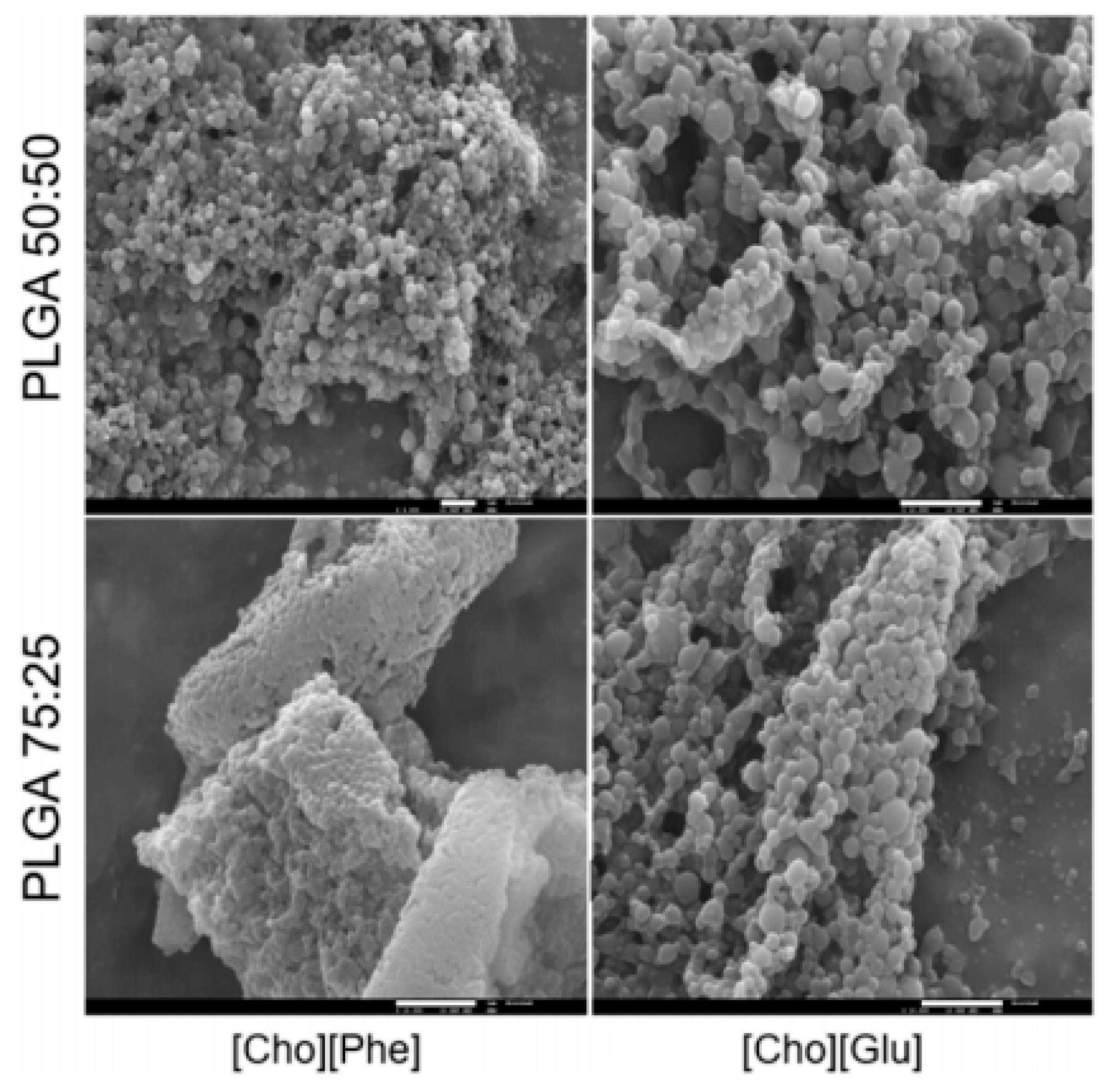
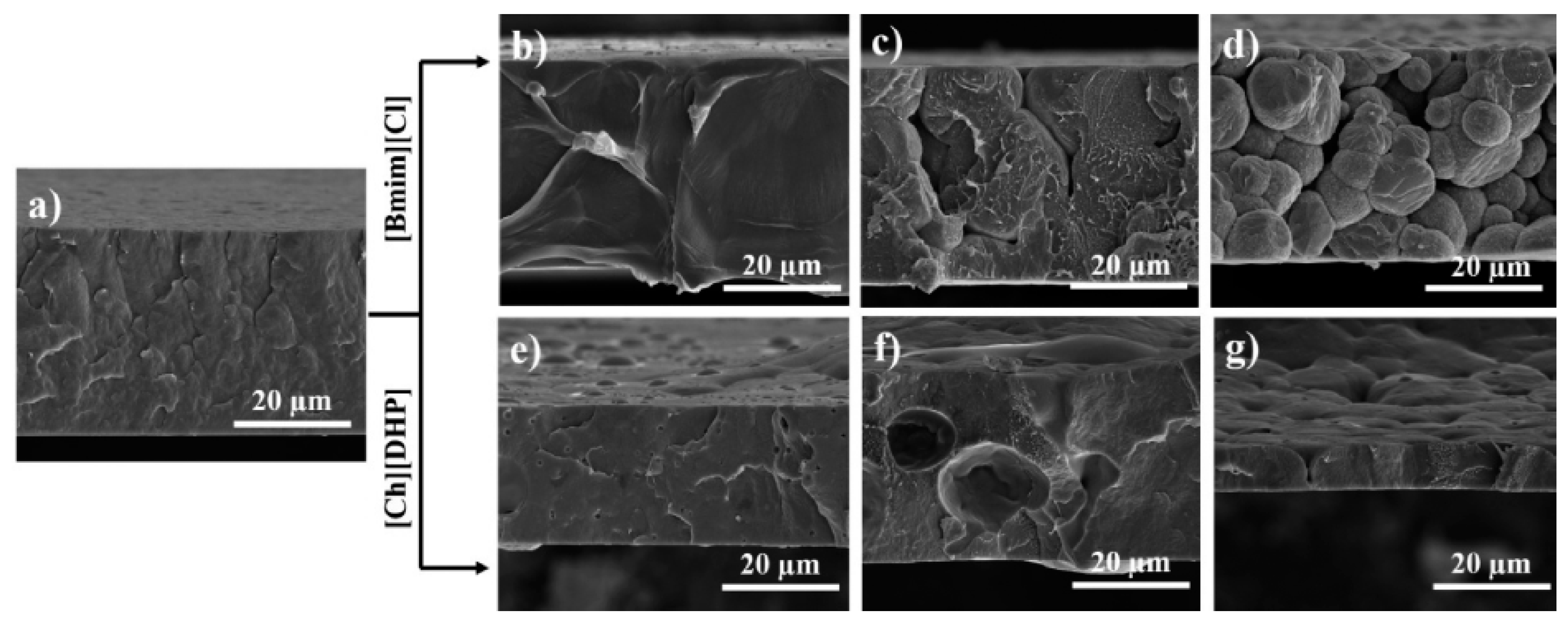
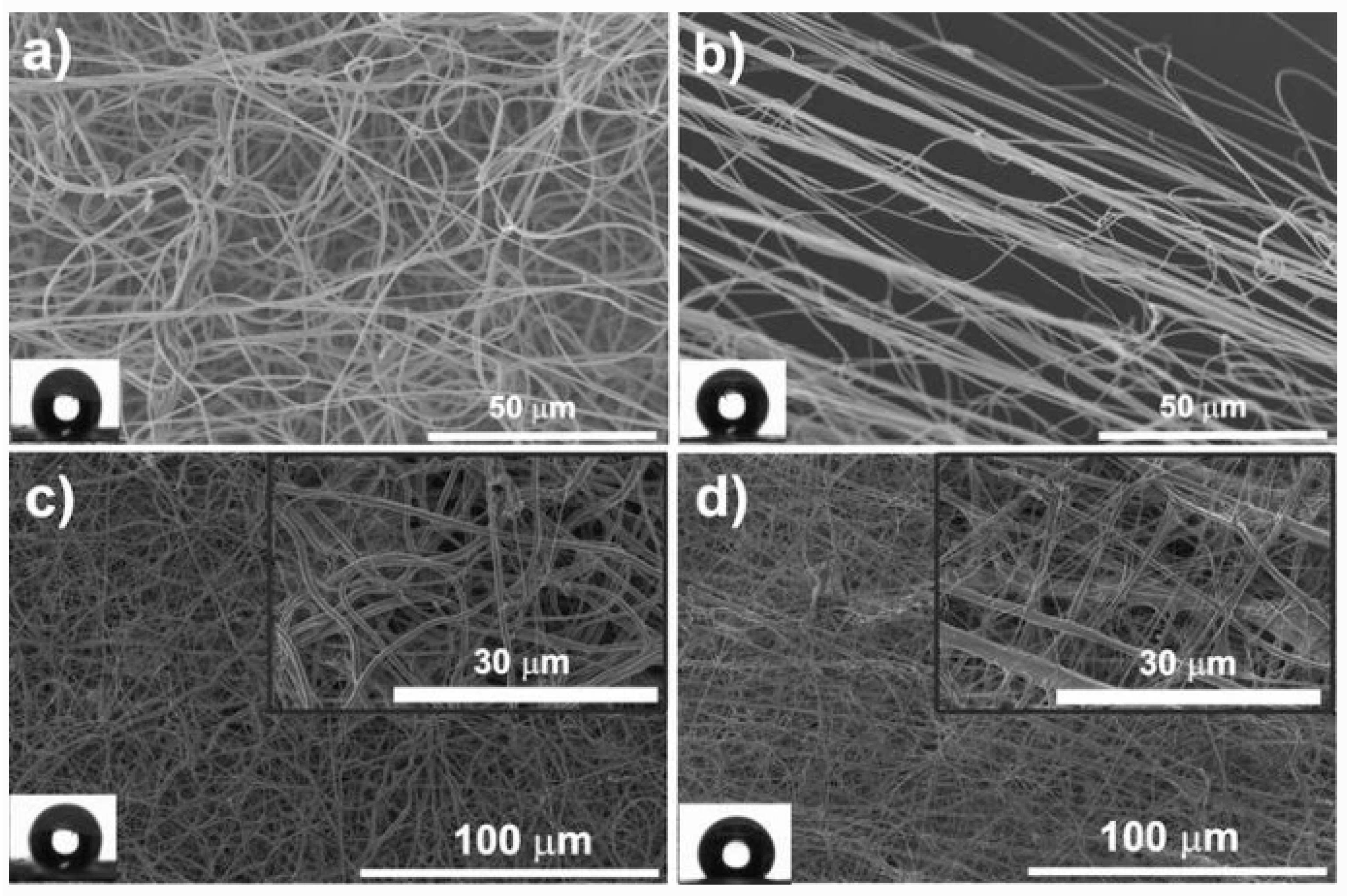



| Materials | Ionic Liquids (ILs) | Biomedical Property | Ref. |
|---|---|---|---|
| Chitosan | [Ch][Cl] | Electrical and pH-sensitive drug delivery | [116] |
| [Ch][DHP] | |||
| Chitin | [C2mim][Ac] | Topical release drug delivery | [119] |
| [Bmim][HSO4] | Sustained drug release application | [120] | |
| [Hmim][HSO4] | |||
| [Chol][HSO4] | |||
| Choline acrylate | |||
| Choline chloride-thiourea | |||
| Cellulose/Chitosan/Keratin | [Bmim][Cl] | Bandage to treat chronic and ulcerous wounds | [121] |
| Cellulose/Fe3O4 NPs/Heparin | [Emim][Ac] | Magnetically responsive drug delivery | [122] |
| Locust bean gum | [Bmim][Cl] | Potential drug delivery system | [123] |
| [Emim][Ac] | |||
| [C2OHmim][Cl] | |||
| Active pharmaceutical ingredient/grafted-PLLA | Choline chloride | Potential drug delivery system | [124] |
| di(2-hydroxyethyl)dimethyl ammonium bromide | |||
| Active pharmaceutical ingredient/grafted-PLLA | 2-hydroxyethyl triethyl ammonium bromide | Drug delivery system | [125] |
| 2-hydroxyethyl tributyl ammonium bromide | |||
| di(2-hydroxyethyl)dibutyl ammonium bromide | |||
| tris(2-hydroxyethyl)butyl ammonium bromide | |||
| pH-sensitive polymer/montmorillonite (MMT) | 3-methyl-1-[2-(2-methyl-acryloxy)ethyl]imidazolium chloride | Colon Specific Drug Delivery System | [126] |
| Cellulose/PNIPAAm | [Bmim][Cl] | Temperature and pH-sensitive drug delivery | [127] |
| IL | Polymer | Cells | Applications | Ref. |
|---|---|---|---|---|
| [Bmim][Cl] | PVDF | C2C12 cells | Skeletal muscle | [115] |
| Silk fibroin | Primary keratinocytes | Tissue engineering scaffold | [129] | |
| Keratin | Murine embryo fibroblast | Tissue engineering matrices | [130] | |
| Cellulose | Human skin fibroblasts | Skin | [135] | |
| [Ch][DHP] | PVDF | C2C12 cells | Skeletal muscle | [115] |
| [C2mim][NTf2] | PVDF | C2C12 cells | Skeletal muscle | [117] |
| [EMIM][Ac] | Collagen | Primary fibroblast | Tissue engineering scaffold | [131] |
| Chitin | Cortical neurons | Neural | [134] | |
| Collagen-based hydrogel | HepG2 and MKN45 cells | Cancer therapy | [141] | |
| [TEA][A] | Collagen-alginate-HA | Rat mesenchymal stem cells (rMSC) | Bone regeneration | [132] |
| [Bmim][OAc] | SAIB and chitin | Human adipose-derived stem cells (hASCs) | Tissue engineering scaffolding | [133] |
| [Amim][Cl] | Cellulose | Mesenchymal stem cells | Tissue engineering scaffold | [136] |
| Choline-based bio-ionic liquid (Bio-IL) | GelMA hydrogel | Co-cultures of primary cardiomyocytes and cardiac fibroblasts | Cardiac tissue repair | [73] |
| Primary rats cardiomyocytes | Cardiac tissue repair | [140] | ||
| [Bmim][Ac] | Chitosan/silk-based hydrogels | Human dermal fibroblasts | Skin tissue engineering | [137] |
| [VAPim][BF4] | KGM hydrogels | L929 cells | Diabetic wound healing | [142] |
| IL | Material or Material/Drug | Cancer Type/Cell Lines | Ref. |
|---|---|---|---|
| [Emim][Sal], [Bmim][Sal], [Hmim][Sal]; ([Emim-OSal][Cl], [Prmim-OSal][Cl], [Emim-OSal][BF4]; [Emim-OSal][Sal] | Salicilic acid | Colorectal adenocarcinoma/human cell line (CaCo-2), CaCo-2 (colorectal adenocarcinoma) and 3215 LS (normal fibroblasts) | [155] |
| [EVPy][DA] | Poly(ionic liquid-co-N-isopropylacrylamide)/doxorubicin | Breat cancer | [156] |
| [Chol-A][5-FU] | Polyacrylate/5-flurouracil (5-FU) | Stomach cancer | [157] |
| [Emim][PF6] | Polydopamine/doxorubicin | Liver cancer/H22 tumor cell lines and HepG2 cells | [158,159] |
| [Emim][Ac] | Human-like and Fish bone collagen hydrogels | Liver and stomach cancer/healthy fibroblasts 3T3-L1 and L929 cells and cancer HepG2 and MKN45 cells | [141] |
| Choline formate | PHEMA/Curcumin | Ovarian Cancer/SKOV-3 cells | [160] |
| Cetpyrsal | Paclitaxel (PTX) | Ovarian and breast and pancreatic Cancer | [161] |
| [VHim][NTf2] | hyaluronic acid grafted poly(ionic liquid)/doxorubicin | Breast and colorectal carcinoma/MCF-7, CT26 | [162] |
| IL | Microorganisms Tested | Effect | Ref. | |
|---|---|---|---|---|
| Cation | Anion | |||
| 1-alkyl-3-methyl imidazolium | Chloride | S. aureus ATCC 29213 | Broad spectrum antibiofilm activity | [167] |
| MRSA (clinical strain 201) | ||||
| Epidemic MRSA strain E-MRSA 15 | ||||
| S.epidermidis ATCC 35984 | ||||
| S. epidermidis ATCC 12228 | ||||
| E. coli NCTC 8196 | ||||
| P. aeruginosa PA01 | ||||
| K. aerogenes NCTC 7427 | ||||
| Burkholderia cenocepacia J2315 | ||||
| Proteus mirabilis NCTC 12442 | ||||
| Candida tropicalis NCTC 7393 | ||||
| 1-alkylquinolinium | Bromide | S. epidermidis ATCC 12228 | Excellent, broad spectrum antibacterial and antifungal activity in both the planktonic and biofilm mode of growth. | [168] |
| Methicillin resistant S. epidermidis (MRSE) ATCC 35984 | ||||
| S. aureus ATCC 29213 | ||||
| Methicillin resistant S. aureus (MRSA) ATCC 43300 | ||||
| E. coli NCTC 8196, | ||||
| K. aerogenes NCTC7427 | ||||
| Bacillus cereus NCTC 2599, | ||||
| P. mirabilis NCTC 12442, | ||||
| P. aeruginosa PA01 | ||||
| C. tropicalis NCTC 7393 | ||||
| Imidazolium-based | Iodide | B. subtilis 168 | Antibacterial activity: the silylalkyl group is useful to generate antibacterial activity to imidazole salts | [190] |
| S. aureus subsp., aureus NBRC 15035 | ||||
| Pyrrolidinium-based | E. coli MG1655, | |||
| Piperidinium-based | P. putida NBRC 14164 | |||
| 1-alkyl-3-methylimidazolium | [AgX2] [CuX4]2 | MRSA | An enhancement of overall IL antimicrobial activity is observed when anions and cations are both inherently antimicrobial | [170] |
| MRSE | ||||
| Imidazo[1,5-a]quinoxalin-4-on-1-yl)-1-pyridinium | Bromide | P. aeruginosa 9027 | Antimicrobial activity and selectivity towards Gram-positive bacteria and yeasts | [191] |
| E. coli F-50 | ||||
| S. aureus 209p | ||||
| B. cereus 8035 | ||||
| Aspergillus niger BKMF-1119 | ||||
| Trichophyton mentagrophytes | ||||
| C. albicans 885-653 | ||||
| Imidazolium-based with different alkyl chain lengths | Bromide | S. aureus ATCC 6538 | The ILs’ antibacterial activities were improved with the increase of the alkyl chain length and higher charge density of imidazolium cations | [188] |
| E. coli ATCC 8099 | ||||
| Imidazolium-based Pyrrolidinonium-based | Cloride Bromide | B. subtilis KCTC1914, | Antibacterial and antifungal properties that improve with longer alkyl chains | [192] |
| S. aureus 209 KCTC1916 | ||||
| S. aureus R209 KCTC1928 | ||||
| E. coli KCTC1924, | ||||
| S. typhimurium KCTC1926 | ||||
| C. albicans KCTC1940 | ||||
| Chllolella regularis (algal bacterium) | ||||
| 1-methyl-3-dodecylimidazolium; | Bromide | E. coli ATCC 25922 | Antimicrobial activity and relatively low hemolytic activity | [193] |
| P. aeruginosa ATCC 27853 | ||||
| 1-dodecyl-methylpyrrolidinium; | S. epidermidis ATCC 35984 | |||
| 1-dodecyl-1-methylpiperidinium | S. aureus ATCC 6538 | |||
| Enterococcus faecalis ATCC 29212 | ||||
| Imidazolium-based Pyridinium-based | Bromide | B. subtilis ATCC6633 | The conjugation of ILs with the acid-based anionic surfactant sodium N-lauroyl sarcosinate result in a strong synergism, resulting in enhanced interfacial and aggregation properties. Broad-spectrum antimicrobial activity against bacteria and fungus | [194] |
| S. aureus ATCC29213 | ||||
| S. epidermidis ATCC12228 | ||||
| Listeria monocytogenes ATCC15313 | ||||
| E. coli ATCC25922 | ||||
| Acinetobacter baumannii ATCC19606 | ||||
| P. aeruginosa ATCC27853 | ||||
| C. albicans ATCC10231 | ||||
| Imidazolium-based | Pyrithione | L. monocytogenes ATCC 13932 | Incorporating pyrithione into an IL resulted in an improvement bactericidal effect, especially against gram-negative bacteria and a high susceptibility against clinically relevant yeast C. tropicalis | [195] |
| B. cereus ATCC 1177 | ||||
| S. aureus ATCC 6538 | ||||
| Trioctylmethyl- based | E. faecalis ATCC 19433 | |||
| Trimethyl-based | Lactobacillus sakei ATCC 15521 | |||
| Lactococcus lactis ATCC 19435 | ||||
| Geobacillus stearothermophilus ATCC 7953 | ||||
| Salmonella enterica ser. Typhimurium ATCC 14028 | Antibacterial, antiviral and antifungal properties | |||
| E. coli ATCC 25922 | ||||
| Citrobacter freundii ATCC 43864 | ||||
| P. mirabilis ATCC 29,906 | ||||
| P. aeruginosa ATCC 27853 | ||||
| Saccharomyces cerevisiae ATCC 4,000,850 | ||||
| C. tropicalis ATCC 750 | ||||
| Imidazolium-based | Chloride | S. aureus | Antimicrobial activity against resistant Gram-positive and Gram-negative bacteria | [196] |
| Bromide | B. subtilis | |||
| K. pneumonia | ||||
| E. coli MC4100 | ||||
| E. coli XL-1 Blue | ||||
| Ammonium salt Phosphonium salt | Chloride | E. faecium 20,477 | Broad spectrum of activities towards Gram-positive and Gram-negative. High cytotoxicity towards human cells | [197] |
| S. aureus CIP 7625 | ||||
| K. pneumonia CIP 82.91 | ||||
| A. Baumannii ATCC 19606 | ||||
| P. aeruginosa 100,720 | ||||
| Bromide | E. aerogenes ATCC 13048 | |||
| Iodide | E. coli CIP 54.8 | |||
| Imidazolium-based | Bromide | S. aureus | The bioactive peptide 3.1-PP4 | [184] |
| E. faecali | (KKLLKWLLKLLKTTKS, C-terminal amide) preserve its potent antibacterial and antibiofilm action, while significantly improving its stability towards an enzyme that is relevant in the skin wound environment | |||
| P. aeruginosa | ||||
| E. coli | ||||
| K. pneumoniae (clinical isolate) | ||||
| Choline; | Ciprofloxacin Norfloxacin | K. pneumoniae | The ILs were not toxic to healthy cell lines he antimicrobial activity against K. pneumoniae was particularly enhanced for the ciprofloxacin-based OSILs | [185] |
| 1-ethyl-3-methylimidazolium; | ||||
| 1-hydroxy-ethyl-3-methylimidazolium; | S. aureus | |||
| 1-(2-hydroxyethyl)-2,3-dimethylimidazolium; | ||||
| 1-(2-methoxyethyl)-3-methylimidazolium; | B. subtilis | |||
| acetylpyridinium | ||||
| Choline | Sarcosinate | E. coli | Lysozyme complexed with ILs, enhanced their antimicrobial activity | [187] |
| Deoxycholate | P. aeruginosa, | |||
| Bacillus thuringiensis | ||||
| Phosphonium-based | Docusate (AOT) | P. aeruginosa | Development of poly(vinyl chloride) materials blended with phosphonium ionic liquids created a slippery, superhydrophilic surface, creating an antifouling surface | [198] |
| S. aureus | ||||
| Alkyl[(1R,2S,5R)-(−)-menthoxymethyl]dimethylammonium (C10-Am-Men) | Chloride | C. albicans | Antifungal activity | [199] |
| IL | Polymer | Application | Ref. |
|---|---|---|---|
| [Bmim][TFSI] | PBA ionogel | Human motion sensing/monitoring | [204] |
| Propylene Carbonate | Ammonia biosensor | [203] | |
| [Bmim][Cl] | Cellulose | Self-healing e-skin sensitive to force and moisture | [211] |
| Silk fibroin | Support normal cell growth and differentiation | [129] | |
| [Bmim][PF6] | Poly- N-succinimidyl acrylate (p-NSA) | Glucose sensor | [212] |
| Propylene Carbonate | Ammonia biosensor | [203] | |
| [Bmim][BF4] | Chitosan | Protein and enzyme sensing platform | [213] |
| Propylene Carbonate | Ammonia biosensor | [203] | |
| 2-[[(butylamino)carbonyl]oxy]ethyl acrylate | Touch sensor | [214] | |
| [Bmim][OTf] | Propylene Carbonate | Ammonia biosensor | [203] |
| [Emim][TFSI] | |||
| [Bmim][PF6] | Graphite powder and others | Detection of rosmarinic acid in plant extracts | [215] |
| [Bmim][BF4] | |||
| [VEIm][DCA] | Silica + ammonium persulfate | Self-healing sensor for Breathing detection/analysis | [216] |
| [Ch][MA] | Levan-Glycerol | Electrolyte-based organic transistor, ECG measurement | [217] |
| [6MQc][TFSI] | None | Intracellular pH monitoring | [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. https://doi.org/10.3390/nano11092401
Correia DM, Fernandes LC, Fernandes MM, Hermenegildo B, Meira RM, Ribeiro C, Ribeiro S, Reguera J, Lanceros-Méndez S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials. 2021; 11(9):2401. https://doi.org/10.3390/nano11092401
Chicago/Turabian StyleCorreia, Daniela Maria, Liliana Correia Fernandes, Margarida Macedo Fernandes, Bruno Hermenegildo, Rafaela Marques Meira, Clarisse Ribeiro, Sylvie Ribeiro, Javier Reguera, and Senentxu Lanceros-Méndez. 2021. "Ionic Liquid-Based Materials for Biomedical Applications" Nanomaterials 11, no. 9: 2401. https://doi.org/10.3390/nano11092401
APA StyleCorreia, D. M., Fernandes, L. C., Fernandes, M. M., Hermenegildo, B., Meira, R. M., Ribeiro, C., Ribeiro, S., Reguera, J., & Lanceros-Méndez, S. (2021). Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials, 11(9), 2401. https://doi.org/10.3390/nano11092401











