Dual-Channel Probe of Carbon Dots Cooperating with Lanthanide Complex Employed for Simultaneously Distinguishing and Sequentially Detecting Tetracycline and Oxytetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of the SiCDs
2.3. Synthesis of Mesoporous SiCDs@mMIPs
2.4. Synthesis of SiCDs@mMIPs-APTES
2.5. Synthesis of SiCDs@mMIPs-cit
2.6. Synthesis of SiCDs@mMIPs-cit-Eu
2.7. Analytical Procedure
2.8. Preparation of Test Paper-Based Ratiometric Fluorescent Sensor Surpported by Smartphone
2.9. Characterization
3. Results and Discussion
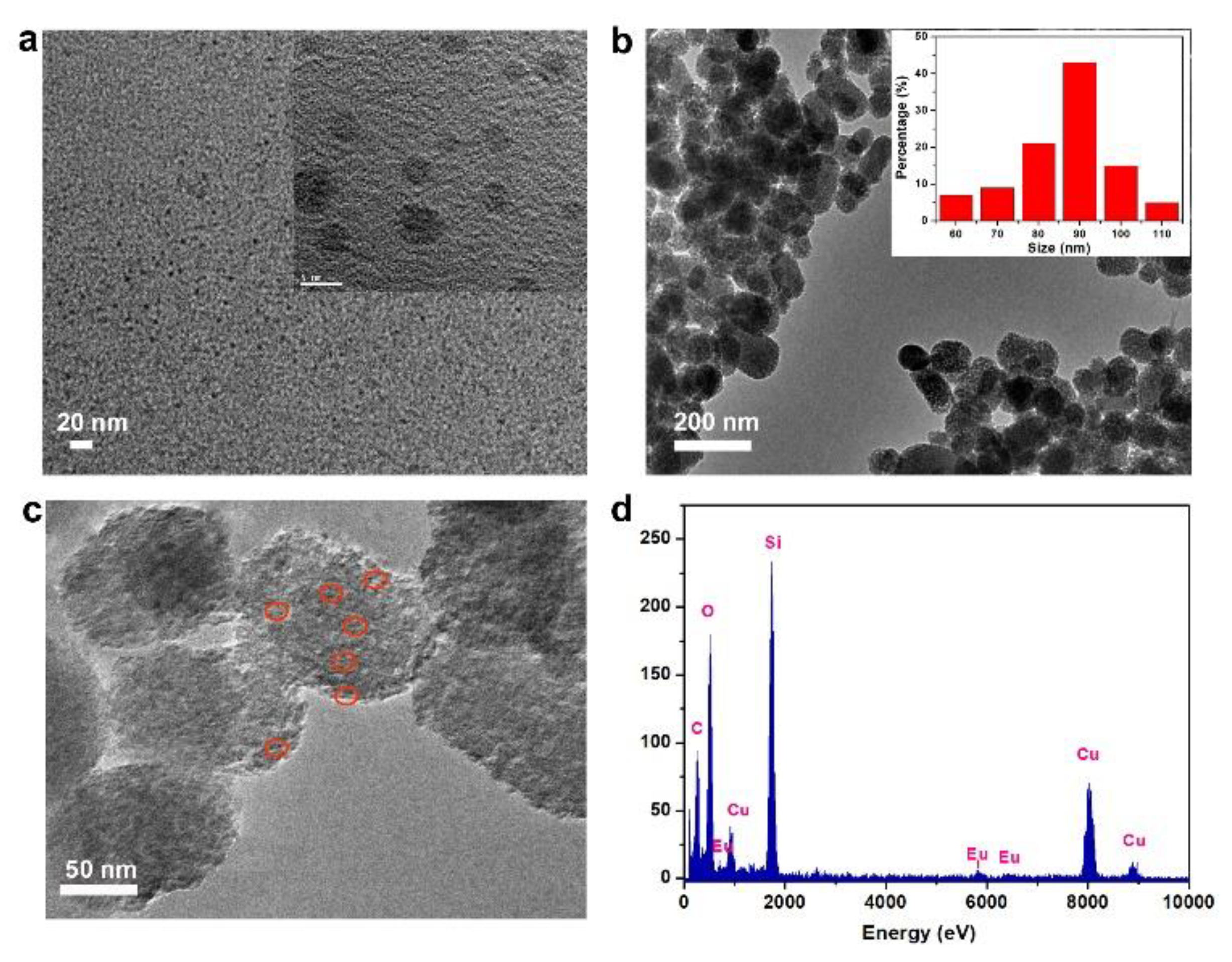
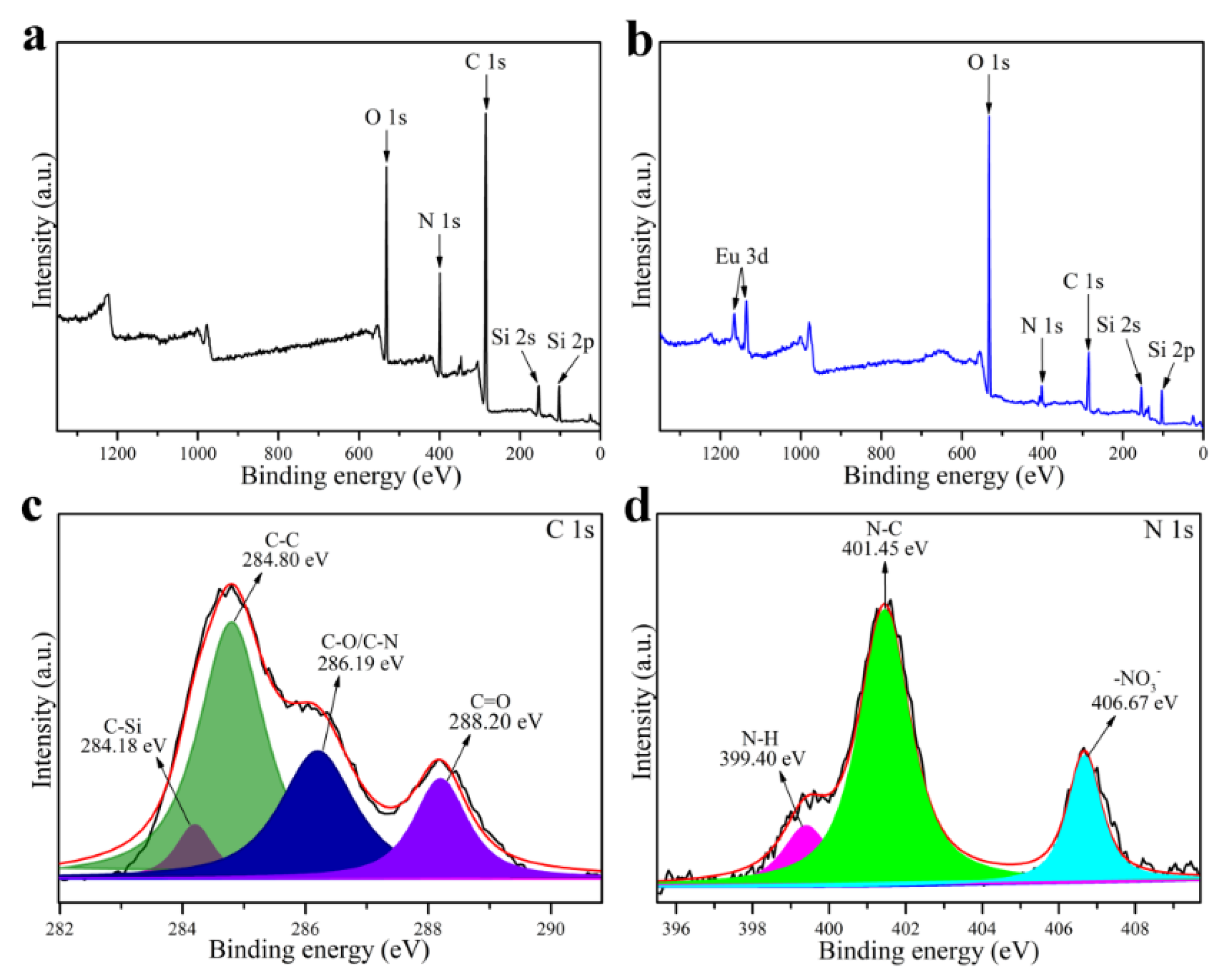
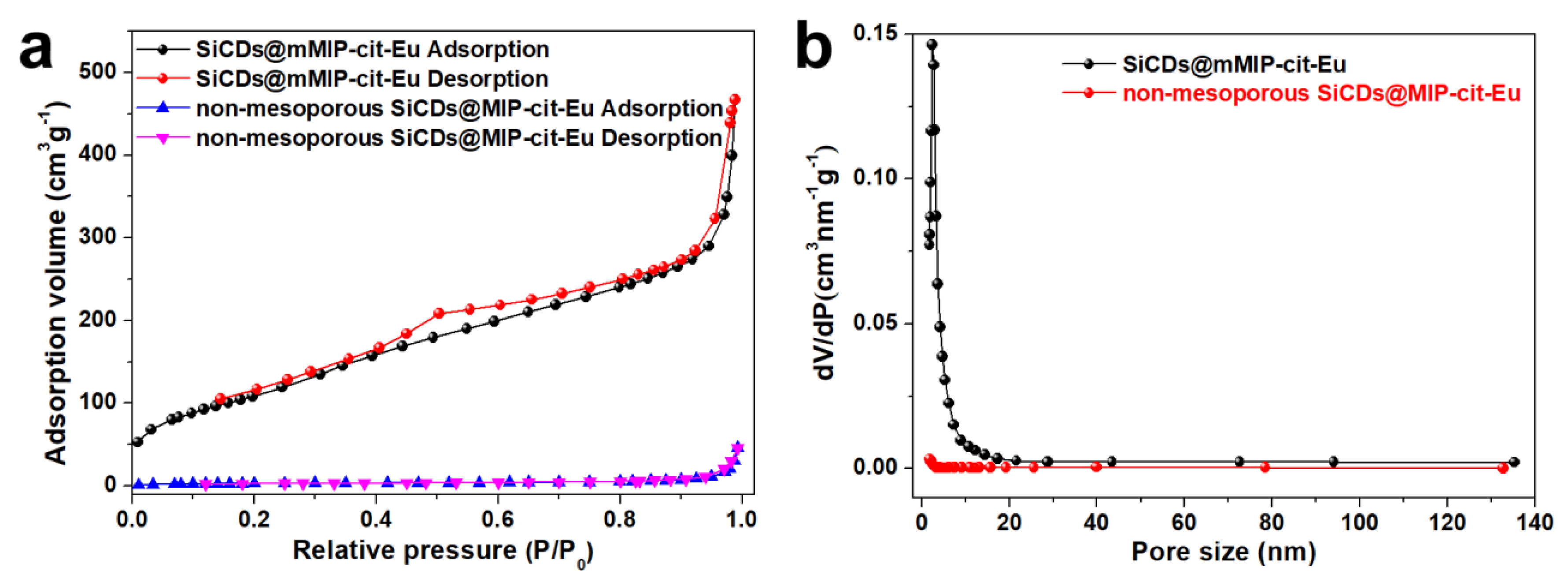
3.1. Preparation and Characterization of SiCDs
3.2. Optimal Detection Conditions of SiCDs@mMIP-cit-Eu Nanoprobe for TC
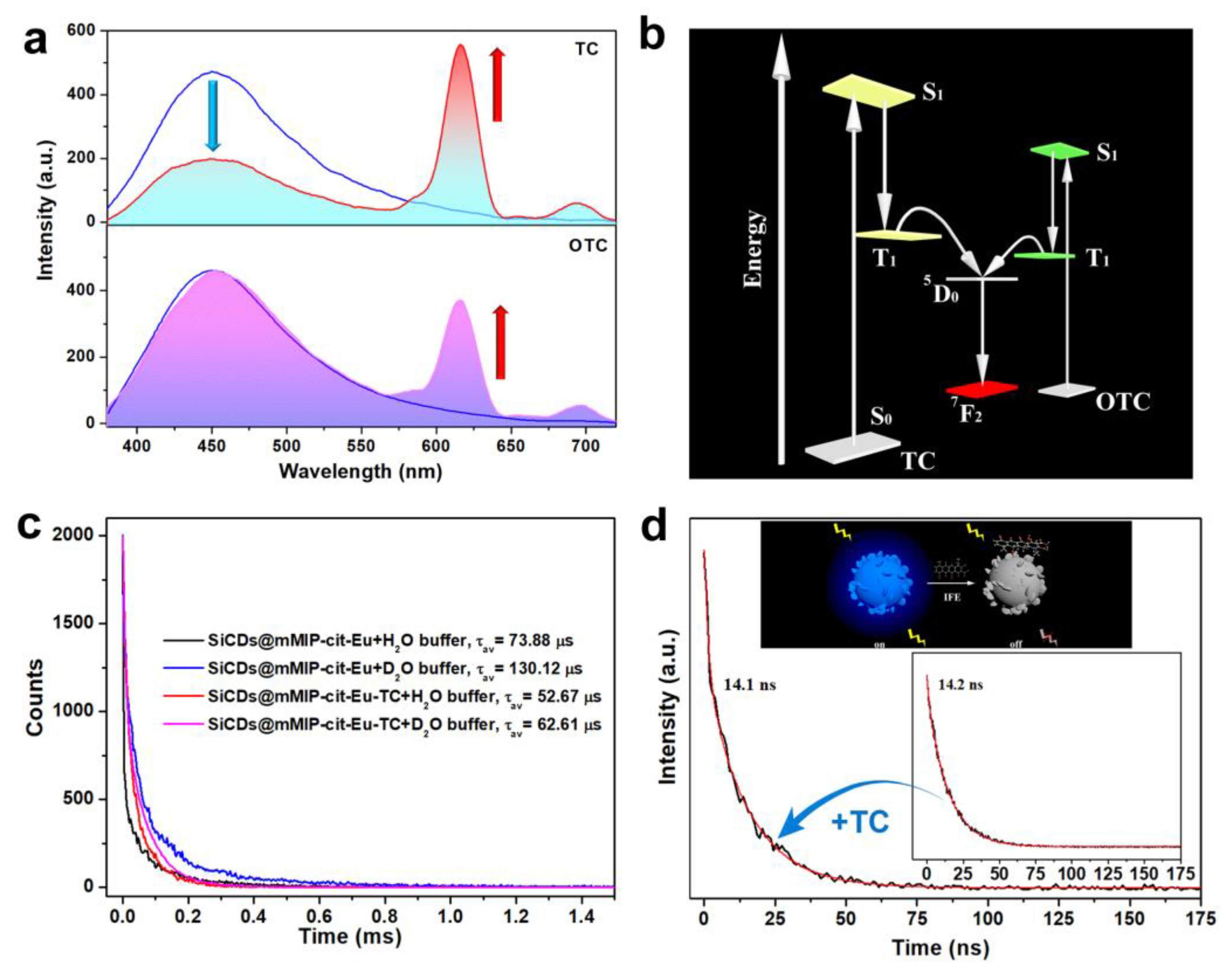
3.3. Sensing and Distinguishing Mechanism
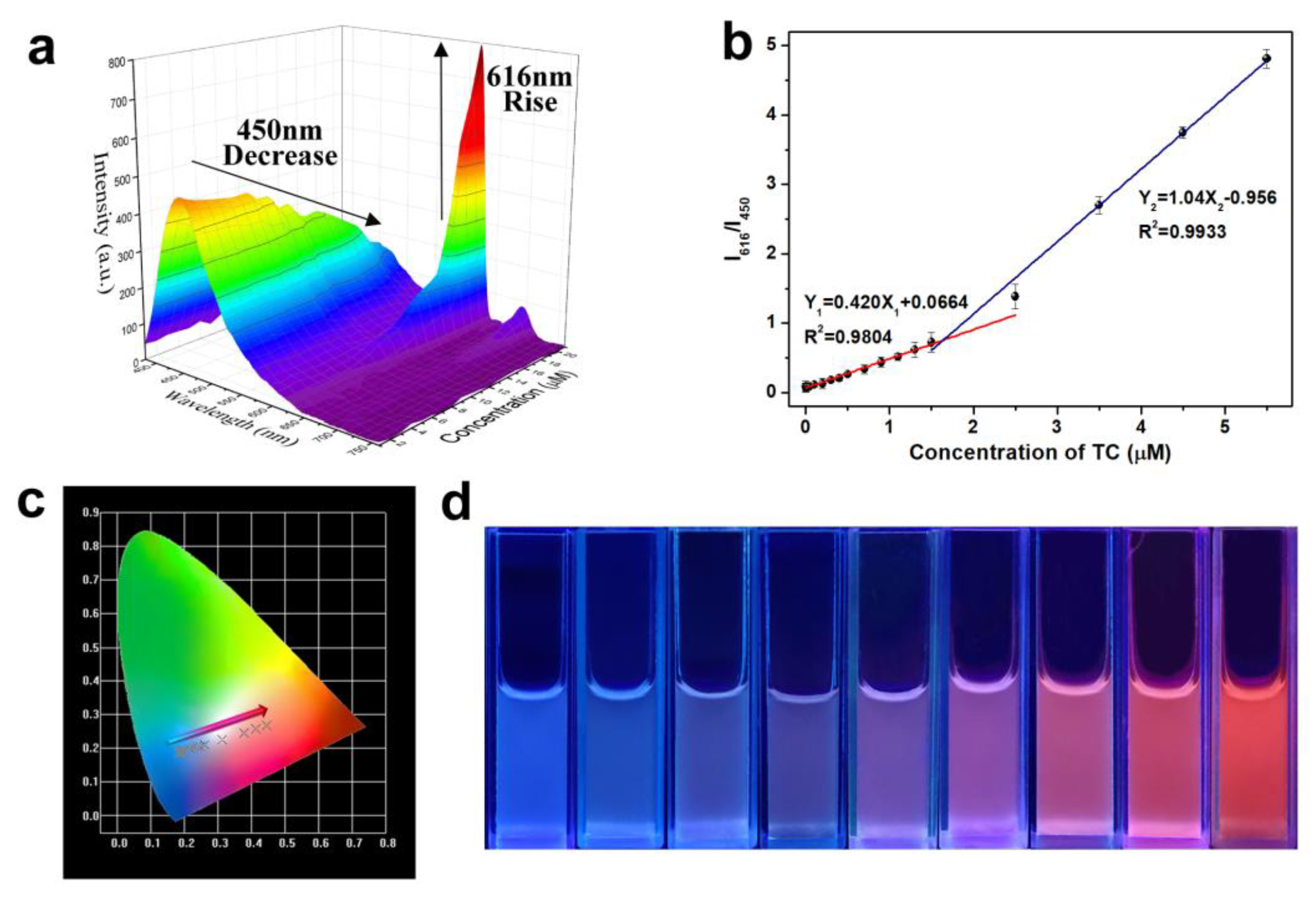
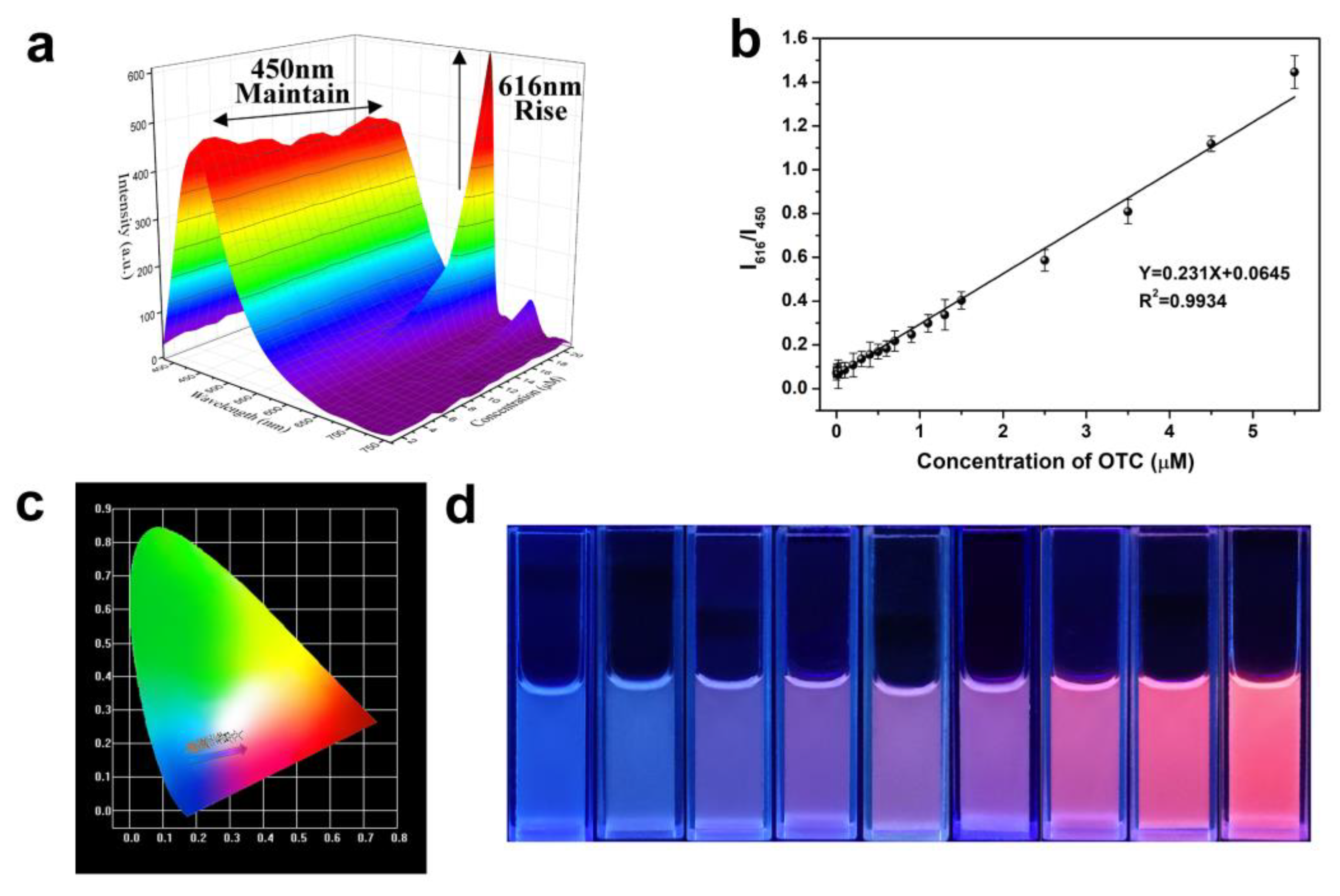
3.4. Sensitive Performance of SiCDs@mMIP-cit-Eu for the Detection of TC and OTC
3.5. Interference of Coexisting Substance
3.6. Detection of TC in Real Samples
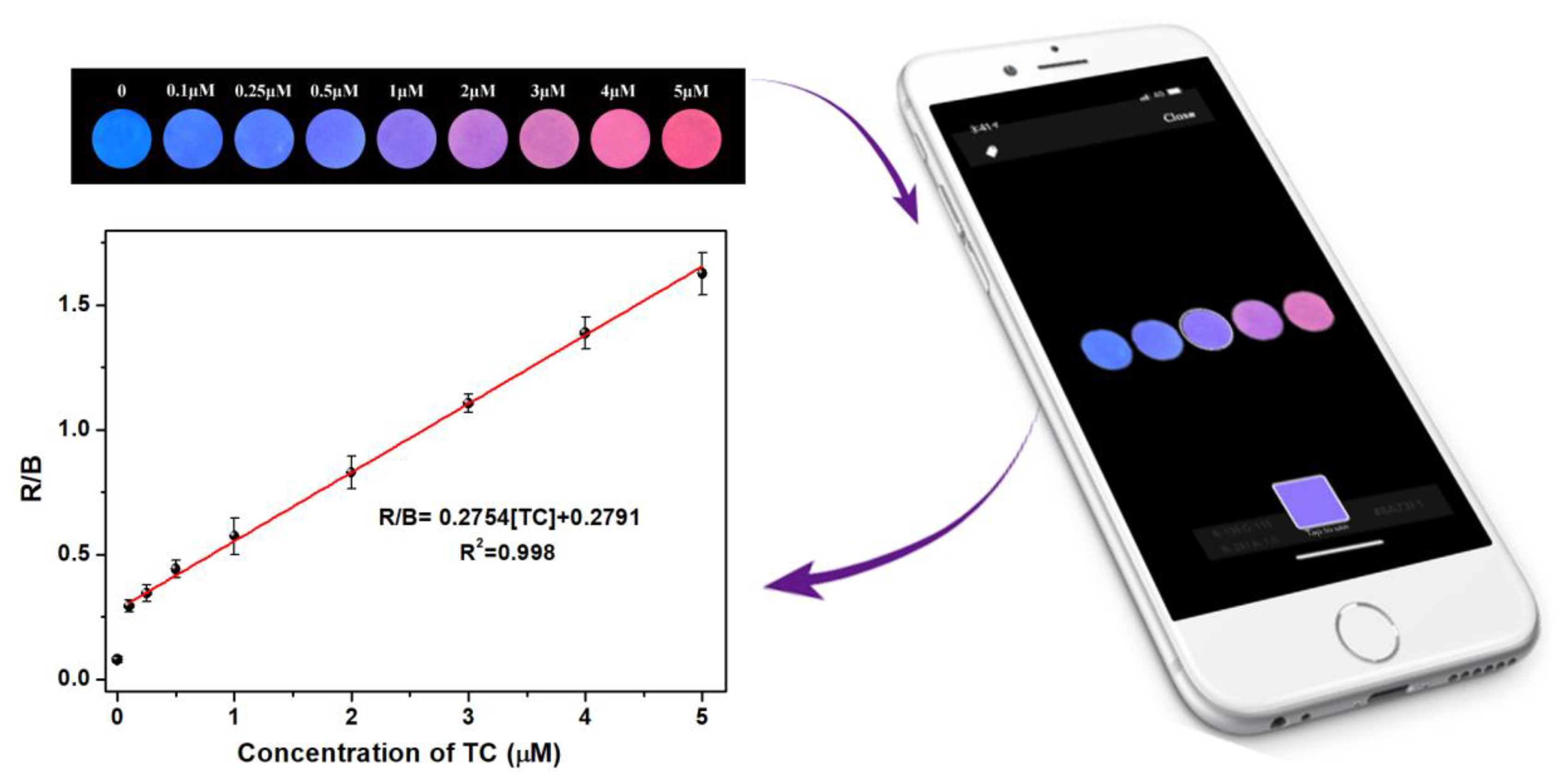
3.7. Smartphone and Test Paper-Based Ratiometric Fluorescent Sensor for Visual Detection of TC
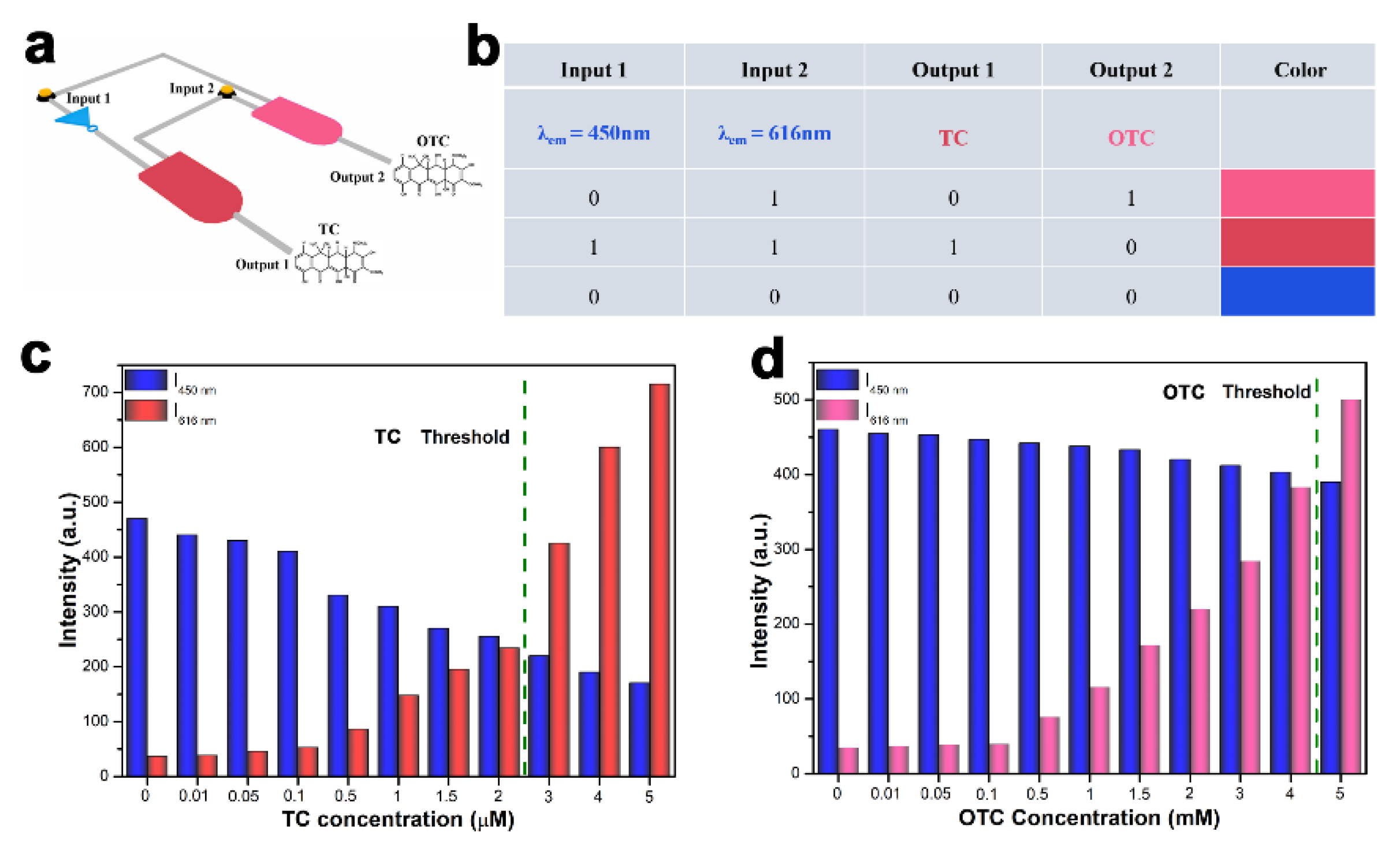
3.8. Ratiometric FL Molecular Logic Gate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, J.; Xie, L.; Ying, Y. Feasibility of Terahertz Time-Domain Spectroscopy to Detect Tetracyclines Hydrochloride in Infant Milk Powder. Anal. Chem. 2014, 86, 11750–11757. [Google Scholar] [CrossRef]
- Malik, A.H.; Iyer, P.K. Conjugated polyelectrolyte based sensitive detection and removal of antibiotics tetracycline from water. ACS Appl. Mater. Interfaces 2017, 9, 4433–4439. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Jia, L.; Bi, N.; Zhao, T. Metal-Enhanced fluorescence detection and degradation of tetracycline by silver nanoparticle-encapsulated halloysite nanolumen. J. Hazard. Mater. 2020, 386, 121630. [Google Scholar] [CrossRef]
- Tan, H.; Ma, C.; Song, Y.; Xu, F.; Chen, S.; Wang, L. Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens. Bioelectron. 2013, 50, 447–452. [Google Scholar] [CrossRef]
- Jeon, M.; Paeng, I.R. Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay. Anal. Chim. Acta 2008, 626, 180–185. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Ryszs, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Yang, S.; Hu, Q.; Cheng, H.; Liu, H.; Qiu, Y. High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp. J. Chromatogr. B 2013, 917, 11–17. [Google Scholar] [CrossRef]
- Hou, H.; Bai, X.; Xing, C.; Gu, N.; Zhang, B.; Tang, J. Aptamer-Based Cantilever Array Sensors for Oxytetracycline Detection. Anal. Chem. 2013, 85, 2010–2014. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The shared antibiotic Resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, J.; Xie, G.; Ren, Y.; Zheng, Y.Q. Ratiometric system based on graphene quantum dots and Eu3+ for selective detection of tetracyclines. Anal. Chim. Acta 2018, 1022, 131–137. [Google Scholar] [CrossRef]
- Lv, Y.K.; Wang, L.M.; Yang, L.; Zhao, C.X.; Sun, H.W. Synthesis and application of molecularly imprinted poly(methacrylic acid)–silica hybrid composite material for selective solid-phase extraction and high-performance liquid chromatography determination of oxytetracycline residues in milk. J. Chromatogr. A 2012, 1227, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.H.; Kim, I.A.; Lee, S.J.; Jurng, J.; Gu, M.B. A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens. Bioelectron. 2010, 26, 1644–1649. [Google Scholar] [CrossRef]
- Moreno-González, D.; Lupión-Enríquez, I.; García-Campaña, A.M. Trace determination of tetracyclines in water samples by capillary zone electrophoresis combining off-line and on-line sample preconcentration. Electrophoresis 2016, 37, 1212–1219. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Development of an ELISA and Immunochromatographic Assay for Tetracycline, Oxytetracycline, and Chlortetracycline Residues in Milk and Honey Based on the Class-Specific Monoclonal Antibody. Food Anal. Methods 2016, 9, 905–914. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Lyu, T.; Wang, Y.; Yang, X.; Wu, X. A facile strategy for the synthesis of water-soluble fluorescent nonconjugated polymer dots and their application in tetracycline detection. J. Mater. Chem. C 2019, 7, 9241–9247. [Google Scholar] [CrossRef]
- Jia, L.; Chen, X.; Xu, J.; Zhang, L.; Guo, S.; Bi, N.; Zhu, T. A smartphone-integrated multicolor fluorescence probe of bacterial spore biomarker: The combination of natural clay material and metal-organic frameworks. J. Hazard. Mater. 2021, 402, 123776. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, J.; Wang, G.; Ye, Z.; Zhang, W.; Jin, D.; Yuan, J.; Piper, J. Development of a visible-light-sensitized europium complex for time-resolved fluorometric application. Anal. Chem. 2010, 82, 2529–2535. [Google Scholar] [CrossRef]
- An, X.; Zhuo, S.; Zhang, P.; Zhu, C. Carbon dots based turn-on fluorescent probes for oxytetracycline hydrochloride sensing. RSC Adv. 2015, 5, 19853–19858. [Google Scholar] [CrossRef]
- Yu, L.; Chen, H.; Yue, J.; Chen, X.; Sun, M.; Tan, H.; Asiri, A.M.; Alamry, K.A.; Wang, X.; Wang, S. Metal–Organic Framework Enhances Aggregation-Induced Fluorescence of Chlortetracycline and the Application for Detection. Anal. Chem. 2019, 91, 5913–5921. [Google Scholar] [CrossRef]
- Le, T.; Yi, S.H.; Zhao, Z.W.; Wei, W. Rapid and sensitive enzyme-linked immunosorbent assay and immunochromatographic assay for the detection of chlortetracycline residues in edible animal tissues. Food Addit. Contam. Part A 2011, 28, 1516–1523. [Google Scholar] [CrossRef]
- Kim, M.; Ko, S.K.; Kim, H.; Shin, I.; Tae, J. Rhodamine cyclic hydrazide as a fluorescent probe for the detection of hydroxyl radicals. Chem. Commun. 2013, 49, 7959–7961. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.R.; Hu, C.W.; Chen, J.L. Comparative syntheses of tetracycline-imprinted polymeric silicate and acrylate on CdTe quantum dots as fluorescent sensors. Biosens. Bioelectron. 2014, 61, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, S.; Cao, W.; Zhang, G.; Yang, X.; Gong, X.; Xing, X. Low-toxicity carbon quantum dots derived from gentamicin sulfate to combat antibiotic resistance and eradicate mature biofilms. Chem. Commun. 2020, 56, 2316–2319. [Google Scholar] [CrossRef]
- Algarra, M.; Perez-Martin, M.; Cifuentes-Rueda, M.; Jimenez-Jimenez, J.; Esteves da Silva, J.C.G.; Bandosz, T.J.; Rodriguez-Castellon, E.; López Navarrete, J.T.; Casado, J. Carbon dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef]
- Yang, K.; Wang, S.; Wang, Y.; Miao, H.; Yang, X. Dual-channel probe of carbon dots cooperating with gold nanoclusters employed for assaying multiple targets. Biosens. Bioelectron. 2017, 91, 566–573. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhu, Z.; Zheng, Z.; Jin, Y.; Di, X.; Xu, Z.; Guan, H. Facile synthesis of highly efficient fluorescent carbon dots for tetracycline detection. Environ. Sci. Pollut. Res. 2020, 27, 4520–4527. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.M.; Poushali, D.S.S.; John, H.T.; Aharon, G. Applications of N-Doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Yu, X.; Li, J.; Wang, Z.; Zhang, Z.; Liu, B. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J. Mater. Chem. C 2018, 6, 9636–9641. [Google Scholar] [CrossRef]
- Liu, H.; Ding, L.; Chen, L.; Chen, Y.; Zhou, T.; Li, H.; Xu, Y.; Zhao, L.; Huang, N. A facile, green synthesis of biomass carbon dots coupled with molecularly imprinted polymers for highly selective detection of oxytetracycline. J. Ind. Eng. Chem. 2019, 69, 455–463. [Google Scholar] [CrossRef]
- Huang, S.; Guo, M.; Tan, J.; Geng, Y.; Wu, J.; Tang, Y.; Su, C.; Lin, C.C.; Liang, Y. Novel Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Highly Selective and Sensitive Detection of Omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.; Wang, X.; Fu, L.; Luo, L.; Chen, L. Rotational Paper-Based Microfluidic-Chip Device for Multiplexed and Simultaneous Fluorescence Detection of Phenolic Pollutants Based on a Molecular-Imprinting Technique. Anal. Chem. 2018, 90, 11827–11834. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Z.; Zhang, C.; Zheng, Y.; Gao, J.; Wang, Q. Ratiometric fluorescence platform based on modified silicon quantum dots and its logic gate performance. Inorg. Chem. 2018, 57, 8866–8873. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, K.; Yang, R.; Du, X.; Qu, B.; Miao, X.; Lu, L. A long lifetime ratiometrically luminescent tetracycline nanoprobe based on Ir(III) complex-doped and Eu3+ -functionalized silicon nanoparticles. J. Hazard. Mater. 2020, 386, 121929. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, M.; Schäferling, M.; Wolfbeis, O.S. Fluorescent imaging of citrate and other intermediates in the citric Acid cycle. Angew. Chem. Int. Edit 2010, 43, 1735–1738. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Q.; Wang, J.; Zhang, C.C. Imaging two targets in live cells based on rational design of lanthanide organic structure appended carbon dots. Carbon 2015, 93, 671–680. [Google Scholar] [CrossRef]
- Lu, C.; Tang, Z.; Liu, C.; Kang, L.; Sun, F. Magnetic-nanobead-based competitive enzyme-linked aptamer assay for the analysis of oxytetracycline in food. Anal. Bioanal. Chem. 2015, 407, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Qi, W.; Song, Y. Luminescent lanthanide graphene for detection of bacterial spores and cysteine. Chem. Comm. 2015, 51, 11022–11025. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr (II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef]
- Gao, F.; Han, J.; Lv, C.; Wang, Q.; Zhang, J.; Li, Q.; Bao, L.; Li, X. Application of Core-shell-structured CdTe@SiO2 quantum dots synthesized via a facile solution method for improving latent fingerprint detection. J. Nanopart. Res. 2012, 14, 1191. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. Molecularly imprinted mesoporous silica embedded with carbon dots and semiconductor quantum dots as a ratiometric fluorescent sensor for diniconazole. Biosens. Bioelectron. 2017, 96, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Wang, Y.; Kong, M. Ratiometric method based on silicon nanodots and Eu3+ system for highly-sensitive detection of tetracyclines. Talanta 2019, 204, 491–498. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Niu, D.; Li, Y.; Shi, J. Large-pore, silica particles with antibody-like, biorecognition sites for efficient protein separation. J. Mater. Chem. B 2017, 5, 4214–4220. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lu, R.; Li, Y.; Wang, Q.; Li, J.; Wang, L. Molecularly imprinted electrospun nanofibers for adsorption of 2,4-dinitrotoluene in water. Analyst 2018, 143, 3465–3471. [Google Scholar] [CrossRef]
- Shurygin, A.V.; Vovna, V.I.; Korochentsev, V.V.; Mirochnik, A.G.; Kalinovskaya, I.V.; Sergienko, V.I. Electronic structure and optical properties of Ln(III) nitrate adducts with 1,10-phenanthroline. Spectrochim. Acta A 2019, 213, 176–183. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L. Fluorescence Probe Based on Hybrid Mesoporous Silica/Quantum Dot/Molecularly Imprinted Polymer for Detection of Tetracycline. ACS Appl. Mater. Interfaces. 2016, 8, 16248–16256. [Google Scholar] [CrossRef]
- Mukherjee, M.; Sardar, P.S.; Ghorai, S.K.; Samanta, S.K.; Roy, A.S.; Dasgupta, S.; Ghosh, S. Interaction of multitryptophan protein with drug: An insight into the binding mechanism and the binding domain by time resolved emission, anisotropy, phosphorescence and docking. J. Photochem. Photobiol. B 2012, 115, 93–104. [Google Scholar] [CrossRef]
- Supkowski, R.M.; Horrocks, W.D. Displacement of Inner-Sphere Water Molecules from Eu3+ Analogues of Gd3+ MRI Contrast Agents by Carbonate and Phosphate Anions: Dissociation Constants from Luminescence Data in the Rapid-Exchange Limit. Inorg. Chem. 1999, 38, 5616–5619. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Bai, Y.; Liu, L.; Liao, D.; Liang, J.; Liu, L.; Han, H. Interaction between fluorescein isothiocyanate and carbon dots: Inner filter effect and fluorescence resonance energy transfer. Spectrochim. Acta A 2017, 171, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Cheng, D.; Mao, L. Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem. 2010, 122, 895–900. [Google Scholar] [CrossRef]
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; A, Y.L. Smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens. Bioelectron. 2020, 148, 111791. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, X.; Jia, L.; Zhang, M.; Zhou, T.; Wei, Y. Facile ratiometric fluorapatite nanoprobes for rapid and sensitive bacterial spore biomarker detection. Biosens. Bioelectron. 2017, 87, 991–997. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.; Jia, L.; Zhu, T.; Chen, X.; Zhao, T. A smartphone-integrated method for visual detection of tetracycline. Chem. Eng. J. 2021, 416, 127741. [Google Scholar] [CrossRef]
- Kurittu, J.; Lönnberg, S.; Virta, M.; Karp, M. A group-specific microbiological test for the detection of tetracycline residues in raw milk. J. Agric. Food Chem. 2000, 48, 3372–3377. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Xu, Z.; Chen, R.; Chen, X.; Xu, J. Dual-Channel Probe of Carbon Dots Cooperating with Lanthanide Complex Employed for Simultaneously Distinguishing and Sequentially Detecting Tetracycline and Oxytetracycline. Nanomaterials 2022, 12, 128. https://doi.org/10.3390/nano12010128
Jia L, Xu Z, Chen R, Chen X, Xu J. Dual-Channel Probe of Carbon Dots Cooperating with Lanthanide Complex Employed for Simultaneously Distinguishing and Sequentially Detecting Tetracycline and Oxytetracycline. Nanomaterials. 2022; 12(1):128. https://doi.org/10.3390/nano12010128
Chicago/Turabian StyleJia, Lei, Zhitao Xu, Rujie Chen, Xiangzhen Chen, and Jun Xu. 2022. "Dual-Channel Probe of Carbon Dots Cooperating with Lanthanide Complex Employed for Simultaneously Distinguishing and Sequentially Detecting Tetracycline and Oxytetracycline" Nanomaterials 12, no. 1: 128. https://doi.org/10.3390/nano12010128
APA StyleJia, L., Xu, Z., Chen, R., Chen, X., & Xu, J. (2022). Dual-Channel Probe of Carbon Dots Cooperating with Lanthanide Complex Employed for Simultaneously Distinguishing and Sequentially Detecting Tetracycline and Oxytetracycline. Nanomaterials, 12(1), 128. https://doi.org/10.3390/nano12010128




