Detection of Oenological Polyphenols via QCM-D Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) Experiments
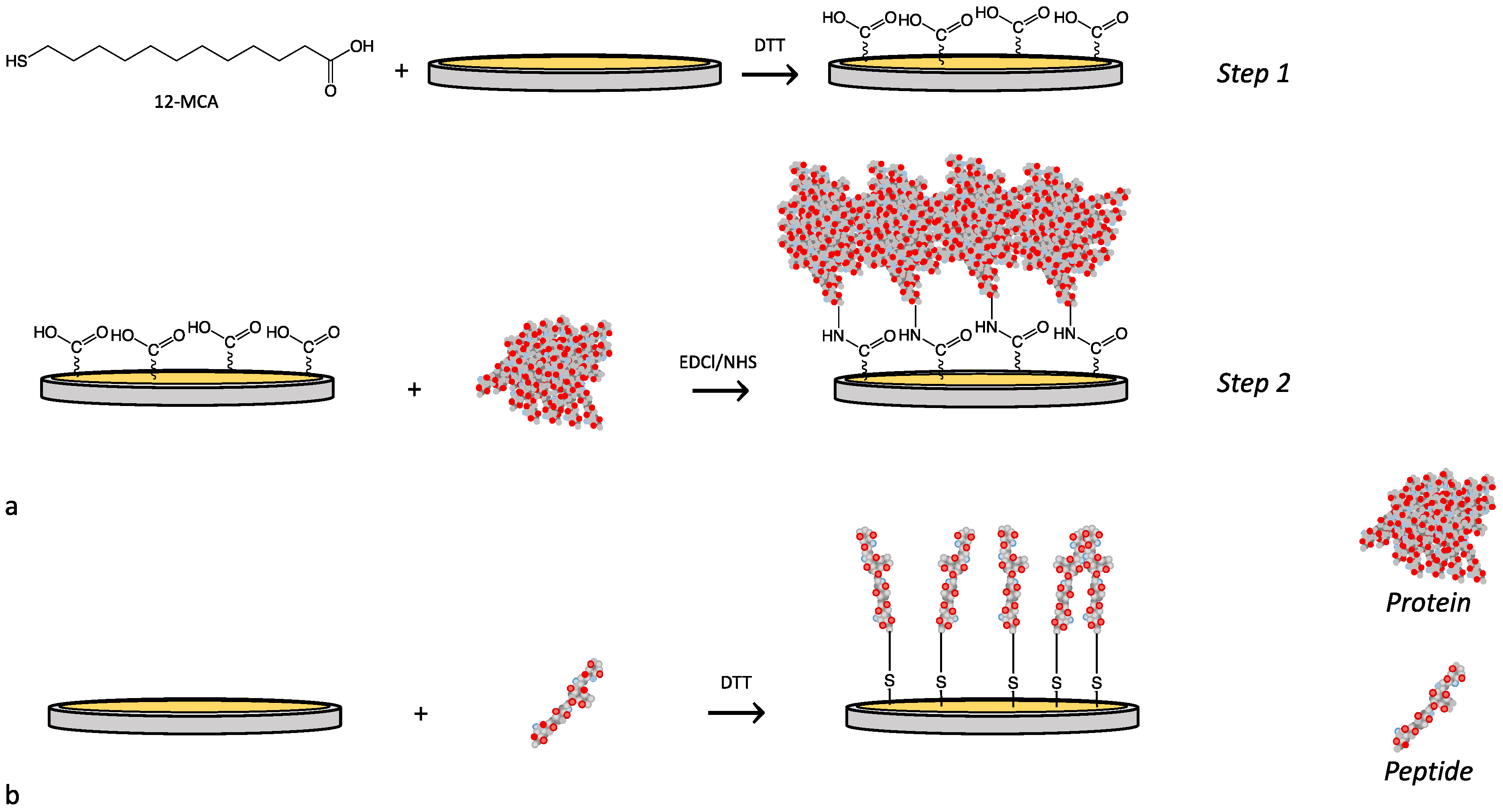
2.3. Sensor Surface Functionalization
2.4. Detection Experiments
2.5. Data Analysis
3. Results
3.1. Functionalization
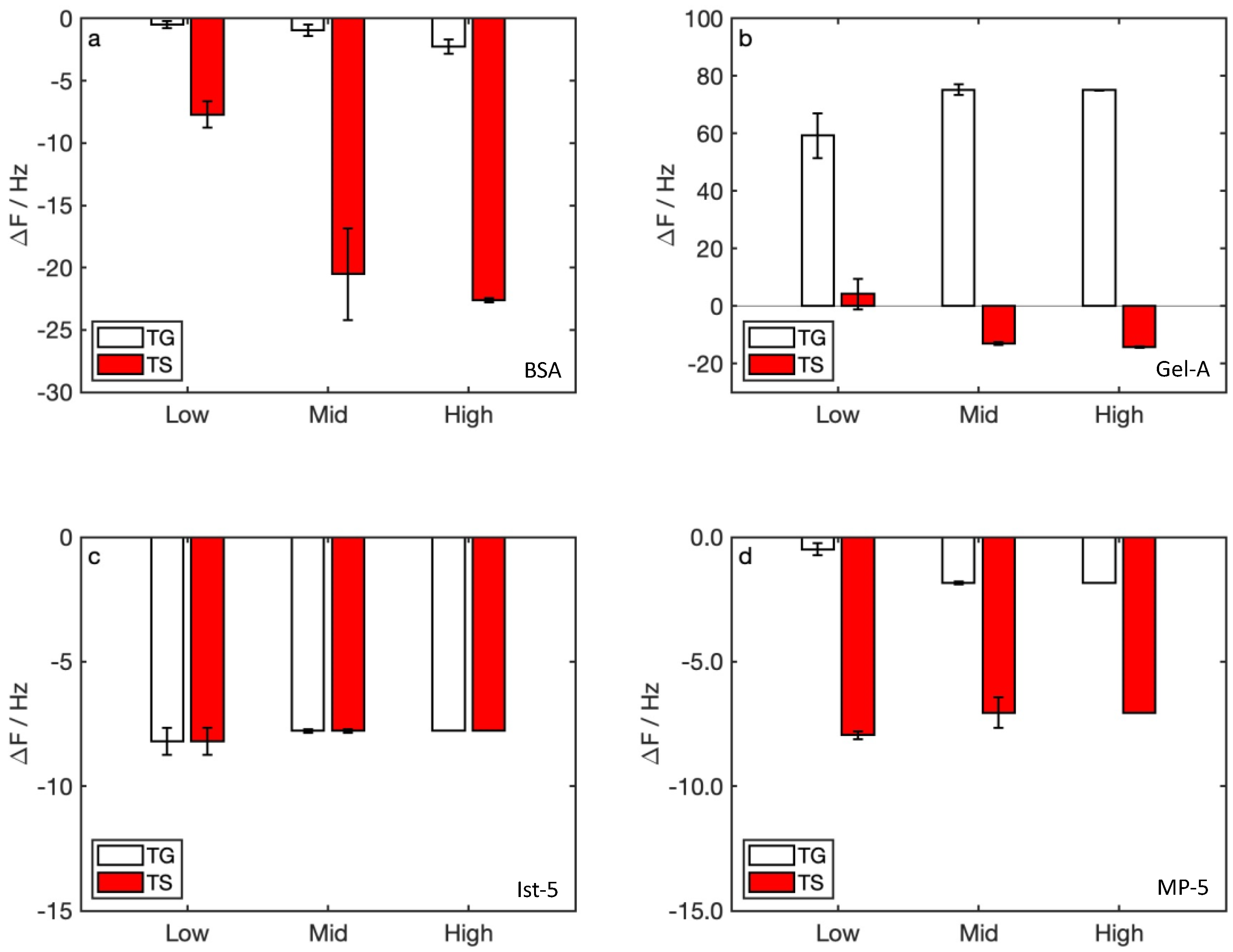
3.2. Watery Samples Detection
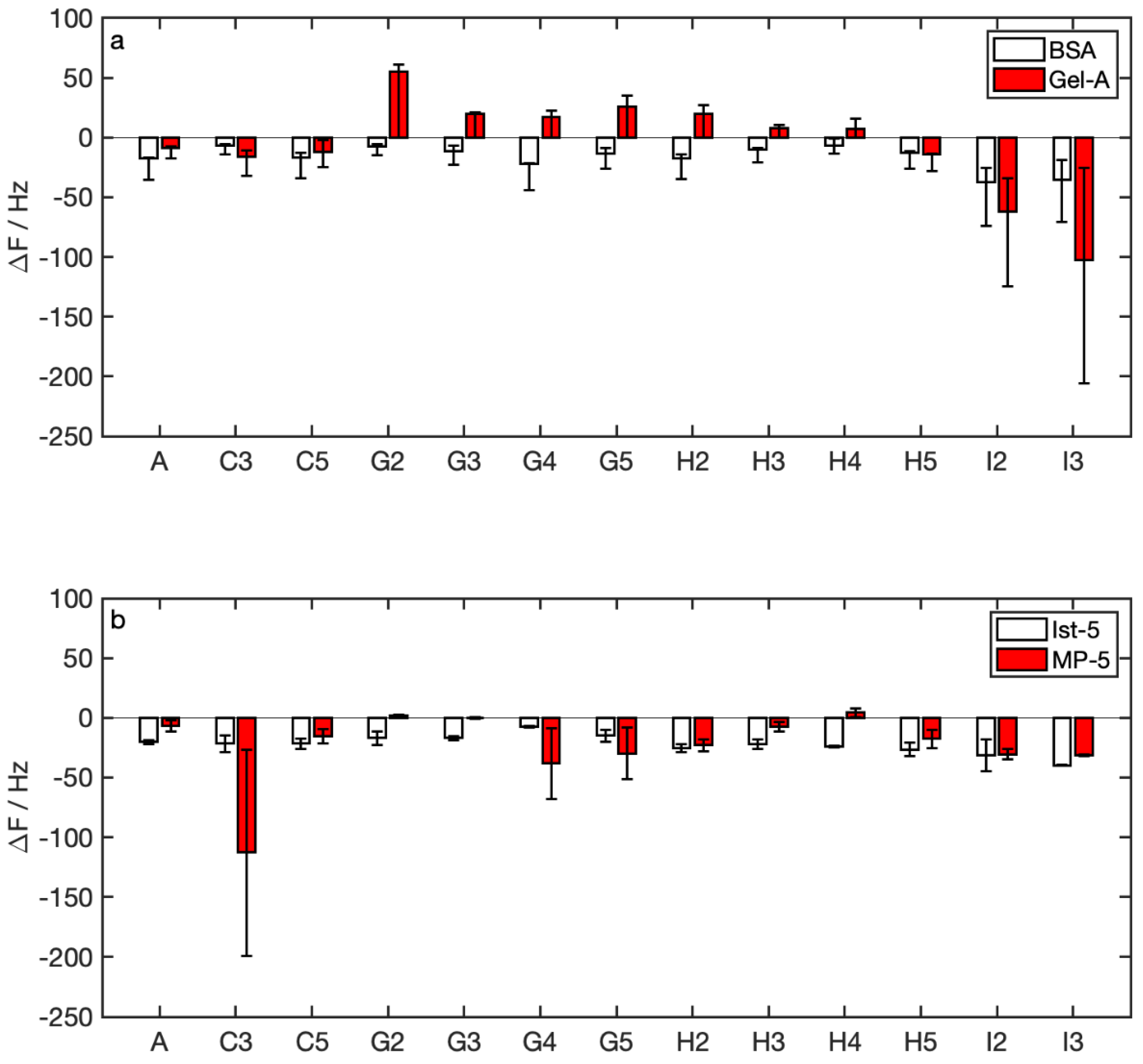
3.3. Winery Samples Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef]
- Sauerbrey, G.Z. Use of quartz vibration for weighing thin films on a microbalance. Z. Phys. 1959, 155, 206–212. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajkumar, R.; Bhargava, K. Introduction to biosensors. In Biosensors and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–68. [Google Scholar]
- Jang, I.R.; Jung, S.I.; Lee, G.; Park, I.; Kim, S.B.; Kim, H.J. Quartz Crystal Microbalance with Thermally-controlled Surface Adhesion for an Efficient Fine Dust Collection and Sensing. J. Haz. Mater. 2021, 424, 127560. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Kamysz, W.; Gębicki, J.; Namieśnik, J. Evaluation of three peptide immobilization techniques on a qcm surface related to acetaldehyde responses in the gas phase. Sensors 2018, 18, 3942. [Google Scholar] [CrossRef] [Green Version]
- Walkowiak, J.; Gradzielski, M.; Zauscher, S.; Ballauff, M. Interaction of Proteins with a Planar Poly (acrylic acid) Brush: Analysis by Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D). Polymers 2021, 13, 122. [Google Scholar] [CrossRef]
- Janshoff, A.; Galla, H.J.; Steinem, C. Piezoelectric mass-sensing devices as biosensors—An alternative to optical biosensors? Angew. Chem. Int. Ed. 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Agostini, M.; Amato, F.; Vieri, M.L.; Greco, G.; Tonazzini, I.; Baroncelli, L.; Cecchini, M. Glial-fibrillary-acidic-protein (GFAP) biomarker detection in serum-matrix: Functionalization strategies and detection by an ultra-high-frequency surface-acoustic-wave (UHF-SAW) lab-on-chip. Biosens. Bioelectron. 2021, 172, 112774. [Google Scholar] [CrossRef]
- Greco, G.; Agostini, M.; Cecchini, M. Ultra-high-frequency Love surface acoustic wave device for real-time sensing applications. IEEE Access 2020, 8, 112507–112514. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Full-SAW microfluidics-based lab-on-a-chip for biosensing. IEEE Access 2019, 7, 70901–70909. [Google Scholar] [CrossRef]
- Piovarci, I.; Hianik, T.; Ivanov, I.N. Detection of chymotrypsin by optical and acoustic methods. Biosensors 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Piovarci, I.; Melikishvili, S.; Tatarko, M.; Hianik, T.; Thompson, M. Detection of Sub-Nanomolar Concentration of Trypsin by Thickness-Shear Mode Acoustic Biosensor and Spectrophotometry. Biosensors 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Dizon, M.; Tatarko, M.; Szabo, K.; Hianik, T. Application of high-resolution ultrasonic spectroscopy for detection of the plasmin activity toward β-casein. Food Chem. 2021, 353, 129373. [Google Scholar] [CrossRef]
- Farokhzad, N.; Tao, W. Materials chemistry-enabled platforms in detecting sexually transmitted infections: Progress towards point-of-care tests. Trends Chem. 2021, 3, 589–602. [Google Scholar] [CrossRef]
- Fracassetti, D.; Di Canito, A.; Bodon, R.; Palmowska, N.; Vigentini, I.; Foschino, R.; Tirelli, A. Light-struck taste in white wine: Reaction mechanisms, preventive strategies and future perspectives to preserve wine quality. Trends Food Sci. Technol. 2021, 112, 547–558. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Matese, A.; Mancin, M.; Primicerio, J.; Palliotti, A. An open-source and low-cost monitoring system for precision enology. Sensors 2014, 14, 23388–23397. [Google Scholar] [CrossRef] [Green Version]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Burgos, E.; Gómez-Serranillos, M.P. Effect of Phenolic Compounds on Human Health. Nutrients 2021, 13, 3922. [Google Scholar] [CrossRef]
- Abreu, T.; Perestrelo, R.; Bordiga, M.; Locatelli, M.; Daniel Coïsson, J.; Câmara, J.S. The Flavor Chemistry of Fortified Wines—A Comprehensive Approach. Foods 2021, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Golokhvast, K. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Shishir, M.R.I.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.W. Red Wine High-Molecular-Weight Polyphenolic Complex: An Emerging Modulator of Human Metabolic Disease Risk and Gut Microbiota. J. Agric. Food Chem. 2021, 69, 10907–10919. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Gonҫalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar]
- Di Egidio, V.; Sinelli, N.; Giovanelli, G.; Moles, A.; Casiraghi, E. NIR and MIR spectroscopy as rapid methods to monitor red wine fermentation. Eur. Food Res. Technol. 2010, 230, 947–955. [Google Scholar] [CrossRef]
- Garcia-Torres, R.; Ramírez-Rodrigues, M.M.; Pérez-Alva, A. Polyphenolic Profile of Orange Wines. Curr. Dev. Nutr. 2021, 5 (Suppl. 2), 1158. [Google Scholar] [CrossRef]
- Fermo, P.; Comite, V.; Sredojević, M.; Ćirić, I.; Gašić, U.; Mutić, J.; Tešić, Ž. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Surface modification influencing adsorption of red wine constituents: The role of functional groups. Appl. Surf. Sci. 2016, 386, 14–23. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Adsorption of wine constituents on functionalized surfaces. Molecules 2016, 21, 1394. [Google Scholar] [CrossRef] [Green Version]
- Karczmarczyk, A.; Haupt, K.; Feller, K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef]
- Wang, S.; Mantilla, S.M.O.; Smith, P.A.; Stokes, J.R.; Smyth, H.E. Astringency sub-qualities drying and pucker are driven by tannin and pH–Insights from sensory and tribology of a model wine system. Food Hydrocol. 2020, 109, 106109. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Teixeira, N.; De Freitas, V.; Sales, M.G.F.; Sutherland, D.S. A saliva molecular imprinted localized surface plasmon resonance biosensor for wine astringency estimation. Food Chem. 2017, 233, 457–466. [Google Scholar] [CrossRef]
- Lei, L.; Tang, Y.; Zheng, J.; Ma, G.; Zhou, Z. Influence of two polyphenols on the structure and lubrication of salivary pellicle: An in vitro study on astringency mechanism. Friction 2021, 10, 167–178. [Google Scholar] [CrossRef]
- Wang, S.; Mantilla, S.M.O.; Smith, P.A.; Stokes, J.R.; Smyth, H.E. Tribology and QCM-D approaches provide mechanistic insights into red wine mouthfeel, astringency sub-qualities and the role of saliva. Food Hydrocol. 2021, 120, 106918. [Google Scholar] [CrossRef]
- Iturmendi, N.; Duràn, D.; Marìn-Arroyo, M.R. Fining of red wines with gluten or yeast extract protein. Int. J. Food Sci. Technol. 2010, 45, 200–207. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-da-Silva, J.M.; Laureano, O. Interactions between protein fining agents and proanthocyanidins in white wine. Food Chem. 2008, 106, 536–544. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H.; Warminski, E.; Liao, H.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Luck, G. Polyphenol complexation. In Phenolic Compounds in Food and Their Effects on Health; Ho, C.T., Lee, C.Y., Huang, M.T., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; pp. 8–50. [Google Scholar]
- Kawamoto, H.; Nakatsubo, F. Effects of environmental factors on two-stage tannin-protein coprecipitation. Phytochem 1997, 46, 379–483. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier., V.; Moutounet, M. Analysis and characterization of wine condensed tannins precipitated by proteins used as fining agent in enology. Am. J. Enol. Viticul. 1999, 50, 81–86. [Google Scholar]
- Wang, X.; Ho, C.T.; Huang, Q. Investigation of adsorption behavior of (−)-epigallocatechin gallate on bovine serum albumin surface using quartz crystal microbalance with dissipation monitoring. J. Agric. Food Chem. 2007, 55, 4987–4992. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Techn. JBT 2008, 19, 151. [Google Scholar]
- Kusuda, M.; Hatano, T.; Yoshida, T. Water-soluble complexes formed by natural polyphenols and bovine serum albumin: Evidences from gel electrophoresis. Biosci. Biotechnol. Biochem. 2006, 70, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Harbertson, J.F.; Kilmister, R.L.; Kelm, M.A.; Downey, M.O. Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem. 2014, 160, 16–21. [Google Scholar] [CrossRef]
- Lei, X.; Zhu, Y.; Wang, X.; Zhao, P.; Liu, P.; Zhang, Q.; Chen, T.; Yuan, H.; Guo, Y. Wine polysaccharides modulating astringency through the interference on interaction of flavan-3-ols and BSA in model wine. Int. J. Biol. Macromol. 2019, 139, 896–903. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.; Yu, Y. Improvement of BSA tannin precipitation assay by reformulation of resuspension buffer. Am. J. Enol. Viticul. 2014, 10, 16–21. [Google Scholar] [CrossRef]
- Jauregi, P.; Olatujoye, J.B.; Cabezudo, I.; Frazier, R.A.; Gordon, M.H. Astringency reduction in red wine by whey proteins. Food Chem. 2016, 199, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Olatujoye, J.B.; Methven, L.; Jauregi, P. Effect of β-lactoglobulin on perception of astringency in red wine as measured by sequential profiling. LWT Food Sci. Technol. 2020, 130, 109611. [Google Scholar] [CrossRef]
- Kaneda, H.; Watari, J.; Takashio, M.; Okahata, Y. Measuring astringency of beverages using a quartz-crystal microbalance. J. Am. Soc. Brew. Chem. 2003, 61, 119–124. [Google Scholar]
- Yan, Q.; Bennick, A. Identification of histatins as tanning-binding proteins in human saliva. Biochem. J. 1995, 311, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Shraberg, J.; Rick, S.W.; Rannulua, N.; Cole, R.B. A study of procyanidin binding to Histatin 5 using Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS) and molecular simulations. Phys. Chem. Chem. Phys. 2015, 17, 12247–12258. [Google Scholar] [CrossRef]
- Wróblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The molecular interaction of human salivary histatins with polyphenolic compounds. Eur. J. Biochem. 2001, 268, 4384–4397. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Rhodes, M.J. Binding affinity of hydrolyzable tannins to parotid saliva and to proline-rich proteins derived from it. J. Agric. Food Chem. 2000, 48, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Saftics, A.; Kurunczi, S.; Peter, B.; Szekacs, I.; Ramsden, J.J.; Horvath, R. Data evaluation for surface-sensitive label-free methods to obtain real-time kinetic and structural information of thin films: A practical review with related software packages. Adv. Coll. Interf. Sci. 2021, 294, 102431. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis. Edition 2021, Volume 1. Available online: https://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts (accessed on 3 November 2021).
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Quartacci, M.F.; Sgherri, C.; Andrich, G.; Zinnai, A. A kinetic approach to describe the time evolution of red wine as a function of packaging conditions adopted: Influence of closure and storage position. Food Packag. Shelf Life 2017, 13, 44–48. [Google Scholar] [CrossRef]
- Asano, K.; Shinagawa, K.; Hashimoto, N. Characterization of haze-forming proteins of beer and their roles in chill haze formation. J. Am. Soc. Brew. Chem. 1982, 40, 147–154. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Motiejunas, D.; Wade, R.C. Structural, Energetic, and Dynamic Aspects of Ligand–Receptor Interactions. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: The Netherlands, 2007; pp. 193–213. [Google Scholar]
- Medda, L.; Monduzzi, M.; Salis, A. The molecular motion of bovine serum albumin under physiological conditions is ion specific. Chem. Comm. 2015, 51, 6663–6666. [Google Scholar] [CrossRef] [Green Version]
- Alihosseini, F. Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawstone, UK, 2016; pp. 155–195. [Google Scholar]
- Semenova, M.G.; Antipova, A.S.; Belyakova, L.E. Food protein interactions in sugar solutions. Curr. Opin. Coll. Interf. Sci. 2002, 7, 438–444. [Google Scholar] [CrossRef]
- Seddon, T.J.; Downey, M.O. Comparison of analytical methods for the determination of condensed tannins in grape skin. Aust. J. Grape Wine Res. 2008, 14, 54–61. [Google Scholar] [CrossRef]
- Sharma, P.; Ghosh, A.; Tudu, B.; Sabhapondit, S.; Baruah, B.; Tamuly, P.; Bandyopadhyay, R. Monitoring the fermentation process of black tea using QCM sensor based electronic nose. Sens. Actuators B Chem. 2015, 219, 146–157. [Google Scholar] [CrossRef]




| Sample Nomenclature | Commercial Tannin Blend Added | Polyphenol Concentration/g L−1 | pH |
|---|---|---|---|
| TG-Low | TANIN GALALCOOL | 0.02 | 4.93 |
| TG-Mid | 0.10 | 4.32 | |
| TG-High | 0.19 | 4.13 | |
| TS-Low | TANIN VR SUPRA | 0.07 | 5.76 |
| TS-Mid | 0.26 | 4.91 | |
| TS-High | 0.52 | 4.91 |
| Sample Nomenclature | Commercial Tannin Blend Added | Polyphenol Concentration (g L−1 Gallic Acid Equivalent) | Proanthocyanidins Concentration /mg L−1 Epicatechin Equivalent | ||
|---|---|---|---|---|---|
| UV/g L−1 | Folin–Ciocalteu | pH (±0.05) | |||
| A | - | 0.14 ± 0.01 | 0.22 ± 0.01 | 50 ± 2 | 3.16 |
| C3 | TB | 2.64 ± 0.02 | 2.21 ± 0.09 | 45 ± 2 | 3.34 |
| C5 | 5.47 ± 0.14 | 4.14 ± 0.07 | 55 ± 2 | 3.15 | |
| G2 | TG | 0.96 ± 0.01 | 0.77 ± 0.01 | 42 ± 1 | 3.20 |
| G3 | 2.06 ± 0.08 | 1.52 ± 0.07 | 39 ± 1 | 3.14 | |
| G4 | 3.12 ± 0.12 | 2.17 ± 0.10 | 38 ± 3 | 2.93 | |
| G5 | 4.26 ± 0.10 | 2.83 ± 0.01 | 64 ± 3 | 3.00 | |
| H2 | TGSP | 1.03 ± 0.03 | 0.91 ± 0.01 | 24 ± 4 | 3.04 |
| H3 | 2.13 ± 0.09 | 1.80 ± 0.09 | 36 ± 2 | 3.11 | |
| H4 | 3.33 ± 0.04 | 2.67 ± 0.07 | 45 ± 6 | 3.14 | |
| H5 | 4.34 ± 0.10 | 3.39 ± 0.04 | 53 ± 5 | 3.15 | |
| I2 | TGR | 0.93 ± 0.03 | 1.74 ± 0.03 | 824 ± 1 | 2.93 |
| I3 | 2.01 ± 0.01 | 3.81 ± 0.11 | 1702 ± 6 | 2.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, M.; Tori, G.; Agostini, M.; Lunardelli, F.; Mencarelli, F.; Sanmartin, C.; Cecchini, M. Detection of Oenological Polyphenols via QCM-D Measurements. Nanomaterials 2022, 12, 166. https://doi.org/10.3390/nano12010166
Gagliardi M, Tori G, Agostini M, Lunardelli F, Mencarelli F, Sanmartin C, Cecchini M. Detection of Oenological Polyphenols via QCM-D Measurements. Nanomaterials. 2022; 12(1):166. https://doi.org/10.3390/nano12010166
Chicago/Turabian StyleGagliardi, Mariacristina, Giorgia Tori, Matteo Agostini, Francesco Lunardelli, Fabio Mencarelli, Chiara Sanmartin, and Marco Cecchini. 2022. "Detection of Oenological Polyphenols via QCM-D Measurements" Nanomaterials 12, no. 1: 166. https://doi.org/10.3390/nano12010166







