Investigation on Transparent, Conductive ZnO:Al Films Deposited by Atomic Layer Deposition Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
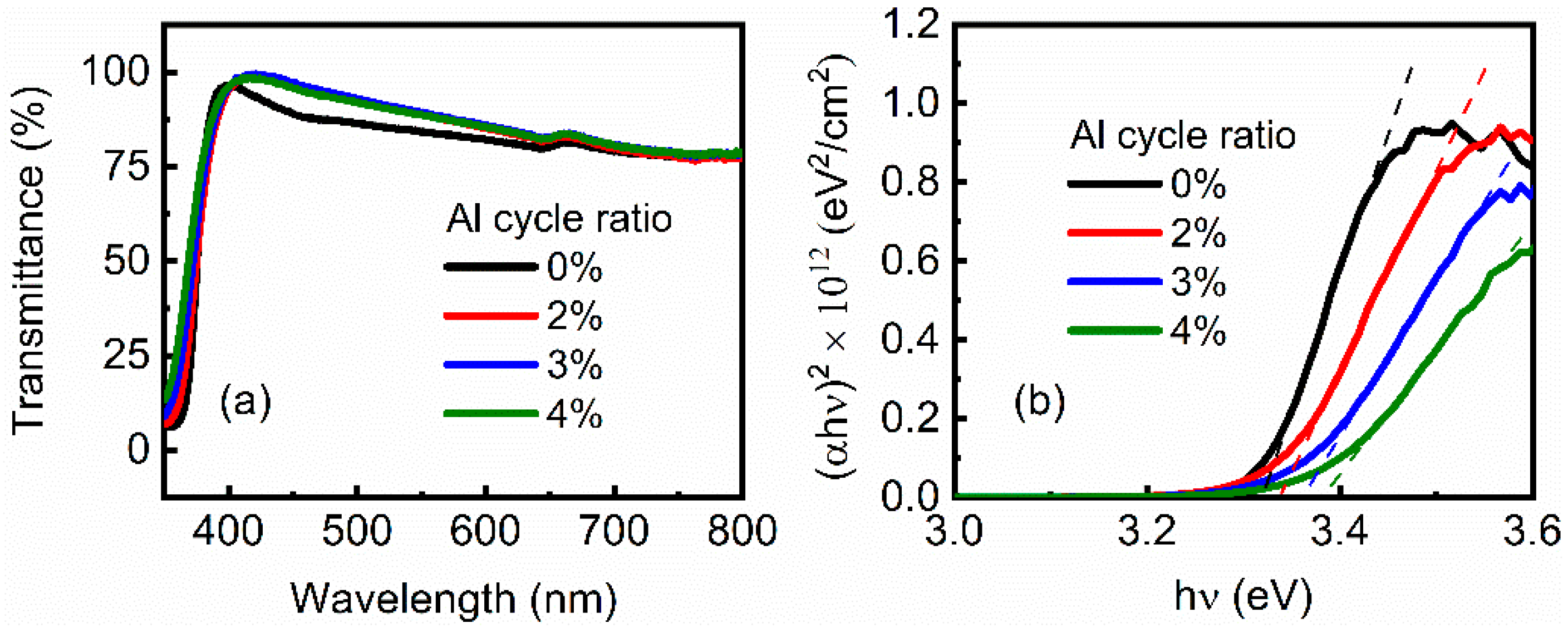
3.1. Resistance and Transmittance of AZO Films
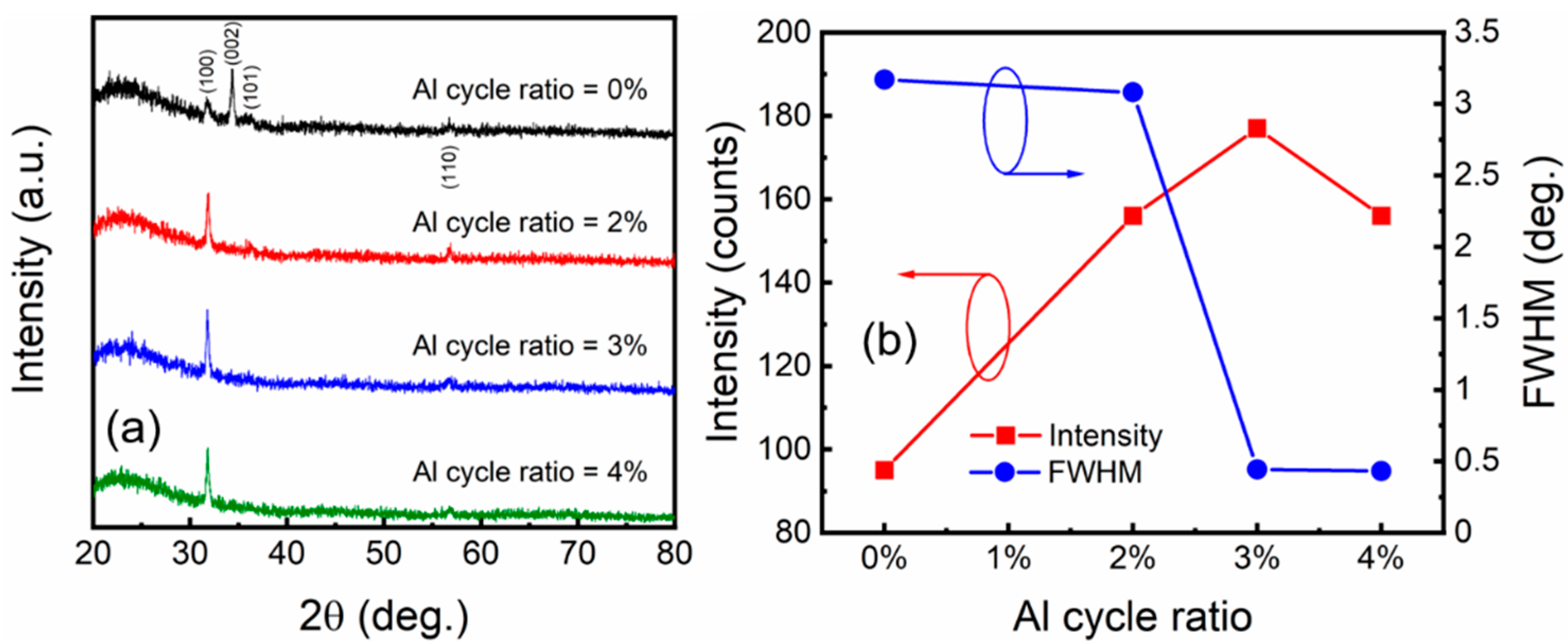
3.2. Crystallinity, Surface Morphology, and Material Components of AZO Films
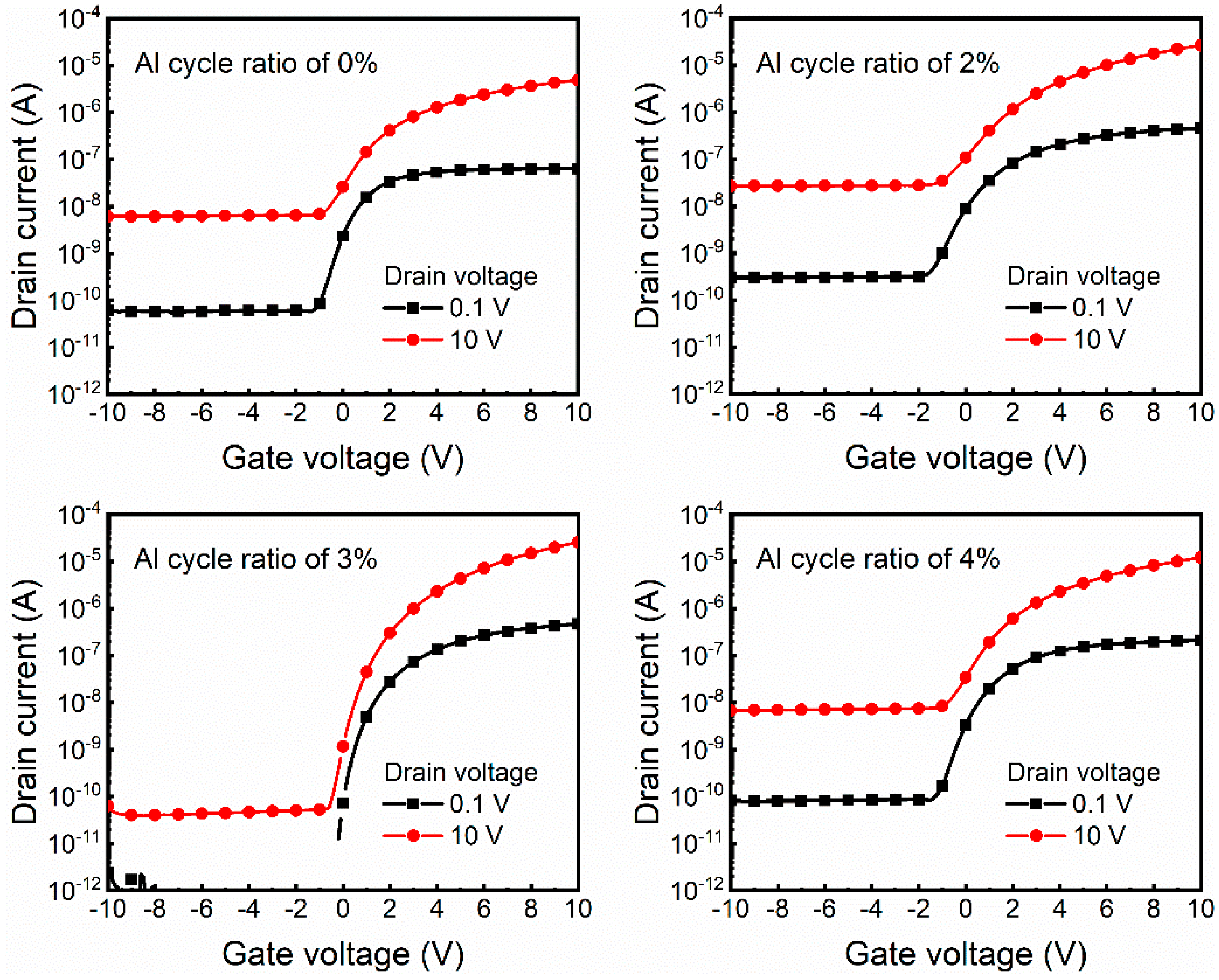
3.3. Performance of Transparent ZnO TFTs with AZO Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, C.S.; Wan, P.; Yang, H.; Shah, S.A.A.; Chen, X. Healable transparent electronic devices. Adv. Funct. Mater. 2017, 27, 1606339. [Google Scholar] [CrossRef]
- Park, J.; Paik, H.; Nomoto, K.; Lee, K.; Park, B.E.; Grisafe, B.; Wang, L.C.; Salahuddin, S.; Datta, S.; Kim, Y.; et al. Fully transparent field-effect transistor with high drain current and on-off ratio. APL Mater. 2020, 8, 011110. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Yao, X.; Ren, Q.; Zheng, C.; Li, S.; Tong, Y.; Shi, B.; Guo, S.; Fan, L.; Ren, H.; et al. Transparent electrode for monolithic perovskite/silicon-heterojunction two-terminal tandem solar cells. Nano Energy 2018, 45, 280–286. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kuwae, H.; Kobayashi, N.; Nobori, A.; Shoji, S.; Mizuno, J. Highly flexible transparent electrodes based on mesh-patterned rigid indium tin oxide. Sci. Rep. 2018, 8, 2825. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Liu, D.; Zhang, C.; Jay Guo, L. Ultrathin-metal-film-based transparent electrodes with relative transmittance surpassing 100. Nat. Commun. 2020, 11, 3367. [Google Scholar] [CrossRef] [PubMed]
- Azani, M.R.; Hassanpour, A.; Torres, T. Benefits, Problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 2020, 10, 2002536. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Zhu, F.; Sun, X.; Sun, Z. Improving temperature-stable AZO-Ag-AZO multilayer transparent electrodes using thin Al layer modification. J. Phys. D Appl. Phys. 2012, 45, 505103. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yan, J.; Li, T.; Zhang, L. Transparent conductive ITO/Cu/ITO films prepared on flexible substrates at room temperature. Appl. Surf. Sci. 2012, 258, 3082–3085. [Google Scholar] [CrossRef]
- Silah, H.; Erkmen, C.; Demir, E.; Uslu, B. Modified indium tin oxide electrodes: Electrochemical applications in pharmaceutical, biological, environmental and food analysis. TrAC Trends Analyt. Chem. 2021, 141, 116289. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Dong, J.; Li, H.; Yi, Z.; Zhang, X.; Wang, Y. The Effects of post annealing process on the electrical performance and stability of Al-Zn-O thin-film transistors. IEEE Electron Device Lett. 2020, 41, 569–572. [Google Scholar] [CrossRef]
- Gil-González, N.; Chen, C.; Akyazi, T.; Zuzuarregui, A.; Rodriguez, A.; Knez, M.; Castaño, E.; Benito-Lopez, F.; Morant-Miñana, M.C. AZO embedded interdigitated electrodes for monitoring stimuli responsive materials. Adv. Funct. Mater. 2018, 28, 1803127. [Google Scholar] [CrossRef]
- Deng, W.; Xiao, X.; Shao, Y.; Song, Z.; Lee, C.Y.; Lien, A.; Zhang, S.D. A back-channel-etched amorphous InGaZnO thin-film transistor technology with Al-doped ZnO as source/drain and pixel electrodes. IEEE Trans. Electron Devices 2016, 63, 2205–2209. [Google Scholar] [CrossRef]
- Novák, P. Possibilities of increasing the usability of sputtered AZO films as a transparent electrode. Phys. Status Solidi A 2019, 216, 1800814. [Google Scholar] [CrossRef]
- Zhao, N.; Han, D.; Chen, Z.; Wu, J.; Cong, Y.; Dong, J.; Zhao, F.; Zhang, S.; Zhang, X.; Wang, Y. High performance Ti-doped ZnO TFTs with AZO/TZO heterojunction S/D contacts. J. Disp. Technol. 2015, 11, 412–416. [Google Scholar] [CrossRef]
- Baek, I.H.; Pyeon, J.J.; Han, S.H.; Lee, G.Y.; Choi, B.J.; Han, J.H.; Chung, T.M.; Hwang, C.S.; Kim, S.K. High-performance thin-film transistors of quaternary indium-zinc-tin oxide films grown by atomic layer deposition. ACS Appl. Mater. Interfaces 2019, 11, 14892–14901. [Google Scholar] [CrossRef]
- Jeong, S.G.; Jeong, H.J.; Choi, W.H.; Kim, K.; Park, J.S. Hydrogen impacts of PEALD InGaZnO TFTs using SiOx gate insulators deposited by PECVD and PEALD. IEEE Trans. Electron Devices 2020, 67, 4250–4255. [Google Scholar] [CrossRef]
- Sheng, J.; Park, E.J.; Shong, B.; Park, J.S. Atomic layer deposition of an indium gallium oxide thin film for thin-film transistor applications. ACS Appl. Mater. Interfaces 2017, 9, 23934–23940. [Google Scholar] [CrossRef]
- Kim, D.; Kang, H.; Kim, J.M.; Kim, H. The properties of plasma-enhanced atomic layer deposition (ALD) ZnO thin films and comparison with thermal ALD. Appl. Surf. Sci. 2011, 257, 3776–3779. [Google Scholar] [CrossRef]
- Dimitrov, D.; Tsai, C.L.; Petrov, S.; Marinova, V.; Petrova, D.; Napoleonov, B.; Blagoev, B.; Strijkova, V.; Hsu, K.Y.; Lin, S.H. Atomic layer-deposited Al-doped ZnO thin films for display applications. Coatings 2020, 10, 539. [Google Scholar] [CrossRef]
- Marques, A.C.; Faria, J.; Perdigao, P.; Faustino, B.M.M.; Ritasalo, R.; Costabello, K.; da Silva, R.C.; Ferreira, I. Stability under humidity, UV-light and bending of AZO films deposited by ALD on Kapton. Sci. Rep. 2019, 9, 17919. [Google Scholar] [CrossRef]
- Li, Y.; Yao, R.; Wang, H.H.; Wu, X.M.; Wu, J.Z.; Wu, X.H.; Qin, W. Enhanced performance in Al-doped ZnO based transparent flexible transparent thin-film transistors due to oxygen vacancy in ZnO film with Zn-Al-O interfaces fabricated by atomic layer deposition. Acs Appl. Mater. Interface 2017, 9, 11711–11720. [Google Scholar] [CrossRef] [PubMed]
- Swatowska, B.; Powroźnik, W.; Czternastek, H.; Lewińska, G.; Stapiński, T.; Pietruszka, R.; Witkowski, B.S.; Godlewski, M. Application properties of ZnO and AZO thin films obtained by the ALD method. Energies 2021, 14, 6271. [Google Scholar] [CrossRef]
- Li, M.; Qian, X.; Li, A.D.; Cao, Y.Q.; Zhai, H.F.; Wu, D. A comparative study of growth and properties of atomic layer deposited transparent conductive oxide of Al doped ZnO films from different Al precursors. Thin Solid Films 2018, 646, 126–131. [Google Scholar] [CrossRef]
- Wu, Y.; Potts, S.E.; Hermkens, P.M.; Knoops, H.C.M.; Roozeboom, F.; Kessels, W.M.M. Enhanced doping efficiency of Al-doped ZnO by atomic layer deposition using dimethylaluminum lsopropoxide as an alternative aluminum precursor. Chem. Mater. 2013, 25, 4619–4622. [Google Scholar] [CrossRef]
- Macco, B.; van de Loo, B.W.H.; Dielen, M.; Loeffen, D.G.J.A.; van Pelt, B.B.; Phung, N.; Melskens, J.; Verheijen, M.A.; Kessels, W.M.M. Atomic-layer-deposited Al-doped zinc oxide as a passivating conductive contacting layer for n+-doped surfaces in silicon solar cells. Sol. Energy Mater. Sol. Cells 2021, 233, 111386. [Google Scholar] [CrossRef]
- Oh, B.Y.; Kim, J.H.; Han, J.W.; Seo, D.S.; Jang, H.S.; Choi, H.J.; Baek, S.H.; Kim, J.H.; Heo, G.S.; Kim, T.W.; et al. Transparent conductive ZnO:Al films grown by atomic layer deposition for Si-wire-based solar cells. Curr. Appl. Phys. 2012, 12, 273–279. [Google Scholar] [CrossRef]
- Mundle, R.; Carvajal, C.; Pradhan, A.K. ZnO/Al:ZnO transparent resistive switching devices grown by atomic layer deposition for memristor applications. Langmuir 2016, 32, 4983–4995. [Google Scholar] [CrossRef] [PubMed]
- Mošková, A.; Moško, M.; Precner, M.; Mikolášek, M.; Rosová, A.; Mičušík, M.; Štrbík, V.; Šoltýs, J.; Gucmann, F.; Dobročka, E.; et al. Doping efficiency and electron transport in Al-doped ZnO films grown by atomic layer deposition. J. Appl. Phys. 2021, 130, 035106. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Liu, Y.; Zhu, J.; Song, J.; Hao, Z.; Zeng, X.; Zhao, X.; Shu, Y.; Chen, J.; et al. Effect of ITO target crystallinity on the properties of sputtering deposited ITO films. Ceram. Int. 2020, 46, 6342–6350. [Google Scholar] [CrossRef]
- Yang, S.; Zhong, J.; Sun, B.; Zeng, X.; Luo, W.; Zhao, X.; Shu, Y.; Chen, J.; He, J. Influence of base pressure on property of sputtering deposited ITO film. J. Mater. Sci. Mater. Electron. 2019, 30, 13005–13012. [Google Scholar] [CrossRef]
- Hamouda, F.; Herth, E.; David, C.; Bayle, F.; Plante, M.P.; Martin, A.; Aassime, A. Electrical and optical properties of sputtered ultra-thin indium tin oxide films using xenon/argon gas. J. Mater. Sci. Mater. Electron. 2019, 30, 8508–8514. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Dong, J.; Yu, W.; Liang, Y.; Luo, Z.; Zhang, S.; Zhang, X.; Wang, Y. Enhanced electrical properties of dual-layer channel ZnO thin film transistors prepared by atomic layer deposition. Appl. Surf. Sci. 2018, 439, 632–637. [Google Scholar] [CrossRef]
- Kang, K.M.; Wang, Y.; Kim, M.; Lee, C.; Park, H.H. Al/F codoping effect on the structural, electrical, and optical properties of ZnO films grown via atomic layer deposition. Appl. Surf. Sci. 2021, 535, 147734. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Dong, J.; Yi, Z.; Zhou, X.; Zhang, S.; Zhang, X.; Wang, Y. Enhanced performance of atomic layer deposited thin-film transistors with high-quality ZnO/Al2O3 interface. IEEE Trans. Electron Devices 2020, 67, 518–523. [Google Scholar] [CrossRef]
- Toan, N.V.; Tuoi, T.T.K.; Inomata, N.; Toda, M.; Ono, T. Aluminum doped zinc oxide deposited by atomic layer deposition and its applications to micro/nano devices. Sci. Rep. 2021, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.; Saadat, I. High-performance ALD Al-doped ZnO thin-film transistors grown on flexible substrates. IEEE Electron Device Lett. 2019, 40, 240–242. [Google Scholar] [CrossRef]
- Teng, L.F.; Liu, P.T.; Lo, Y.J.; Lee, Y.J. Effects of microwave annealing on electrical enhancement of amorphous oxide semiconductor thin film transistor. Appl. Phys. Lett. 2012, 101, 132901. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Chong, E.; Hyung Kim, D.; Woo Jeon, Y.; Hwan Kim, D.; Yeol Lee, S. Origin of threshold voltage shift by interfacial trap density in amorphous InGaZnO thin film transistor under temperature induced stress. Appl. Phys. Lett. 2011, 99, 062108. [Google Scholar] [CrossRef]
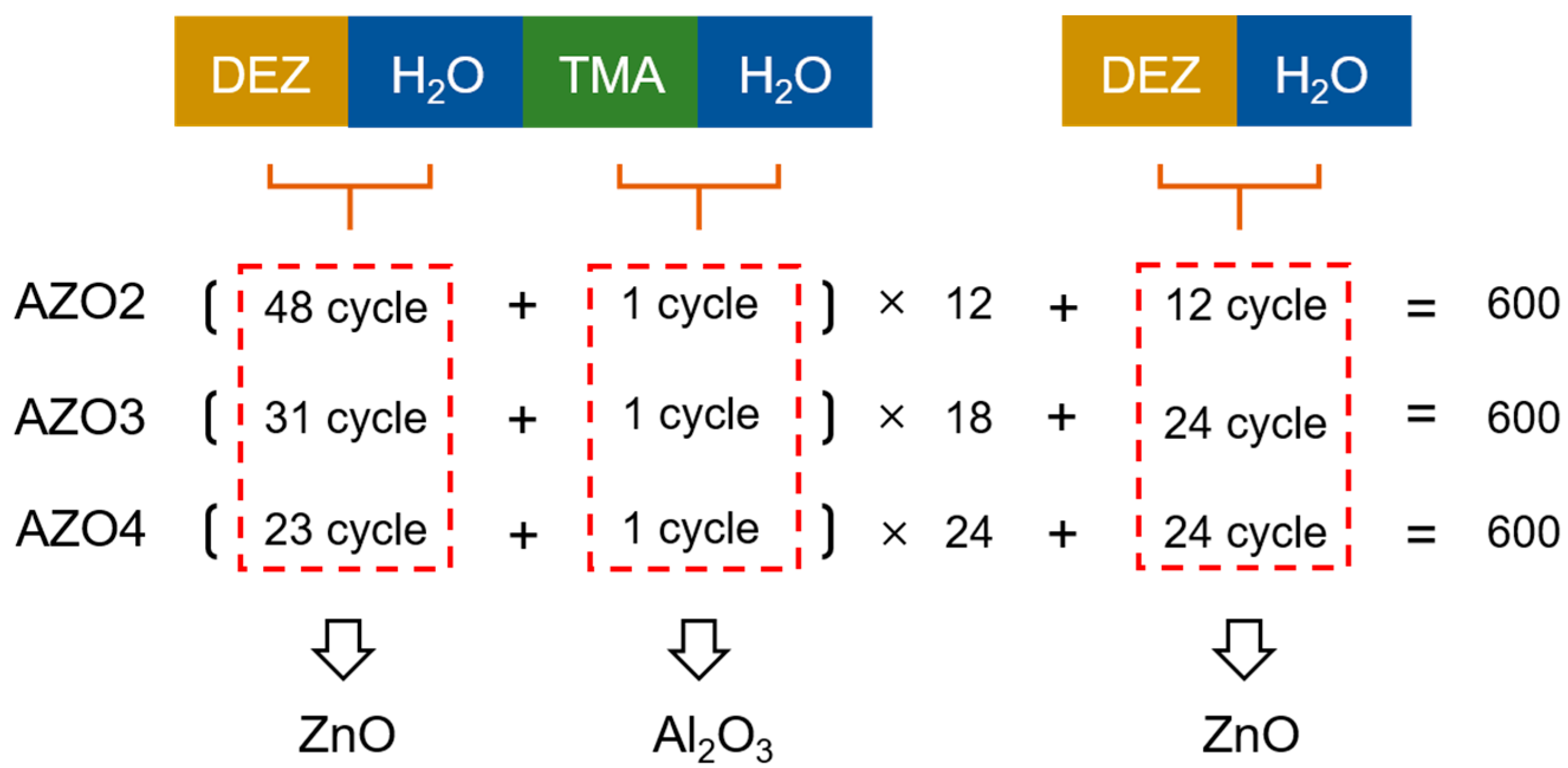

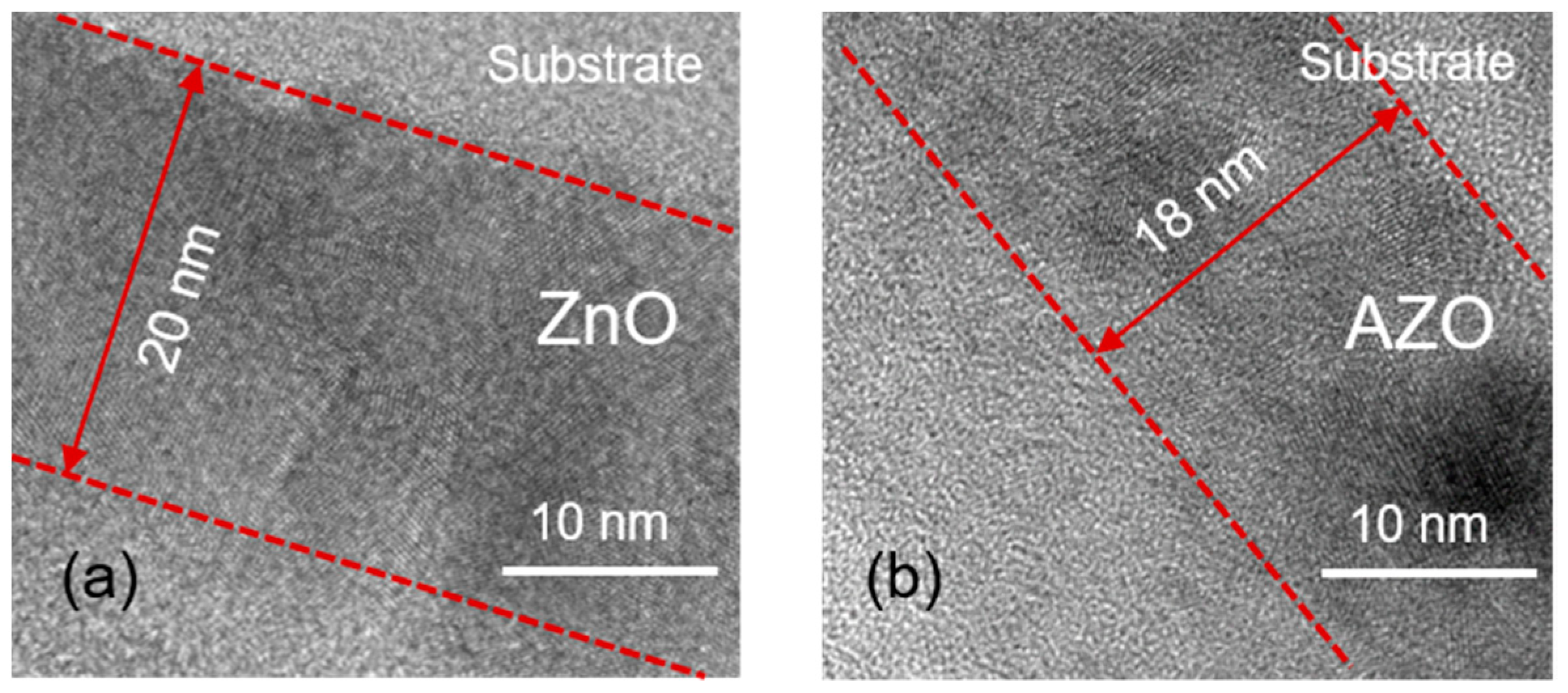


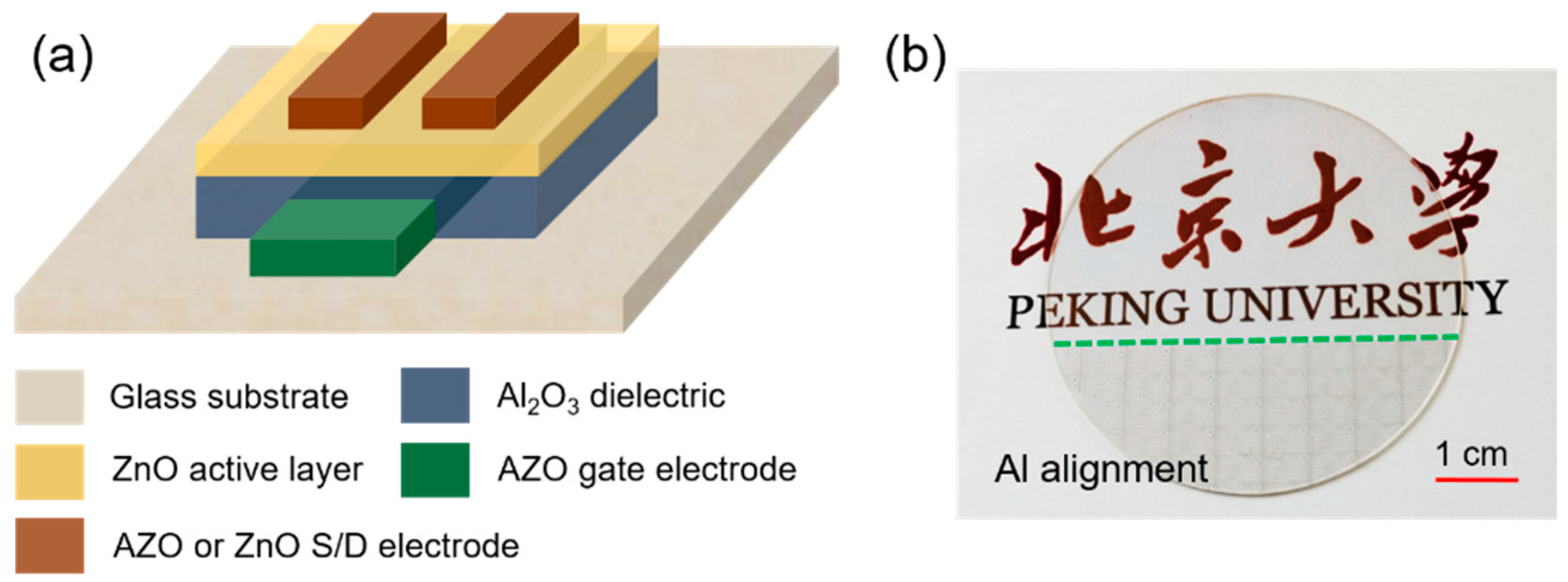

| Al Cycle RATIO (%) | 0 | 2 | 3 | 4 |
|---|---|---|---|---|
| D (nm) | 2.61 | 2.68 | 18.61 | 19.16 |
| Al Cycle Ratio (%) | µFE (cm2V−1s−1) | SS (mV/decade) | VON (V) | ION/IOFF |
|---|---|---|---|---|
| 0 | 1.07 | 627.97 | −1.3 | 1.08 × 103 |
| 2 | 3.64 | 960.93 | −1.9 | 1.46 × 103 |
| 3 | 4.07 | 194.94 | −0.2 | 7.34 × 105 |
| 4 | 2.29 | 696.54 | −1.5 | 2.55 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Xie, J.; Zhao, Y.; Han, D.; Wang, Y.; Liu, B.; Dong, J. Investigation on Transparent, Conductive ZnO:Al Films Deposited by Atomic Layer Deposition Process. Nanomaterials 2022, 12, 172. https://doi.org/10.3390/nano12010172
Zhao K, Xie J, Zhao Y, Han D, Wang Y, Liu B, Dong J. Investigation on Transparent, Conductive ZnO:Al Films Deposited by Atomic Layer Deposition Process. Nanomaterials. 2022; 12(1):172. https://doi.org/10.3390/nano12010172
Chicago/Turabian StyleZhao, Kai, Jingye Xie, Yudi Zhao, Dedong Han, Yi Wang, Bin Liu, and Junchen Dong. 2022. "Investigation on Transparent, Conductive ZnO:Al Films Deposited by Atomic Layer Deposition Process" Nanomaterials 12, no. 1: 172. https://doi.org/10.3390/nano12010172





