Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation
Abstract
:1. Introduction
2. Materials and Methods
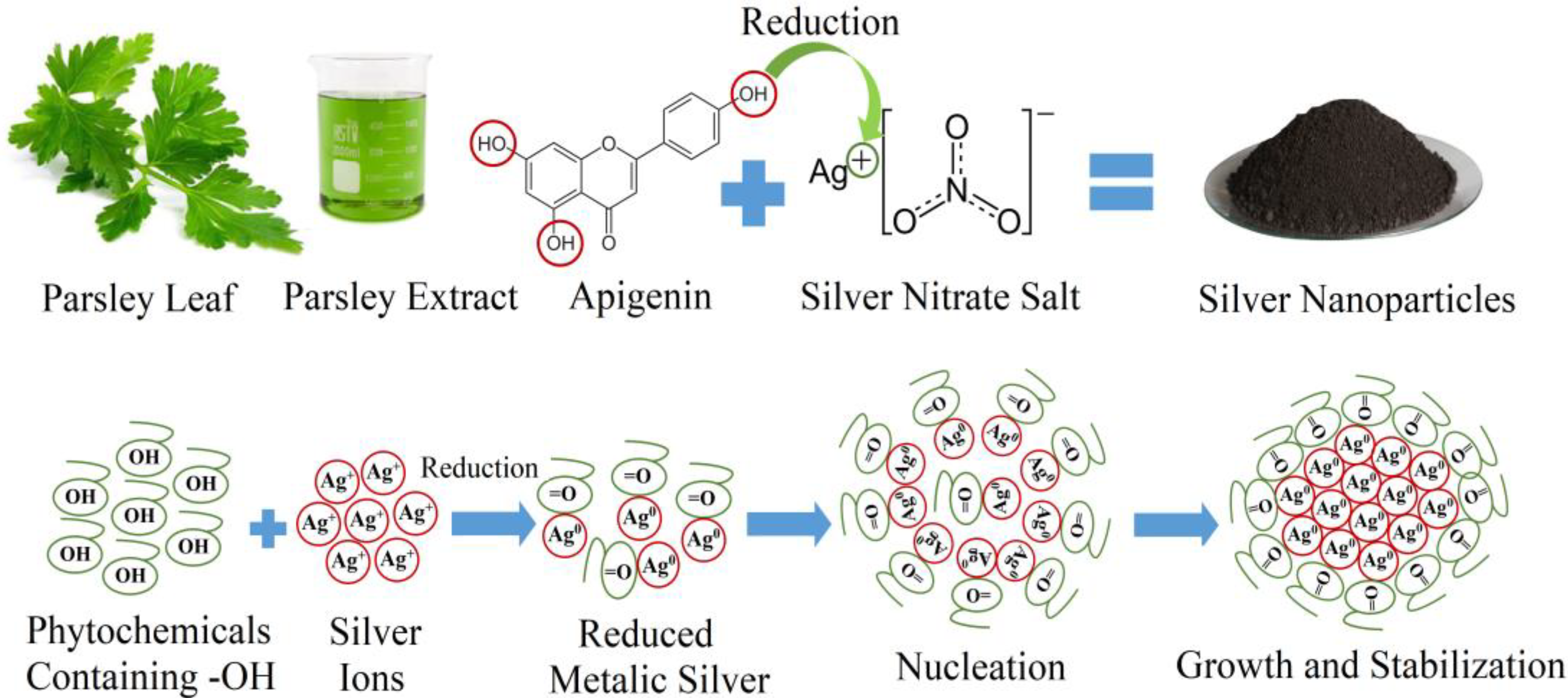
2.1. Preparation of the Parsley Extract
2.2. Synthesis of Silver Nanoparticles
2.3. Characterization of Ag NPs
2.4. Increasing Temperature during Irradiation of the System
3. Results and Discussion
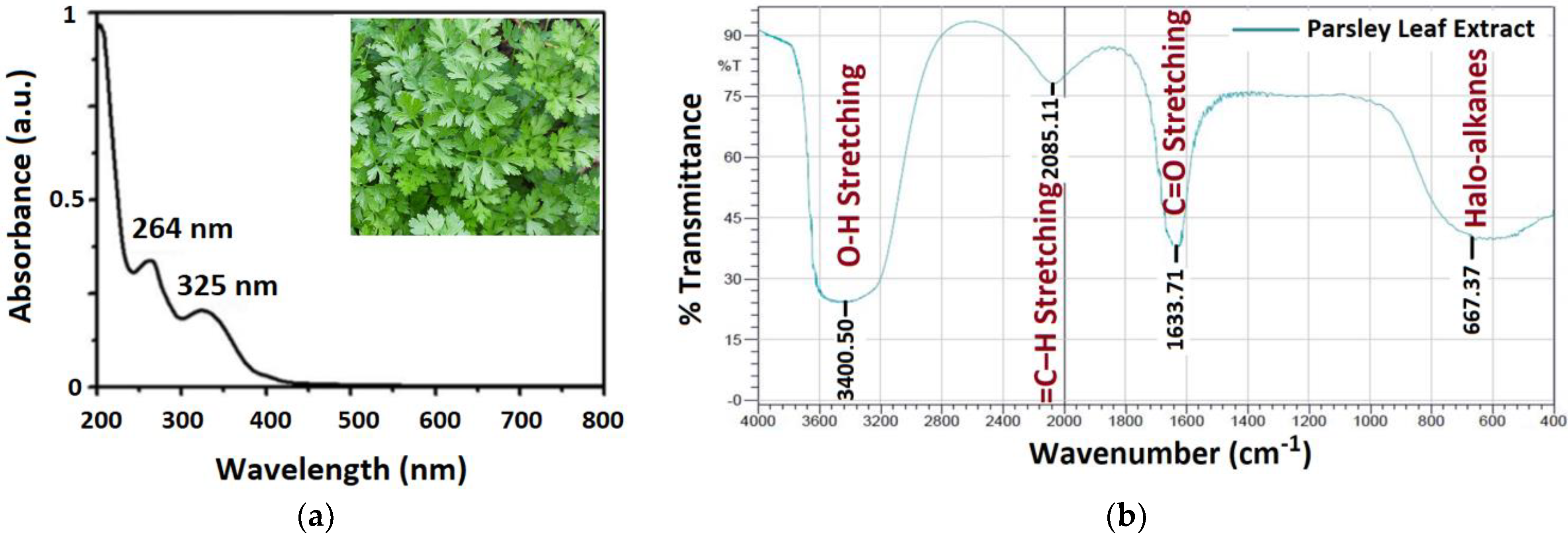
3.1. Characterization of Parsley Leaf Extract
3.2. Characterization of Ag NPs
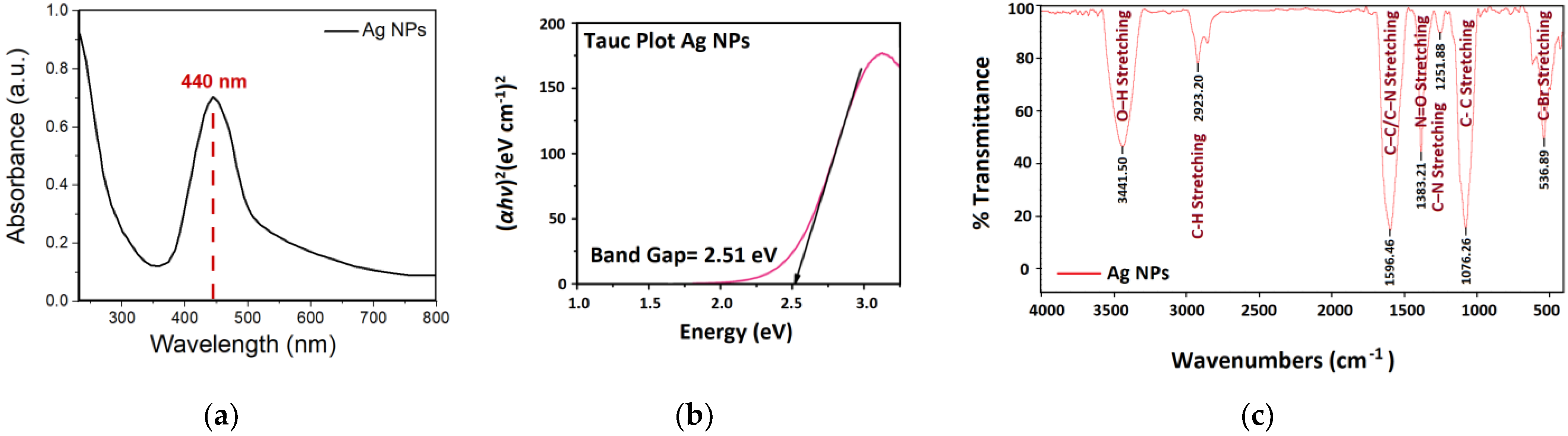
3.2.1. UV-Vis Spectrum of the Biosynthesized Ag NPs
3.2.2. FTIR Spectrum Analysis for Ag NPs
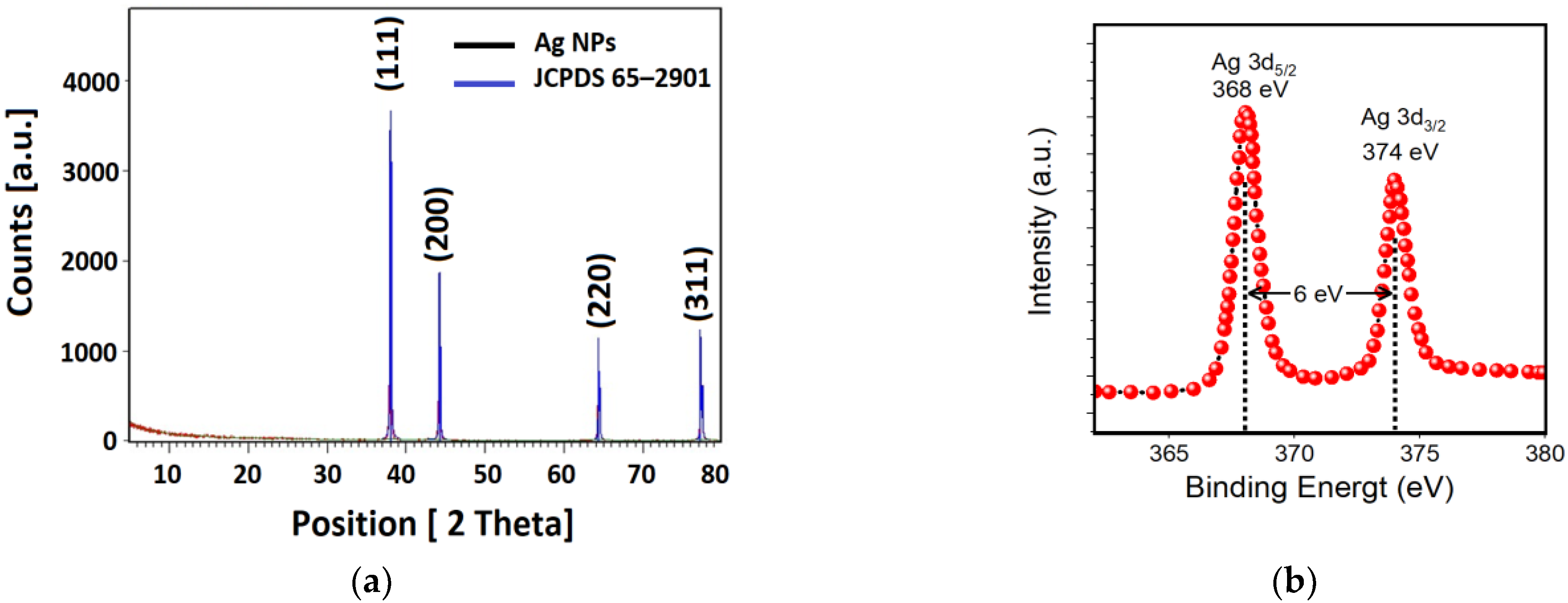
3.2.3. X-ray Diffraction (XRD) Pattern and X-ray Photoelectron Spectroscopy (XPS) of Ag NPs
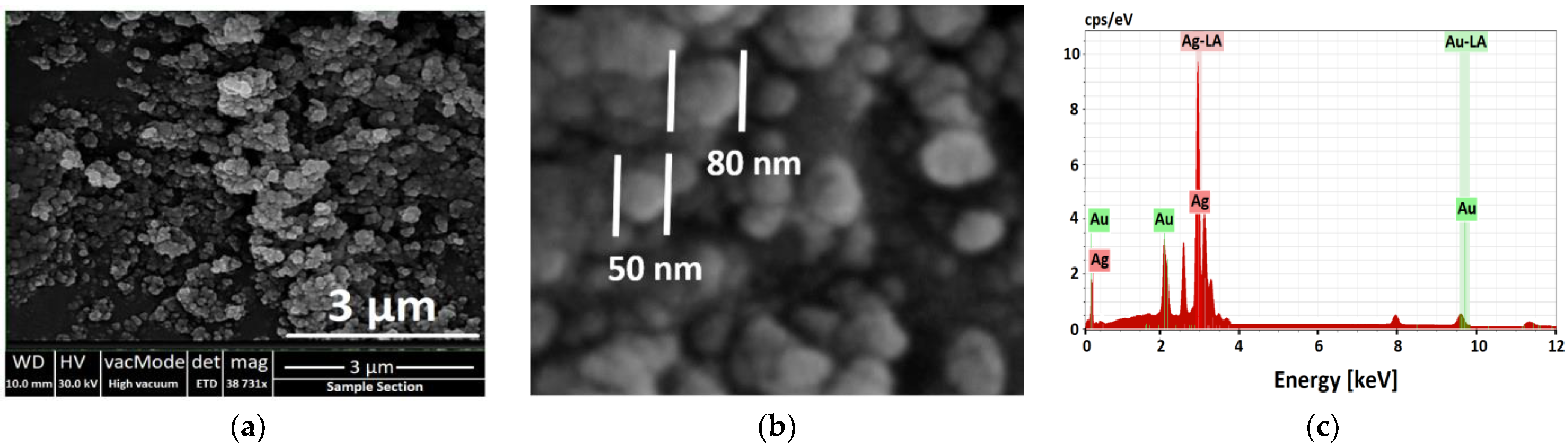
3.2.4. SEM and EDX Analysis of Ag NPs
3.2.5. Transmission Electron Microscopy Analysis of Ag NPs
3.2.6. TGA–DSC Analysis
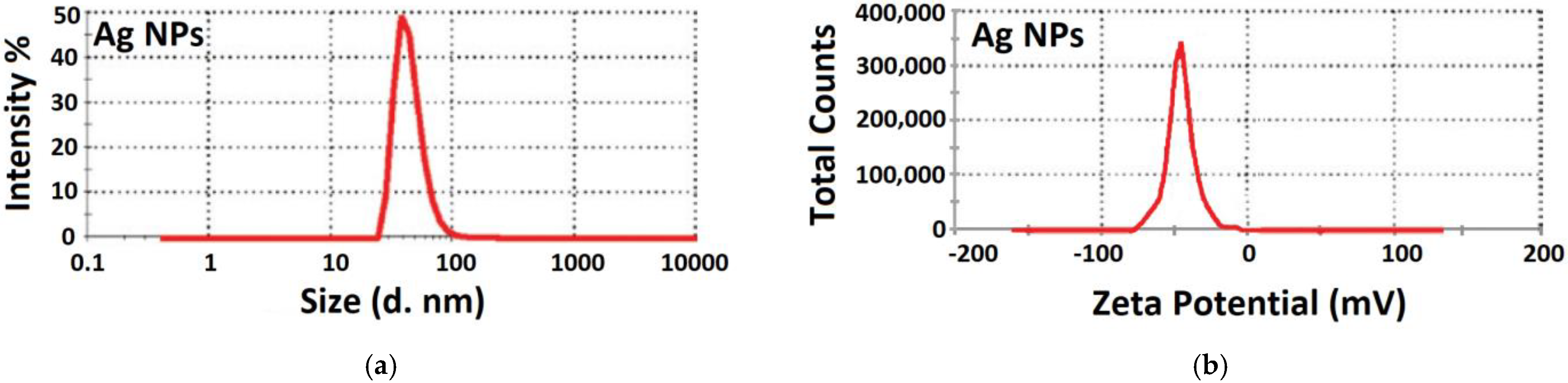
3.2.7. Particle Size Distribution and Zeta Potential Measurement
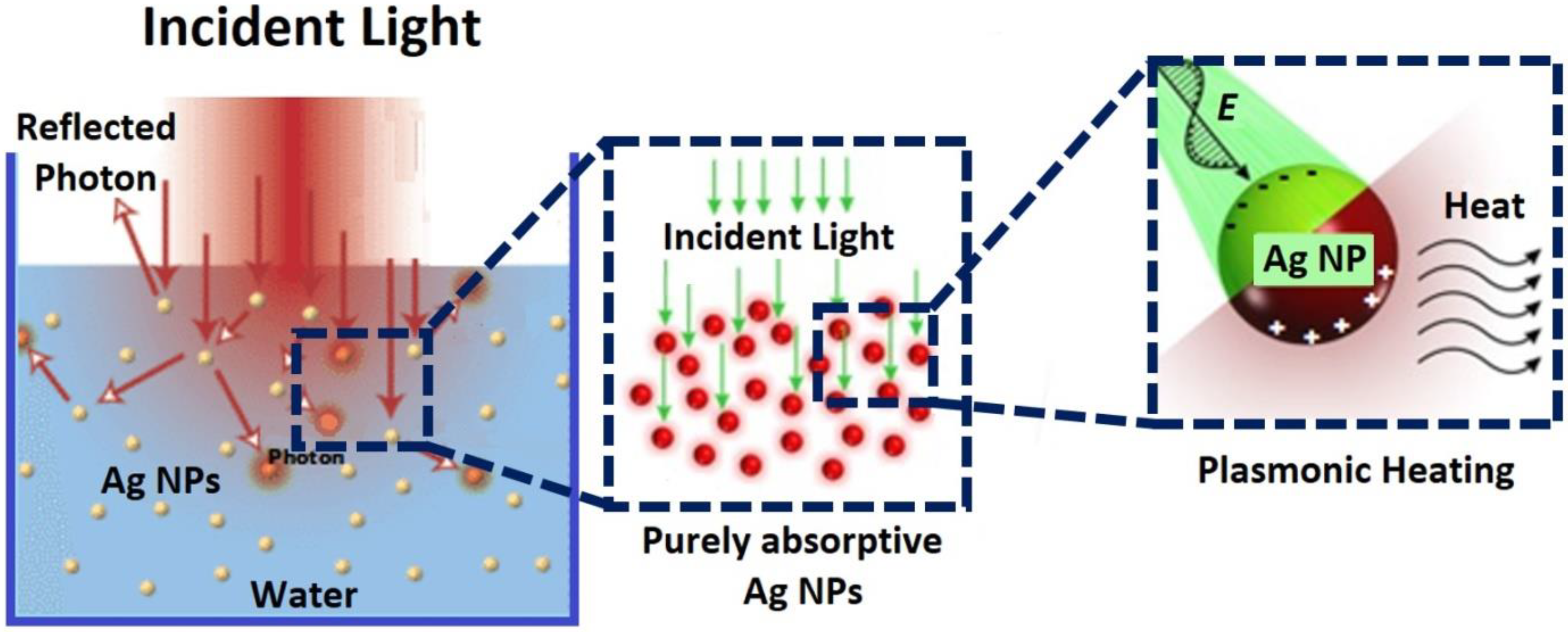
3.3. Solar Energy Harvesting Using Ag NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burger, J.R.; Brown, J.H.; Day, J.W.; Flanagan, T.P.; Roy, E.D. The Central Role of Energy in the Urban Transition: Global Challenges for Sustainability. Biophys. Econ. Resour. Qual. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Honcharuk, I.; Babyna, O. Dominant trends of innovation and investment activities in the development of alternative energy sources. Wschod. Czas. Nauk. 2020, 54, 6–12. [Google Scholar]
- Dupont, E.; Koppelaar, R.; Jeanmart, H. Global available solar energy under physical and energy return on investment constraints. Appl. Energy 2019, 257, 113968. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Vijila, C.; Jose, R.; Uddin, A.; Ramakrishna, S. Metal oxide semiconducting interfacial layers for photovoltaic and photocatalytic applications. Mater. Renew. Sustain. Energy 2015, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Wang, H.; Wang, C.; Luo, H. Advanced Nanomaterials and Nanotechnologies for Solar Energy. Int. J. Photoenergy 2019, 2019, 8437964. [Google Scholar] [CrossRef]
- Ibrahim, K.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Coronado, E.A.; Encina, E.R.; Stefani, F. Optical properties of metallic nanoparticles: Manipulating light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S.; Fisher, J. Effect of metallic nanoparticles on the biotribocorrosion behaviour of Metal-on-Metal hip prostheses. Wear 2009, 267, 683–688. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.D., Jr.; Alam, T.E.; Kamal, R.; Goswami, D.Y.; Stefanakos, E. Nitrate salts doped with CuO nanoparticles for thermal energy storage with improved heat transfer. Appl. Energy 2016, 165, 225–233. [Google Scholar] [CrossRef]
- Kamat, P. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Element Res. 2020, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Hvolbæk, B.; Janssens, T.V.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Eastman, J. Novel Thermal Properties of Nanostructured Materials; Argonne National Lab.: Lemont, IL, USA, 1999.
- Li, Y.; Shakeriaski, F.; Barzinjy, A.A.; Dara, R.N.; Shafee, A.; Tlili, I. Nanomaterial thermal treatment along a permeable cylinder. J. Therm. Anal. Calorim. 2019, 139, 3309–3315. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef] [Green Version]
- Löberg, J.; Holmberg, J.P.; Mattisson, I.; Arvidsson, A.; Ahlberg, E. Electronic Properties of TiO2 Nanoparticles Films and the Effect on Apatite-Forming Ability. Int. J. Dent. 2013, 2013, 139615. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Shabat, M.M.; Schaadt, D.M. Analytical modeling of power transfer via metallic nanoparticles in a solar cell absorber. J. Quant. Spectrosc. Radiat. Transf. 2019, 243, 106807. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, K.; Jaggi, N. Localized Surface Plasmonic Properties of Au and Ag Nanoparticles for Sensors: A Review. Plasmonics 2021, 16, 981–999. [Google Scholar] [CrossRef]
- Islam, A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef]
- Tulinski, M.; Jurczyk, M. Nanomaterials synthesis methods. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Wiley: Weinheim, Germany, 2017; pp. 75–98. [Google Scholar]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Biswas, K. Characterization of Nanomaterials by Physical Methods. Annu. Rev. Anal. Chem. 2009, 2, 435–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modan, E.M.; Plăiașu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. “Dunarea de Jos” Univ. Galati Fascicle IX Met. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Amin, S.; Solangi, A.R.; Hassan, D.; Hussain, N.; Ahmed, J.; Baksh, H. Recent Trends in Development of Nanomaterials Based Green Analytical Methods for Environmental Remediation. Curr. Anal. Chem. 2021, 17, 438–448. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2020, 264, 128580. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Shamaila, S.; Sajjad, A.K.L.; Ryma, N.-U.; Farooqi, S.A.; Jabeen, N.; Majeed, S.; Farooq, I. Advancements in nanoparticle fabrication by hazard free eco-friendly green routes. Appl. Mater. Today 2016, 5, 150–199. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [Green Version]
- Keshavamurthy, M.; Srinath, B.S.; Rai, V.R. Phytochemicals-mediated green synthesis of gold nanoparticles using Pterocarpus santalinus L. (Red Sanders) bark extract and their antimicrobial properties. Part. Sci. Technol. 2017, 36, 785–790. [Google Scholar] [CrossRef]
- Zarei, Z.; Razmjoue, D.; Karimi, J. Green Synthesis of Silver Nanoparticles from Caralluma tuberculata Extract and its Antibacterial Activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4606–4614. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, D.; Okram, G.S. Influence of surfactant, particle size and dispersion medium on surface plasmon resonance of silver nanoparticles. J. Phys. Condens. Matter 2019, 32, 145302. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, L.R.; Hess, O.; Lischner, J. Single plasmon hot carrier generation in metallic nanoparticles. Commun. Phys. 2019, 2, 47. [Google Scholar] [CrossRef] [Green Version]
- Zada, A.; Muhamma, P.; Ahmad, W.; Hussain, Z.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Surface Plasmonic-Assisted Photocatalysis and Optoelectronic Devices with Noble Metal Nanocrystals: Design, Synthesis, and Applications. Adv. Funct. Mater. 2020, 30, 1906744. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Schuller, J.A.; Barnard, E.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, J.; Jiang, Y.; Jiang, L.; Chen, X. Plasmonic Enhanced Optoelectronic Devices. Plasmonics 2014, 9, 859–866. [Google Scholar] [CrossRef]
- Magnozzi, M.; Ferrera, M.; Mattera, L.; Canepa, M.; Bisio, F. Plasmonics of Au nanoparticles in a hot thermodynamic bath. Nanoscale 2019, 11, 1140–1146. [Google Scholar] [CrossRef]
- Tong, L.; Wei, H.; Zhang, S.; Xu, H. Recent Advances in Plasmonic Sensors. Sensors 2014, 14, 7959–7973. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Azeeza, H.H.; Barzinjya, A.A. Biosynthesis zinc oxide nanoparticles using Apium graveolens L. leaf extract and its use in removing the organic pollutants in water. Desalination Water Treat. 2020, 190, 179–192. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Abdul, D.A.; Hussain, F.H.S.; Hamad, S.M. Green synthesis of the magnetite (Fe3O4) nanoparticle using Rhus coriaria extract: A reusable catalyst for efficient synthesis of some new 2-naphthol bis-Betti bases. Inorg. Nano-Metal. Chem. 2020, 50, 620–629. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Hamad, S.M.; Abdulrahman, A.F.; Biro, S.J.; Ghafor, A.A. Biosynthesis, Characterization and Mechanism of Formation of ZnO Nanoparticles Using Petroselinum Crispum Leaf Extract. Curr. Org. Synth. 2020, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Barzinjy, A.A.; Hamad, S.M.; Esmaeel, M.M.; Aydın, S.K.; Hussain, F.H.S. Biosynthesis and characterisation of zinc oxide nanoparticles from Punica granatum (pomegranate) juice extract and its application in thin films preparation by spin-coating method. Micro Nano Lett. 2020, 15, 415–420. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Hamad, S.M.; Aydın, S.; Ahmed, M.H.; Hussain, F.H.S. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 11303–11316. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Maham, M.; Sajadi, S.M.; Barzinjy, A.A. Biosynthesis of the palladium/sodium borosilicate nanocomposite using Euphorbia milii extract and evaluation of its catalytic activity in the reduction of chromium(VI), nitro compounds and organic dyes. Mater. Res. Bull. 2018, 102, 24–35. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Kolo, K.; Hamad, S.M.; Mahmud, S.A.; Barzinjy, A.A.; Hussein, S.M. Green Synthesis of the Ag/Bentonite Nanocomposite Using Euphorbia larica Extract: A Reusable Catalyst for Efficient Reduction of Nitro Compounds and Organic Dyes. ChemistrySelect 2018, 3, 12274–12280. [Google Scholar] [CrossRef]
- Barzinjy, A.; Mustafa, S.; Ismael, H. Characterization of ZnO NPs Prepared from Green Synthesis Using Euphorbia Petiolata Leaves. EAJSE 2019, 4, 74–83. [Google Scholar]
- Sarwar, S.; Ayyub, M.A.; Rezgui, M.; Nisar, S.; Jilani, M.I. Parsley: A review of habitat, phytochemistry, ethnopharmacology and biological activities. Int. J. Chem. Biochem. Sci 2019, 9, 49–55. [Google Scholar]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Makarov, V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Sarmanovna, T.Z. Phytochemical Study of Odorous Celery Root (Apium graveolens L.) Grown in the North Caucasus. Pharmacogn. J. 2019, 11, 527–530. [Google Scholar] [CrossRef] [Green Version]
- Mencherini, T.; Cau, A.; Bianco, G.; Della Loggia, R.; Aquino, R.P.; Autore, G. An extract of Apium graveolens var. dulce leaves: Structure of the major constituent, apiin, and its anti-inflammatory properties. J. Pharm. Pharmacol. 2007, 59, 891–897. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Kósa, A.; Boldizsár, I.; Ruszkai, A.; Balogh, E.; Klebovich, I.; Antal, I. Pharmaceutical and formulation aspects of Petroselinum crispum extract. Acta Pharm. Hung. 2012, 82, 3–14. [Google Scholar]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef]
- Sameh, B.; Ibtissem, B.; Mahmoud, A.; Boukef, K.; Boughattas, N.A. Antioxidant activity of Apium graveolens extracts. J. Biol. Act. Prod. Nat. 2011, 1, 340–343. [Google Scholar]
- Liu, G.; Zhuang, L.; Song, D.; Lu, C.; Xu, X. Isolation, purification, and identification of the main phenolic compounds from leaves of celery (Apium graveolens L. var. dulce Mill./Pers.). J. Sep. Sci. 2017, 40, 472–479. [Google Scholar] [PubMed]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H.M. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal. Agric. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Isaac, R.; Sakthivel, G.; Murthy, C. Green Synthesis of Gold and Silver Nanoparticles Using Averrhoa bilimbi Fruit Extract. J. Nanotechnol. 2013, 2013, 906592. [Google Scholar] [CrossRef] [Green Version]
- Sathyavathi, R.; Krishna, M.B.; Rao, S.V.; Saritha, R.; Rao, D.N. Biosynthesis of Silver Nanoparticles Using Coriandrum Sativum Leaf Extract and Their Application in Nonlinear Optics. Adv. Sci. Lett. 2010, 3, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Niraimathi, K.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Umadevi, M.; Shalini, S.; Bindhu, M. Synthesis of silver nanoparticle using D. carota extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025008. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Magara, H.; Herbani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021–1024. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Peethambar, S.K.; Rajeshwara, A.N.; Satyanarayan, N.D. Green Synthesis of Silver Nanoparticles Using Acacia farnesiana (Sweet Acacia) Seed Extract Under Microwave Irradiation and Their Biological Assessment. J. Clust. Sci. 2013, 24, 1081–1092. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, P.; Kumari, P.; Aarti, C.; Renganathan, A. Synthesis and Characterization of Silver Nanoparticles Using Cannonball Leaves and Their Cytotoxic Activity against MCF-7 Cell Line. J. Nanotechnol. 2013, 2013, 598328. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, S.; Masumeh, G.S.; Abbas, S.S.; Soheila, S. Comparative study on silver nanoparticles properties produced by green methods. Iran. J. Biotechnol. 2012, 10, 191–197. [Google Scholar]
- Fu, M.; Li, Q.; Sun, D.; Lu, Y.; He, N.; Deng, X.; Wang, H.; Huang, J. Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin. J. Chem. Eng. 2006, 14, 114–117. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic Conversion of Ag+ to highly Stable Ag0 Nanoparticles by Wild Type and Cell Wall Deficient Strains of Chlamydomonas reinhardtii. Molecules 2018, 24, 98. [Google Scholar] [CrossRef] [Green Version]
- Kuntyi, O.I.; Kytsya, A.R.; Mertsalo, I.P.; Mazur, A.S.; Zozula, G.I.; Bazylyak, L.; Topchak, R.V. Electrochemical synthesis of silver nanoparticles by reversible current in solutions of sodium polyacrylate. Colloid Polym. Sci. 2019, 297, 689–695. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F. Phytofabrication of Silver Nanoparticles (AgNPs) with Pharmaceutical Capabilities Using Otostegia persica (Burm.) Boiss. Leaf Extract. Nanomaterials 2021, 11, 1045. [Google Scholar] [CrossRef]
- Dar, M.A.; Ingle, A.; Rai, M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 105–110. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size-and shape-controlled silver nanoparticles. Met. Nanomater. Part B 2018, 1B, 1–116. [Google Scholar]
- Aziz, A.; Khalid, M.; Akhtar, M.S.; Nadeem, M.; Gilani, Z.A.; Ul Huda Khan Asghar, H.M.N.; Rehman, J.; Ullah, Z.; Saleem, M. Structural, morphological and optical investigations of silver nanoparticles synthesized by sol-gel auto-combustion method. Dig. J. Nanomater. Biostruct. 2018, 13, 679–683. [Google Scholar]
- Das, A.J.; Kumar, R.; Goutam, S.P. Sunlight Irradiation Induced Synthesis of Silver Nanoparticles using Glycolipid Bio-surfactant and Exploring the Antibacterial Activity. J. Bioeng. Biomed. Sci. 2016, 6, 5. [Google Scholar] [CrossRef]
- Mistry, H.; Thakor, R.; Patil, C.; Trivedi, J.; Bariya, H. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol. Lett. 2020, 43, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, L.; Arunachalam, J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef] [PubMed]
- Prakasham, R.S. Characterization of Silver Nanoparticles Synthesized by Using Marine Isolate Streptomyces albidoflavus. J. Microbiol. Biotechnol. 2012, 22, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Jemal, K.; Sandeep, B.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 4213275. [Google Scholar] [CrossRef] [Green Version]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Structural and Optical Properties of Ag Nanoparticles Synthesized by Thermal Treatment Method. Materials 2017, 10, 402. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2015, 9, 385–406. [Google Scholar]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Eltarahony, M.; Ibrahim, A.; El-Shall, H.; Ibrahim, E.; Althobaiti, F.; Fayad, E. Antibacterial, Antifungal and Antibiofilm Activities of Silver Nanoparticles Supported by Crude Bioactive Metabolites of Bionanofactories Isolated from Lake Mariout. Molecules 2021, 26, 3027. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.; Hassan, S.E.-D.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar] [CrossRef]
- Bakhtiari-Sardari, A.; Mashreghi, M.; Eshghi, H.; Behnam-Rasouli, F.; Lashani, E.; Shahnavaz, B. Comparative evaluation of silver nanoparticles biosynthesis by two cold-tolerant Streptomyces strains and their biological activities. Biotechnol. Lett. 2020, 42, 1985–1999. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-Mediated Green Synthesis of Silver Nanoparticles Exhibiting Antioxidant and Anticancer Activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Mollick, M.M.R.; Bhowmick, B.; Maity, D.; Mondal, D.; Bain, M.K.; Bankura, K.; Rana, D.; Acharya, K.; Chattopadhyay, D. Green synthesis of silver nanoparticles using Paederia foetida L. leaf extract and assessment of their antimicrobial activities. Int. J. Green Nanotechnol. 2012, 4, 230–239. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Ahani, M.; Khatibzadeh, M. Optimisation of significant parameters through response surface methodology in the synthesis of silver nanoparticles by chemical reduction method. Micro Nano Lett. 2017, 12, 705–710. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.; Rego, A. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kądzioła-Długołęcka, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef] [Green Version]
- Ghaemi, F.; Abdullah, L.C.; Kargarzadeh, H.; Abdi, M.M.; Azli, N.F.W.M.; Abbasian, M. Comparative Study of the Electrochemical, Biomedical, and Thermal Properties of Natural and Synthetic Nanomaterials. Nanoscale Res. Lett. 2018, 13, 112. [Google Scholar] [CrossRef]
- Arabshahi-D, S.; Devi, D.V.; Urooj, A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 2007, 100, 1100–1105. [Google Scholar] [CrossRef]
- Traiwatcharanon, P.; Timsorn, K.; Wongchoosuk, C. Flexible room-temperature resistive humidity sensor based on silver nanoparticles. Mater. Res. Express 2017, 4, 085038. [Google Scholar] [CrossRef]
- Manam, V.K. Biosynthesis and Characterization of Silver Nanoparticles From Marine Macroscopic Red Seaweed Halymenia Porphyroides Boergesen (Crypton) and Its Antifungal Efficacy Against Dermatophytic and Non-Dermatophytic Fungi. Asian J. Pharm. Clin. Res. 2020, 174–181. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Bharti, A.; Meena, V.K. Structural, thermal, zeta potential and electrical properties of disaccharide reduced silver nanoparticles. J. Mater. Sci. Mater. Electron. 2014, 25, 3747–3752. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Bobbu, P.; Gaddam, S.A.; Tartte, V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti-microbial studies. 3 Biotech 2016, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.P.; Lahtinen, M.; Särkkä, H.; Sillanpää, M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B Biointerfaces 2010, 80, 26–33. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Panoiu, N.C.; Sha, W.E.I.; Lei, D.Y.; Li, G.-C. Nonlinear optics in plasmonic nanostructures. J. Opt. 2018, 20, 083001. [Google Scholar] [CrossRef] [Green Version]
- Boriskina, S.V.; Ghasemi, H.; Chen, G. Plasmonic materials for energy: From physics to applications. Mater. Today 2013, 16, 375–386. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; O’Donnell, S.; Sang, D.L.; Maggard, P.A.; Wang, G. Harnessing Plasmon-Induced Hot Carriers at the Interfaces with Ferroelectrics. Front. Chem. 2019, 7, 299. [Google Scholar] [CrossRef] [Green Version]
- De Aberasturi, D.J.; Serrano-Montes, A.B.; Liz-Marzán, L.M. Modern Applications of Plasmonic Nanoparticles: From Energy to Health. Adv. Opt. Mater. 2015, 3, 602–617. [Google Scholar] [CrossRef]
- Okonkwo, E.C.; Wole-Osho, I.; Almanassra, I.W.; Abdullatif, Y.M.; Al-Ansari, T. An updated review of nanofluids in various heat transfer devices. J. Therm. Anal. Calorim. 2020, 145, 2817–2872. [Google Scholar] [CrossRef]
- Zhang, Z.; Van Steendam, K.; Maji, S.; Balcaen, L.; Anoshkina, Y.; Zhang, Q.; Vanluchene, G.; De Rycke, R.; Van Haecke, F.; Deforce, D.; et al. Tailoring Cellular Uptake of Gold Nanoparticles Via the Hydrophilic-to-Hydrophobic Ratio of their (Co)polymer Coating. Adv. Funct. Mater. 2015, 25, 3433–3439. [Google Scholar] [CrossRef]
- Xiong, Q.; Ayani, M.; Barzinjy, A.A.; Dara, R.N.; Shafee, A.; Nguyen-Thoi, T. Modeling of heat transfer augmentation due to complex-shaped turbulator using nanofluid. Phys. A Stat. Mech. Its Appl. 2020, 540. [Google Scholar] [CrossRef]
- Tlili, I.; Alkanhal, T.A.; Barzinjy, A.A.; Dara, R.N.; Shafee, A.; Li, Z. Investigation of thermal characteristics of carbon nanotubes: Measurement and dependence. J. Mol. Liq. 2019, 294, 111564. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, H.; Shan, X.; Di, Y.; Zhao, A.; Hu, Y.; Gan, Z. Solar steam generation based on the photothermal effect: From designs to applications, and beyond. J. Mater. Chem. A 2019, 7, 19203–19227. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Yu, J.; Zhou, L.; Zhu, J. Plasmon-enhanced solar vapor generation. Nanophotonics 2019, 8, 771–786. [Google Scholar] [CrossRef]
- Beicker, C.L.; Amjad, M.; Filho, E.P.B.; Wen, D. Experimental study of photothermal conversion using gold/water and MWCNT/water nanofluids. Sol. Energy Mater. Sol. Cells 2018, 188, 51–65. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Zeng, S.; Zheng, Z. Experimental investigation on photothermal properties of nanofluids for direct absorption solar thermal energy systems. Energy Convers. Manag. 2013, 73, 150–157. [Google Scholar] [CrossRef]
- Campos, C.; Vasco, D.; Angulo-Pineda, C.; Burdiles, P.A.; Cardemil, J.; Palza, H. About the relevance of particle shape and graphene oxide on the behavior of direct absorption solar collectors using metal based nanofluids under different radiation intensities. Energy Convers. Manag. 2018, 181, 247–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]





| Water mL | Ag NPs Concentration | Temp. (2 min) °C | Temp. (4 min) °C | Temp. (6 min) °C | Temp. (8 min) °C |
|---|---|---|---|---|---|
| 50 | 0.0% | 14 | 14.3 | 14.5 | 14.8 |
| 50 | 0.3% | 14.8 | 16.7 | 16.5 | 17.4 |
| 50 | 0.5% | 14 | 14.4 | 15.6 | 16.3 |
| 50 | 0.7% | 14.5 | 17 | 17.1 | 17.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talabani, R.F.; Hamad, S.M.; Barzinjy, A.A.; Demir, U. Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials 2021, 11, 2421. https://doi.org/10.3390/nano11092421
Talabani RF, Hamad SM, Barzinjy AA, Demir U. Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials. 2021; 11(9):2421. https://doi.org/10.3390/nano11092421
Chicago/Turabian StyleTalabani, Rebwar Faiq, Samir Mustafa Hamad, Azeez Abdullah Barzinjy, and Usame Demir. 2021. "Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation" Nanomaterials 11, no. 9: 2421. https://doi.org/10.3390/nano11092421
APA StyleTalabani, R. F., Hamad, S. M., Barzinjy, A. A., & Demir, U. (2021). Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials, 11(9), 2421. https://doi.org/10.3390/nano11092421








