Modeling of Self-Assembled Peptide Nanotubes and Determination of Their Chirality Sign Based on Dipole Moment Calculations
Abstract
:1. Introduction
2. Models Details and Computational Methods
2.1. Main Models and Used Software
2.2. Models of FF Nanotubes
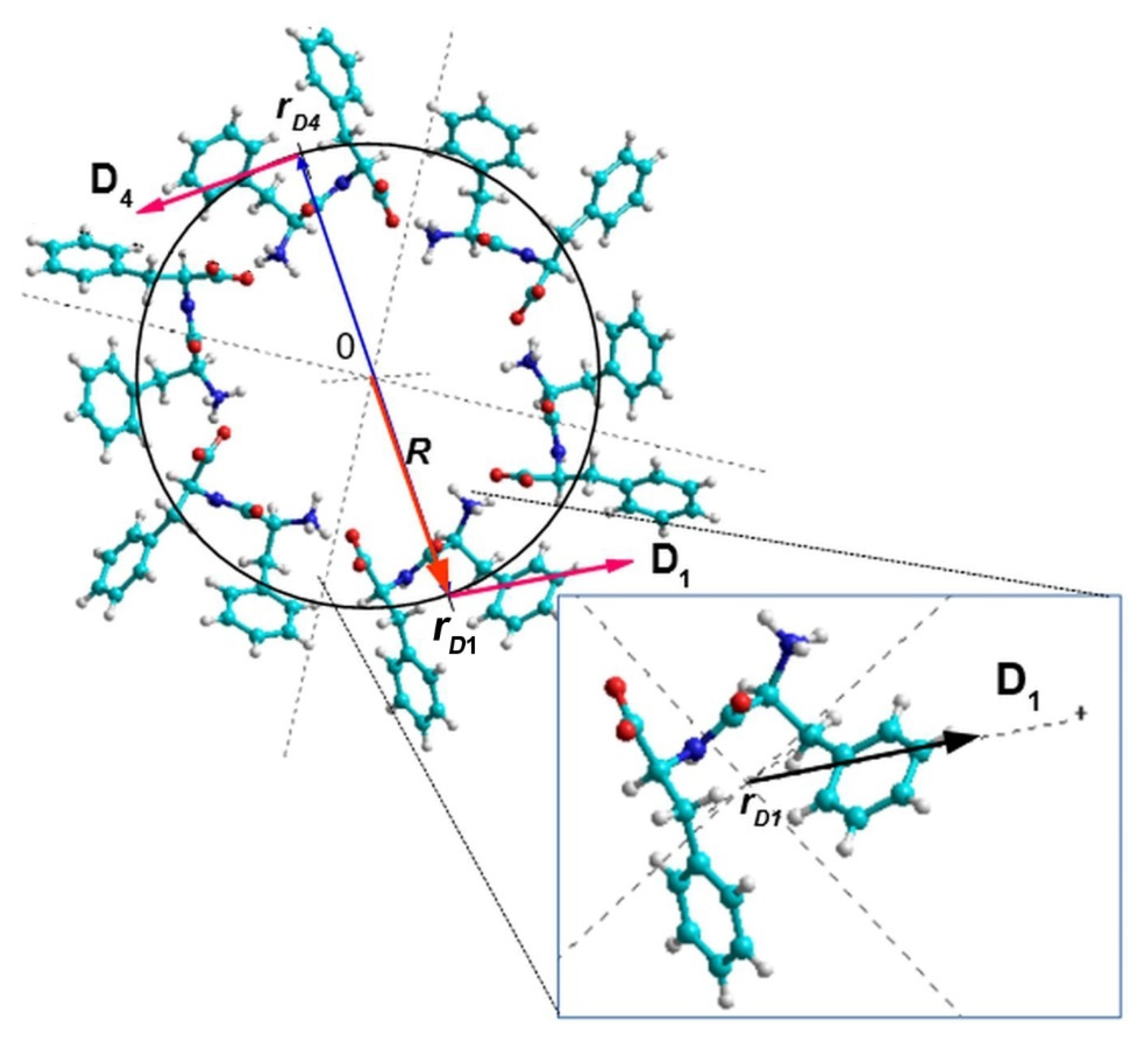
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehninger, A.L. Biochemistry—The Molecular Basis of Cell Structure and Function; Worth Publishers, Inc.: New York, NY, USA, 1972. [Google Scholar]
- Stock, D.; Leslie, A.G.; Walker, J.E. Molecular architecture of the rotary motor in ATP synthase. Science 1999, 286, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Ban, N.; McPherson, A. The structure of satellite panicum mosaic virus at 1.9 Å resolution. Nat. Struct. Biol. 1995, 2, 882–890. [Google Scholar] [CrossRef]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechn. Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Mendes, A.C.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-assembly in nature: Using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 582–612. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Shirazi, A.N.; Parang, K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 2014, 12, 3544–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tverdislov, V.A. Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 2013, 58, 128–132. [Google Scholar] [CrossRef]
- Malyshko, E.V.; Tverdislov, V.A. Chirality as a physical aspect of structure formation in biological macromolecular systems. IOP J. Phys. Conf. Ser. 2016, 741, 012065. [Google Scholar] [CrossRef] [Green Version]
- Tverdislov, V.A.; Malyshko, E.V. Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Tverdislov, V.A.; Malyshko, E.V. On regularities in the spontaneous formation of structural hierarchies in chiral systems of nonliving and living matter. Phys.-Uspekhi 2019, 62, 354–363. [Google Scholar] [CrossRef]
- Buda, A.B.; der Heyde, T.A.; Mislow, K. On Quantifying Chirality. Angew. Chem. Int. Ed. Engl. 1992, 31, 989–1007. [Google Scholar] [CrossRef]
- Weinberg, N.; Mislow, K. On chirality measures and chirality properties. Can. J. Chem. 2000, 78, 41–45. [Google Scholar] [CrossRef]
- Randic, M. Shape recognition and chirality measure: Reestablishing the link between similarity and dissimilarity in discrete space. Chemom. Intell. Lab. Syst. 1991, 10, 213–225. [Google Scholar] [CrossRef]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous symmetry measures. J. Am. Chem. Soc. 1992, 114, 7843–7851. [Google Scholar] [CrossRef]
- Mezey, P.G. Chirality Measures and Graph Representations. Comput. Math. Appl. 1997, 34, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Pinsky, M.; Dryzun, C.; Casanova, D.; Alemany, P.; Avnir, D. Analytical methods for calculating Continuous Symmetry Measures and the Chirality Measure. J. Comput. Chem. 2008, 29, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, M. Chirality and Symmetry Measures: A Transdisciplinary Review. Entropy 2003, 5, 271–312. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Long, H.; Xu, L. Graph Theoretical Representation of Atomic Asymmetry and Molecular Chirality of Benzenoids in Two-Dimensional Space. PLoS ONE 2014, 9, e102043. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Malyshko, E.V.; Kotov, A.R.; Tverdislov, V.A.; Ustinin, M.N. Quantitative Criteria of Chirality in Hierarchical Protein Structures. Biophysics 2019, 64, 155–166. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Levashova, N.T.; Malyshko, E.V.; Tverdislov, V.A. Autowave Self-Organization in the Folding of Proteins. Mosc. Univ. Phys. Bull. 2019, 74, 213–226. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Lutsenko, A.O.; Shpigun, D.K.; Malyshko, E.V.; Tverdislov, V.A. Methods for Determining the Chirality sign of the Helical and Superhelical Protein Structures. Biophysics 2021, 66, 421. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Malyshko, E.V.; Lutsenko, A.O.; Shpigun, D.K.; Bagrova, O.E. Protein Helical Structures: Defining Handedness and Localization Features. Symmetry 2021, 13, 879. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org (accessed on 30 August 2021).
- Yuan, C.; Ji, W.; Xing, R.; Li, J.; Gazit, E.; Yan, X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3, 567. [Google Scholar] [CrossRef]
- Hamley, I.W. Peptide Nanotubes. Angew. Chem. Int. Ed. 2014, 53, 6866–6881. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Zhulyabina, O.A.; Tverdislov, V.A. Chiral peculiar properties of self-organization of diphenylalanine peptide nanotubes: Modeling of structure and properties. Math. Biol. Bioinform. 2019, 14, 94–124. [Google Scholar] [CrossRef] [Green Version]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chem. Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer′s b-amyloid polypeptide. Chem. Commun. 2006, 1, 2332–2334. [Google Scholar] [CrossRef]
- Kim, J.; Han, T.E.; Kim, Y.; Park, J.S.; Choi, J.; Churchill, D.G.; Kim, S.O.; Ihee, H. Role of water in directing diphenylalanine assembly into nanotubes and nanowires. Adv. Mater. 2010, 22, 583–587. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Bdikin, I.; Heredia, A.; Pullar, R.C.; Mishina, E.; Sigov, A.; Kholkin, A.L. Piezoelectricity and Ferroelectricity in biomaterials: From proteins to self-assembled peptide nanotubes. In Piezoelectric Nanomaterials for Biomedical Applications; Ciofani, G., Menciassi, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 187–211. [Google Scholar]
- Bystrov, V.S. Computer Simulation Nanostructures: Bioferroelectric Peptide Nanotubes. Bioferroelectricity: Peptide Nanotubes; LAP Lambert Academic Publishing: Saarbruecken, Germay, 2016. [Google Scholar]
- Bystrov, V.S.; Paramonova, E.V.; Bdikin, I.K.; Kopyl, S.; Heredia, A.; Pullar, R.C.; Kholkin, A.L. Bioferroelectricity: Diphenylalanine peptide nanotubes computational modeling and ferroelectric properties at the nanoscale. Ferroelectrics 2012, 440, 3–24. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Zhulyabina, O.A.; Tverdislov, V.A. Molecular modeling and computational study of the chiral-dependent structures and properties of the self-assembling diphenylalanine peptide nanotubes. J. Mol. Modeling 2019, 25, 199. [Google Scholar] [CrossRef]
- Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Arkhipov, S.G.; Vasilev, S.G.; Bystrov, V.S.; Gruzdev, D.A.; Waliszek, M.; Svitlyk, V.; Shur, V.Y.; et al. Chirality-Dependent Growth of Self-Assembled Diphenylalanine Microtubes. Cryst. Growth Des. 2019, 19, 6414–6421. [Google Scholar] [CrossRef]
- Nuraeva, A.; Vasilev, S.; Vasileva, D.; Zelenovskiy, P.; Chezganov, D.; Esin, A.; Kopyl, S.; Romanyuk, K.; Shur, V.Y.; Kholkin, A.L. Evaporation-Driven Crystallization of Diphenylalanine Microtubes for Microelectronic Applications. Cryst. Growth Des. 2016, 16, 1472–1479. [Google Scholar] [CrossRef]
- Yuan, T.; Xu, Y.; Fei, J.; Xue, H.; Li, X.; Wang, C.; Fytas, G.; Li, J. The Ultrafast Assembly of a Dipeptide Supramolecular Organogel and its Phase Transition from Gel to Crystal. Angew. Chem. Int. Ed. 2019, 58, 11072–11077. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Filho, T.; Martins, T.C.; Ferreira, F.F.; Alves, W.A.; Rocha, A.R. Water-driven stabilization of diphenylalanine nanotube structures. Theor. Chem. Acc. 2016, 135, 185. [Google Scholar] [CrossRef] [Green Version]
- Bystrov, V.S.; Kopyl, S.A.; Zelenovskiy, P.; Zhulyabina, O.A.; Tverdislov, V.A.; Salehli, F.; Ghermani, N.E.; Shur, V.Y.; Kholkin, A.L. Investigation of physical properties of diphenylalanine peptide nanotubes having different chiralities and embedded water molecules. Ferroelectrics 2018, 525, 168–177. [Google Scholar] [CrossRef]
- Dayarian, S.; Kopyl, S.; Bystrov, V.; Correia, M.R.; Ivanov, M.S.; Pelegova, E.; Kholkin, A. Effect of the Chloride Anions on the Formation of Self-Assembled Diphenylalanine Peptide Nanotubes. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1563–1570. [Google Scholar] [CrossRef]
- Zelenovskiy, P.; Yuzhakov, V.; Nuraeva, A.; Kornev, M.; Shur, V.Y.; Kopyl, S.; Kholkin, A.; Vasilev, S.; Tofail, S.A.M. The Effect of Water Molecules on Elastic and Piezoelectric Properties of Diphenylalanine Microtubes. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1474–1477. [Google Scholar] [CrossRef]
- Guo, C.; Luo, Y.; Zhou, R.; Wei, G. Probing the Self-Assembly Mechanism of Diphenylalanine-Based Peptide Nanovesicles and Nanotubes. ACS Nano 2012, 6, 3907–3918. [Google Scholar] [CrossRef]
- Kholkin, A.; Amdursky, N.; Bdikin, I.; Gazit, E.; Rosenman, G. Strong piezoelectricity in bioinspired peptide nanotubes. ACS Nano 2010, 4, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Hereida, A.; Bdikin, I.; Kopyl, S.; Mishina, E.; Semin, S.; Sigov, A.; German, K.; Bystrov, V.; Gracio, J.; Kholkin, A.L. Temperature-driven phase transformation in self-assembled diphenylalanine peptide nanotubes. J. Phys. D Appl. Phys. 2010, 43, 462001. [Google Scholar] [CrossRef] [Green Version]
- Bdikin, I.; Bystrov, V.S.; Delgadillo, I.; Gracio, J.; Kopyl, S.; Wojtas, M.; Mishina, E.; Sigov, A.; Kholkin, A.L. Polarization switching and patterning in self-assembled peptide tubular structures. J. Appl. Phys. 2012, 111, 074104. [Google Scholar] [CrossRef] [Green Version]
- Reches, M.; Gazit, E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat. Nanotechnol. 2006, 1, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Araújo, D.R.; Silva, E.R.; Ando, R.A.; Alves, W.A. L-diphenylalanine microtubes as a potencial drug-delivery system: Characterization, release kinetics, and cytotoxicity. Langmuir 2013, 29, 10205–10212. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.; Zhu, R.; Jenkins, K.; Yang, R. Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat. Commun. 2016, 7, 13566. [Google Scholar] [CrossRef] [Green Version]
- Emtiazi, G.; Zohrabi, T.; Lee, L.Y.; Habibi, N.; Zarrabi, A. Covalent diphenylalanine peptide nanotube conjugated to folic acid/magnetic nanoparticles for anti-cancer drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 90–98. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, I.; Sharma, A.K.; Kumar, P. Ultrashort Peptide Self-Assembly: Front-Runners to Transport Drug and Gene Cargos. Front. Bioeng. Biotechnol. 2020, 8, 504. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [Green Version]
- Bystrov, V.S.; Coutinho, J.; Zelenovskiy, P.; Nuraeva, A.; Kopyl, S.; Zhulyabina, O.; Tverdislov, V. Structures and properties of the self-assembling diphenylalanine peptide nanotubes containing water molecules: Modeling and data analysis. Nanomaterials 2020, 10, 1999. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Coutinho, J.; Zhulyabina, J.A.; Kopyl, S.A.; Zelenovskiy, P.S.; Nuraeva, A.S.; Tverdislov, V.A.; Filippov, S.V.; Kholkin, A.L.; Shur, V.Y. Modeling and physical properties of diphenylalanine peptide nanotubes containing water molecules. Ferroelectrics 2021, 574, 78–91. [Google Scholar] [CrossRef]
- Tamamis, P.; Adler-Abramovich, L.; Reches, M.; Marshall, K.; Sikorski, P.; Serpell, L.; Gazit, E.; Archontis, G. Self-assembly of phenylalanine oligopeptides: Insights from experiments and simulations. Biophys. J. 2009, 96, 5020–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Mao, K.; Chen, S.; Zhu, H. Chirality Effects in Peptide Assembly Structures. Front. Bioeng. Biotechnol. 2021, 9, 703004. [Google Scholar] [CrossRef] [PubMed]
- Müller, U. Symmetry Relationships between Crystal Structures. Applications of Crystallographic Group Theory in Crystal Chemistry; University Press: Oxford, UK, 2013. [Google Scholar]
- Hahn, T. (Ed.) International Tables for Crystallography. Volume A: Space-Group Symmetry; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- HyperChem 8. Tools for Molecular Modeling. Professional Edition for Windows AC Release 8.0 USB (on CD); Hypercube. Inc.: Gainesville, FL, USA, 2011. [Google Scholar]
- The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk/ (accessed on 30 August 2021).
- VASP (Vienna Ab initio Simulation Package). Available online: https://www.vasp.at/ (accessed on 30 August 2021).
- CCDC Mercury. Available online: https://www.ccdc.cam.ac.uk/solutions/csd-system/components/mercury/ (accessed on 30 August 2021).
- Open Babel. Available online: https://openbabel.org/docs/dev/Installation/install.html (accessed on 30 August 2021).
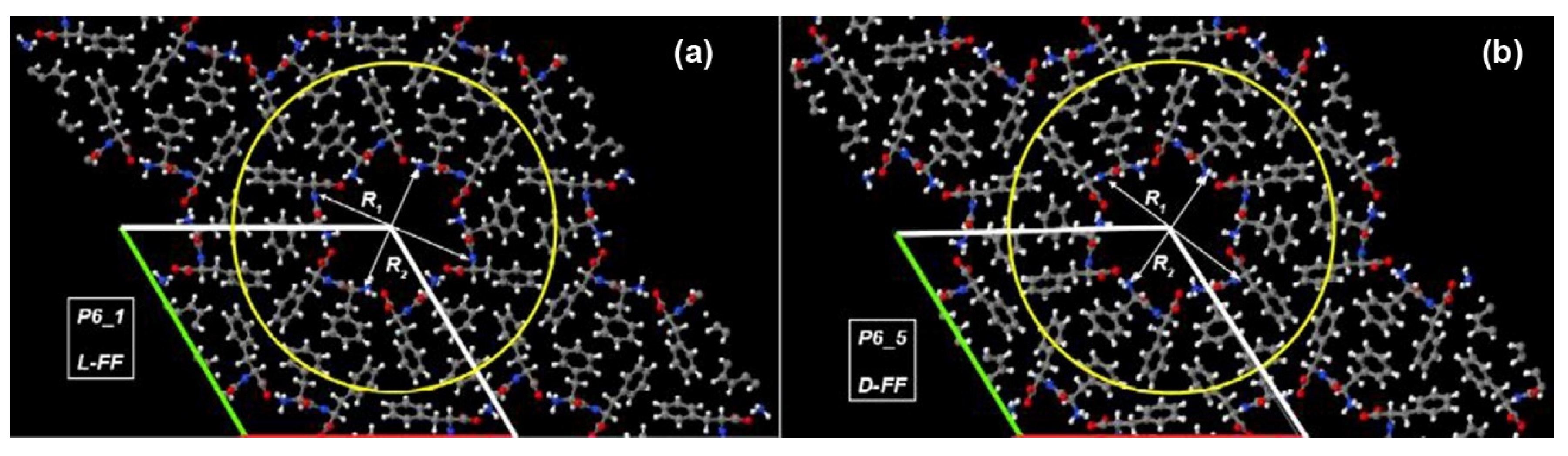
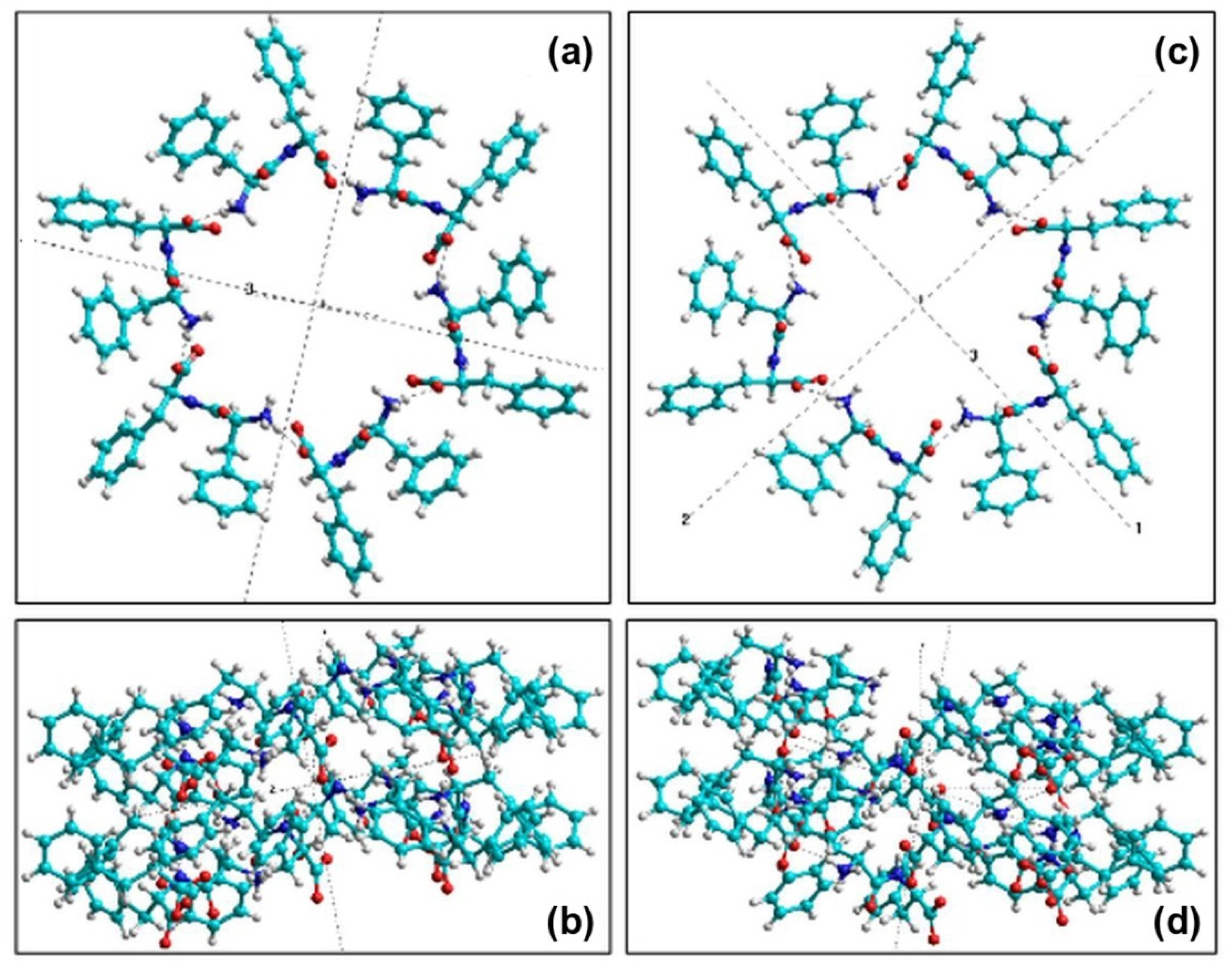
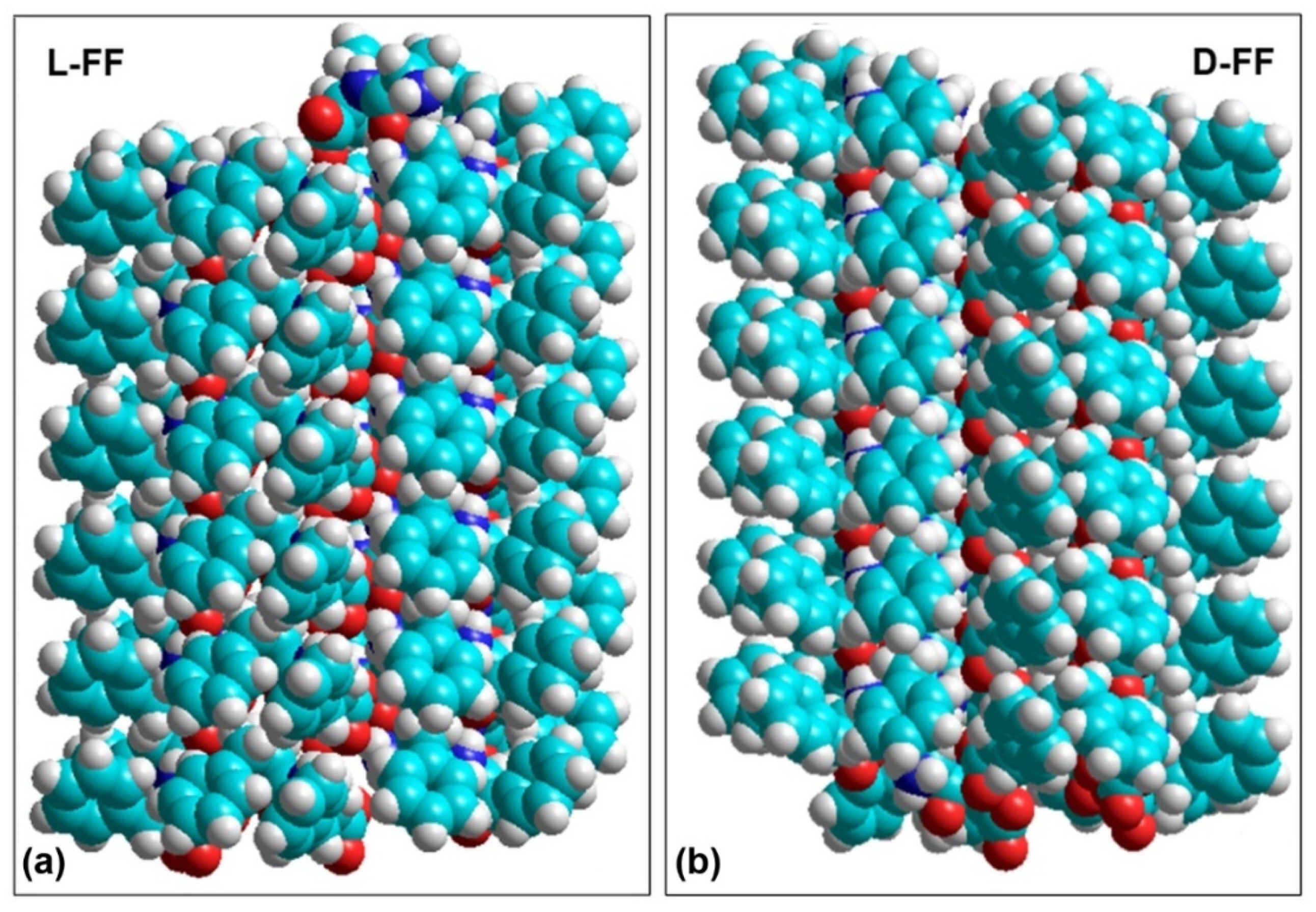

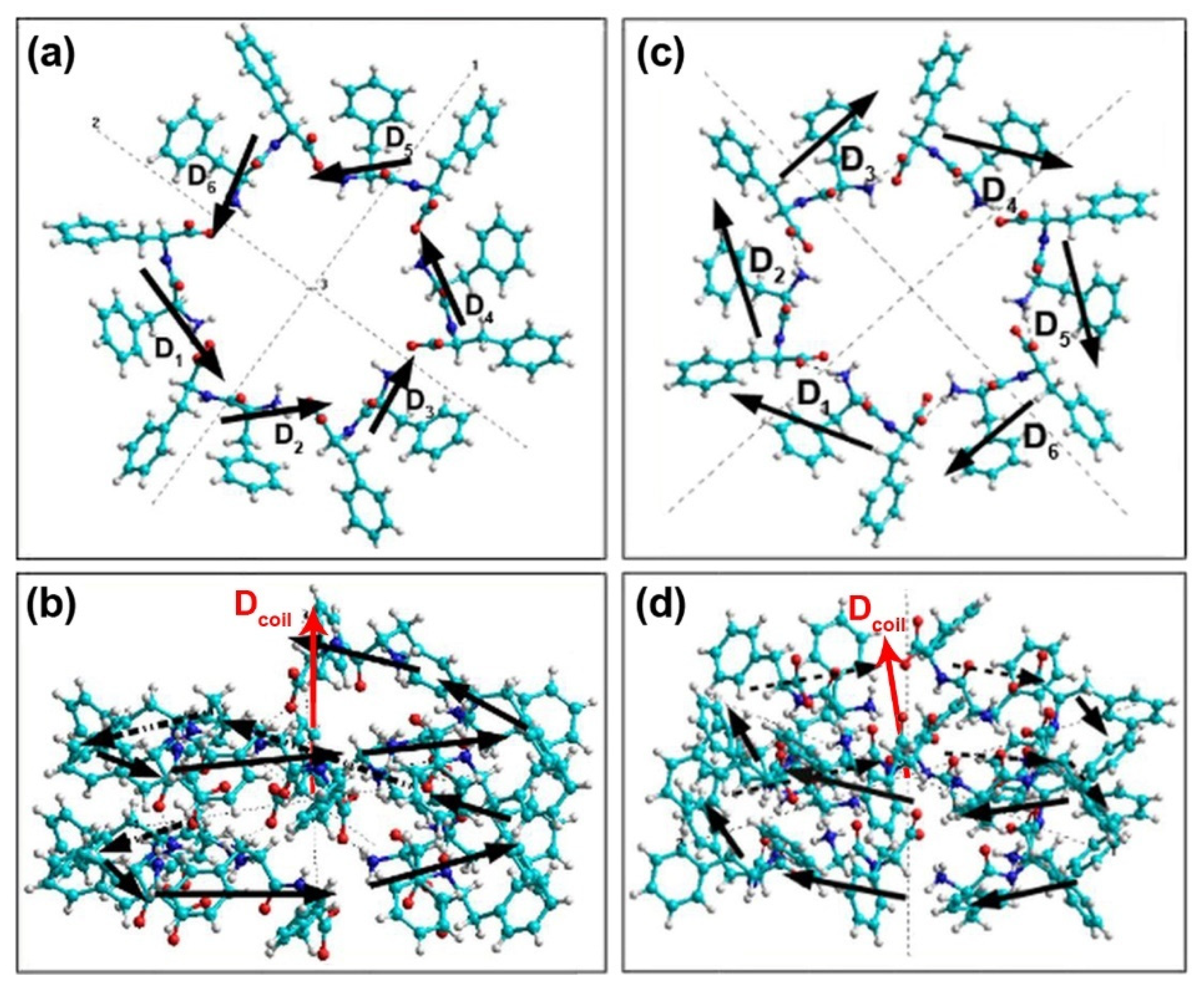
| L-FF | D-FF | |
| Space group | P61 | P65 |
| a, Å | 24.0709 | 23.9468 |
| b, Å | 24.0709 | 23.9468 |
| c, Å | 5.4560 | 5.4411 |
| V, Å3 | 2737.7 | 2702.2 |
| R1, Å | 15.3 | 15.2 |
| R2, Å | 12.2 | 12.1 |
| Molecule | Dx, Debye | Dy, Debye | Dz, Debye | Dtot, Debye | Van der Waals Volume, Å3 | Polarization, C/m2 | Total Energy, a.u. | RMS Gradient, a.u./Å |
|---|---|---|---|---|---|---|---|---|
| L-FF | 11.645 | 1.115 | 0.899 | 11.733 | 291.919 | 0.134 | −133.959 | ~0.06 |
| D-FF | −11.630 | 1.052 | 1.113 | 11.730 | 291.977 | 0.134 | −133.959 | ~0.07 |
| i | PM3 RHF | Amber | ||||||
|---|---|---|---|---|---|---|---|---|
| Di | Dx | Dy | Dz | Di | Dx | Dy | Dz | |
| 1 | 24.022 | 14.576 | −15.421 | −11.261 | 23.458 | 14.901 | −15.250 | −9.781 |
| 2 | 22.549 | −6.313 | −18.923 | −10.513 | 21.734 | −6.280 | −18.879 | −8.748 |
| 3 | 22.389 | −18.646 | −3.636 | −11.849 | 21.545 | −18.698 | −3.629 | −10.070 |
| 4 | 22.381 | −11.564 | 14.461 | −12.573 | 21.530 | −11.695 | 14.495 | −10.801 |
| 5 | 22.441 | 7.555 | 17.308 | −12.123 | 21.578 | 7.397 | 17.408 | −10.384 |
| 6 | 22.587 | 18.767 | 2.568 | −12.303 | 21.638 | 18.581 | 2.745 | −10.742 |
| Dcoil | 70.851 | 4.376 | −3.643 | −70.622 | 60.752 | 4.206 | −3.109 | −60.526 |
| Dav | 22.728 | 0.729 | −0.607 | −11.770 | 21.914 | 0.701 | −0.518 | −10.088 |
| i | PM3 RHF | Amber | ||||||
|---|---|---|---|---|---|---|---|---|
| Di | Dx | Dy | Dz | Di | Dx | Dy | Dz | |
| 1 | 22.523 | −12.228 | −15.267 | −11.167 | 21.707 | −12.299 | −15.170 | −9.475 |
| 2 | 22.340 | 7.302 | −18.014 | −11.072 | 21.527 | 7.210 | −17.995 | −9.360 |
| 3 | 22.372 | 19.234 | −2.597 | −11.125 | 21.520 | 19.168 | −2.656 | −9.416 |
| 4 | 22.475 | 11.905 | 15.905 | −11.290 | 21.625 | 11.914 | 15.274 | −9.612 |
| 5 | 22.629 | −6.613 | 17.478 | −12.761 | 21.703 | −6.487 | 17.386 | −11.256 |
| 6 | 23.855 | −19.820 | 4.382 | −12.531 | 23.271 | −19.893 | 4.727 | −11.112 |
| Dcoil | 69.971 | −0.218 | 1.888 | −69.945 | 60.253 | −0.387 | 1.565 | −60.231 |
| Dav | 22.704 | −0.036 | 0.315 | −11.658 | 21.892 | −0.064 | 0.261 | −10.038 |
| Type of PNT | L-FF | D-FF | ||
|---|---|---|---|---|
| Calculating Method | PM3 | Amber | PM3 | Amber |
| ctotal, Debye3 | 16,034.82 | 13,870.71 | −14,497.03 | −12,161.23 |
| cnorm | 1.37 | 1.32 | −1.23 | −1.16 |
| Chirality sign | positive | positive | negative | negative |
| Chirality symbol | D | D | L | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bystrov, V.; Sidorova, A.; Lutsenko, A.; Shpigun, D.; Malyshko, E.; Nuraeva, A.; Zelenovskiy, P.; Kopyl, S.; Kholkin, A. Modeling of Self-Assembled Peptide Nanotubes and Determination of Their Chirality Sign Based on Dipole Moment Calculations. Nanomaterials 2021, 11, 2415. https://doi.org/10.3390/nano11092415
Bystrov V, Sidorova A, Lutsenko A, Shpigun D, Malyshko E, Nuraeva A, Zelenovskiy P, Kopyl S, Kholkin A. Modeling of Self-Assembled Peptide Nanotubes and Determination of Their Chirality Sign Based on Dipole Moment Calculations. Nanomaterials. 2021; 11(9):2415. https://doi.org/10.3390/nano11092415
Chicago/Turabian StyleBystrov, Vladimir, Alla Sidorova, Aleksey Lutsenko, Denis Shpigun, Ekaterina Malyshko, Alla Nuraeva, Pavel Zelenovskiy, Svitlana Kopyl, and Andrei Kholkin. 2021. "Modeling of Self-Assembled Peptide Nanotubes and Determination of Their Chirality Sign Based on Dipole Moment Calculations" Nanomaterials 11, no. 9: 2415. https://doi.org/10.3390/nano11092415






