Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics
Abstract
:1. Introduction
Local Drug Delivery Systems and Titanium Implants
2. Antibacterial Effects
2.1. Anodizing Titania Nanotubes and Vancomycin
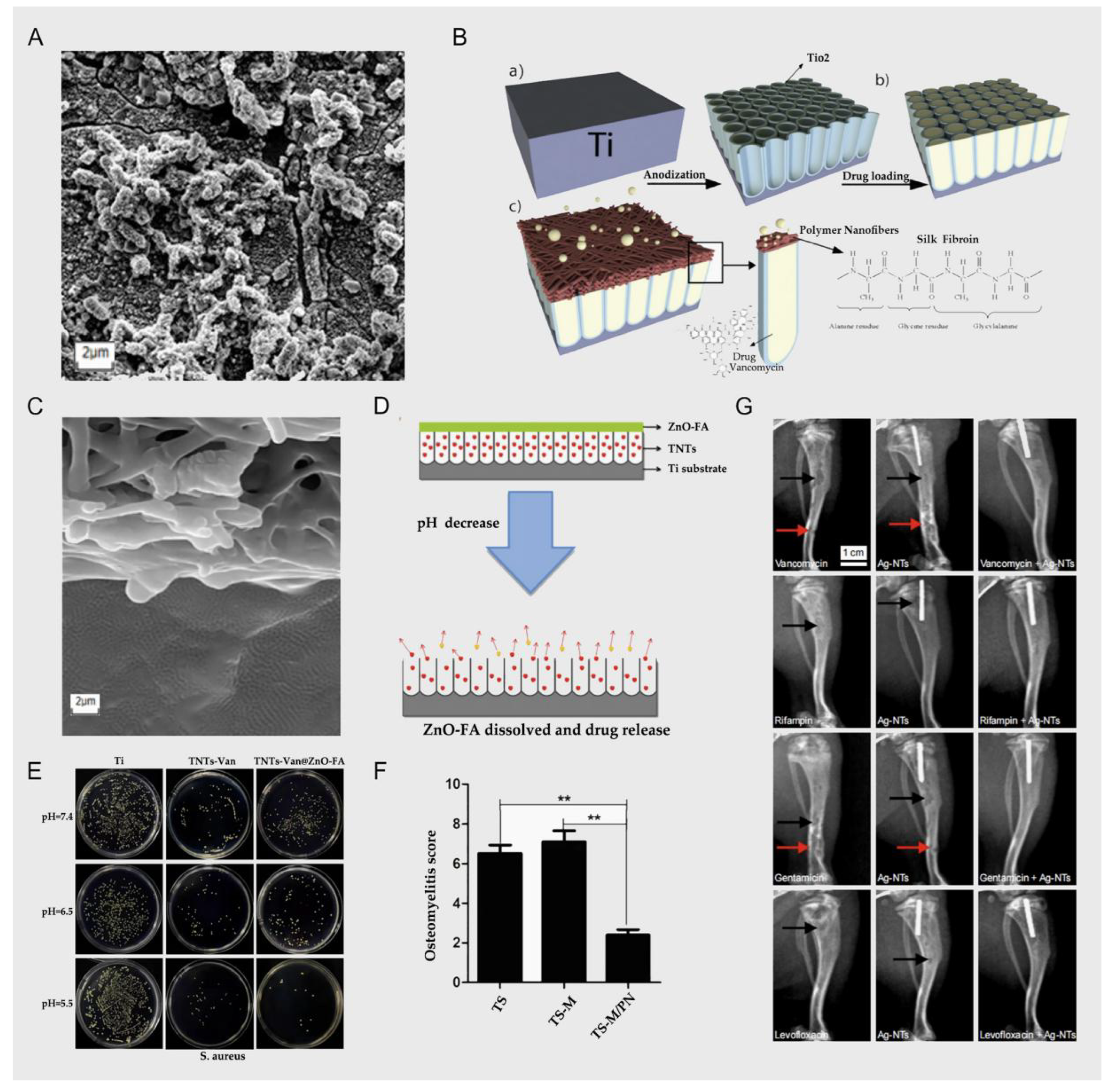
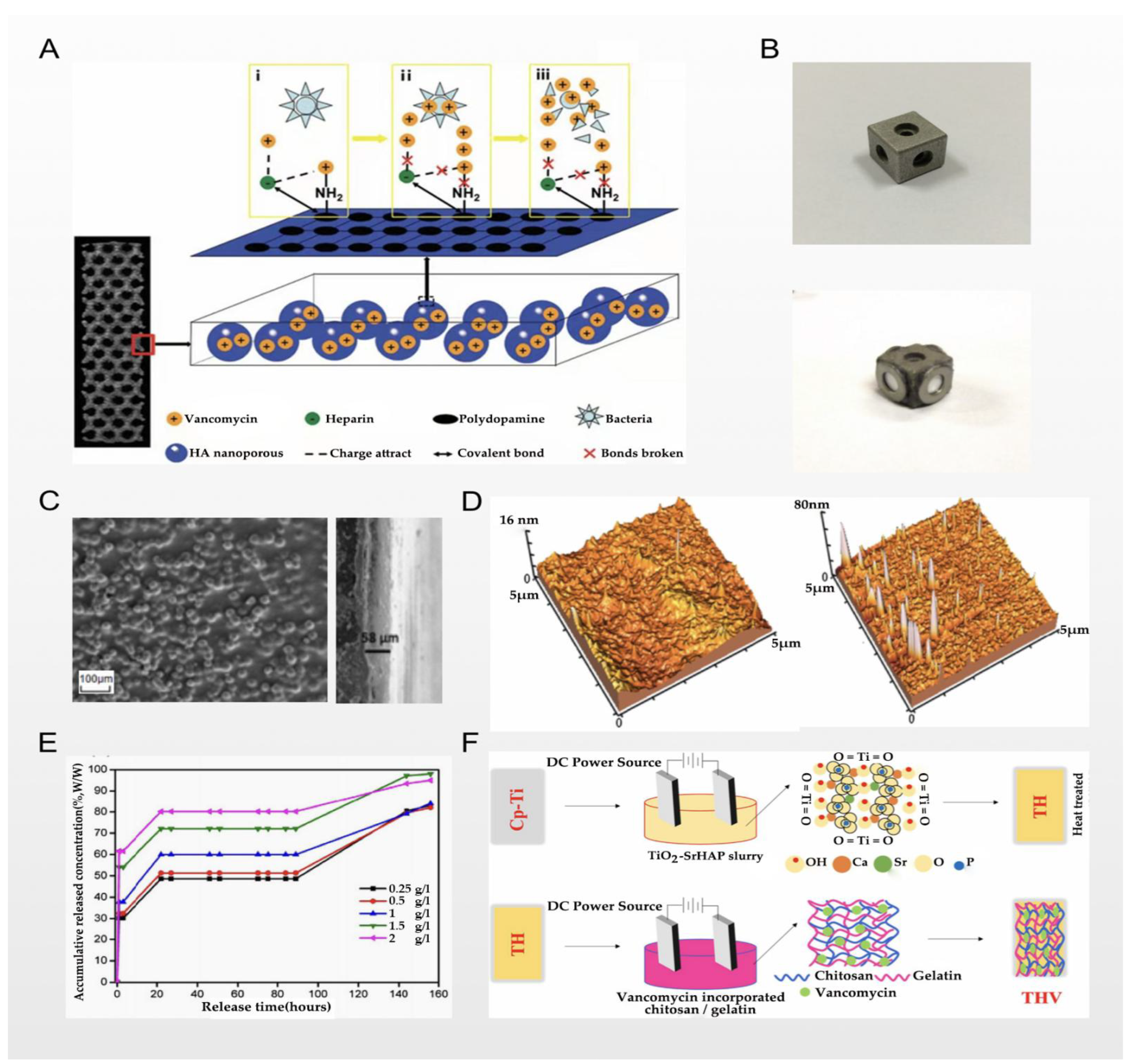
2.2. Electrochemical Deposition and Vancomycin
2.3. Chemical Coating and Vancomycin
3. Antitumor Effects
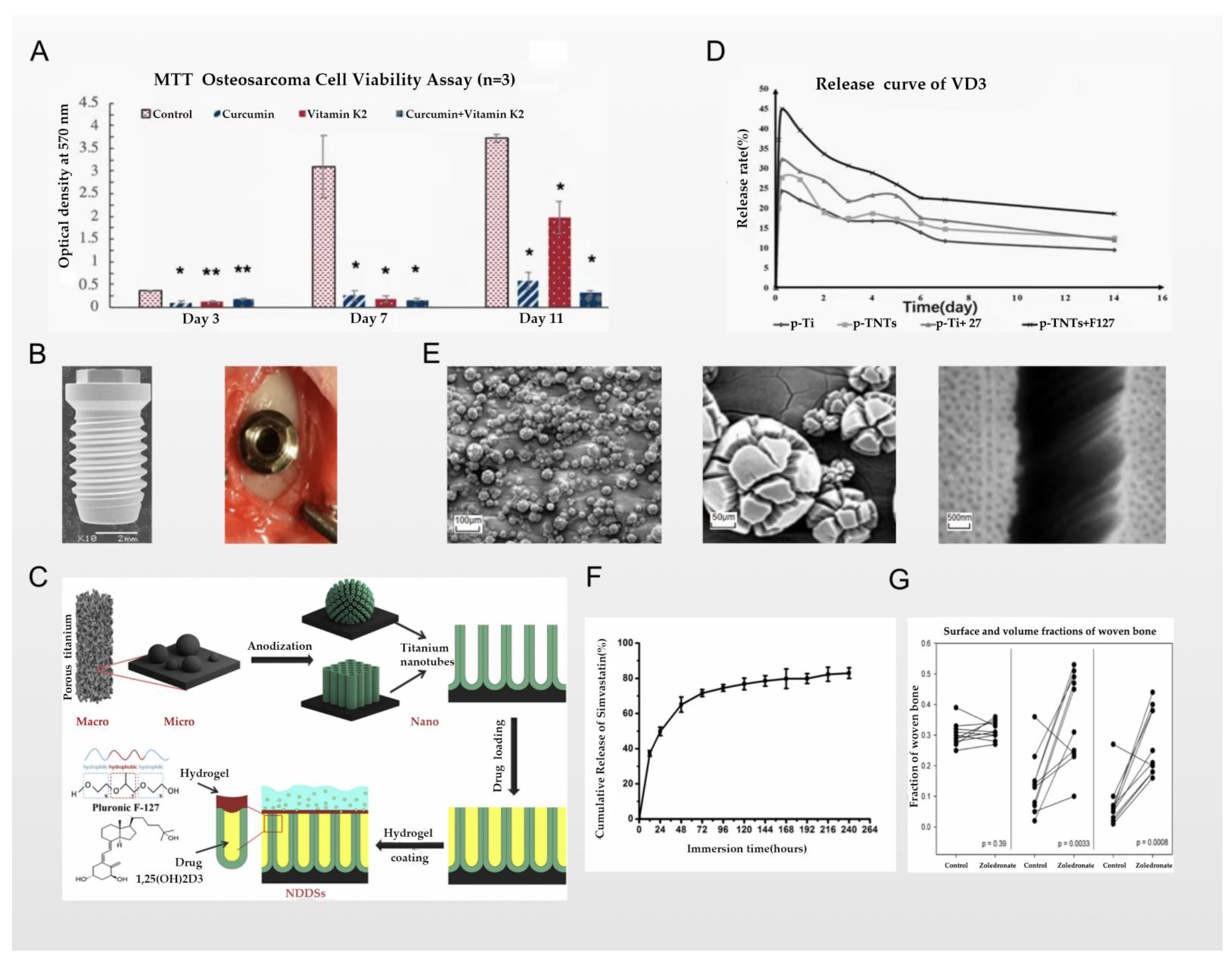
3.1. Titanium Nano/Micro-Surface Modification and Antitumor Activity
3.2. Chemical Coating and Antitumor Activity
4. Osseointegration Effect
4.1. Titanium Nano/Micro-Scale Surface Modification and Osseointegration
4.2. Chemical Coatings on Titanium Surfaces and Osseointegration
5. Discussion
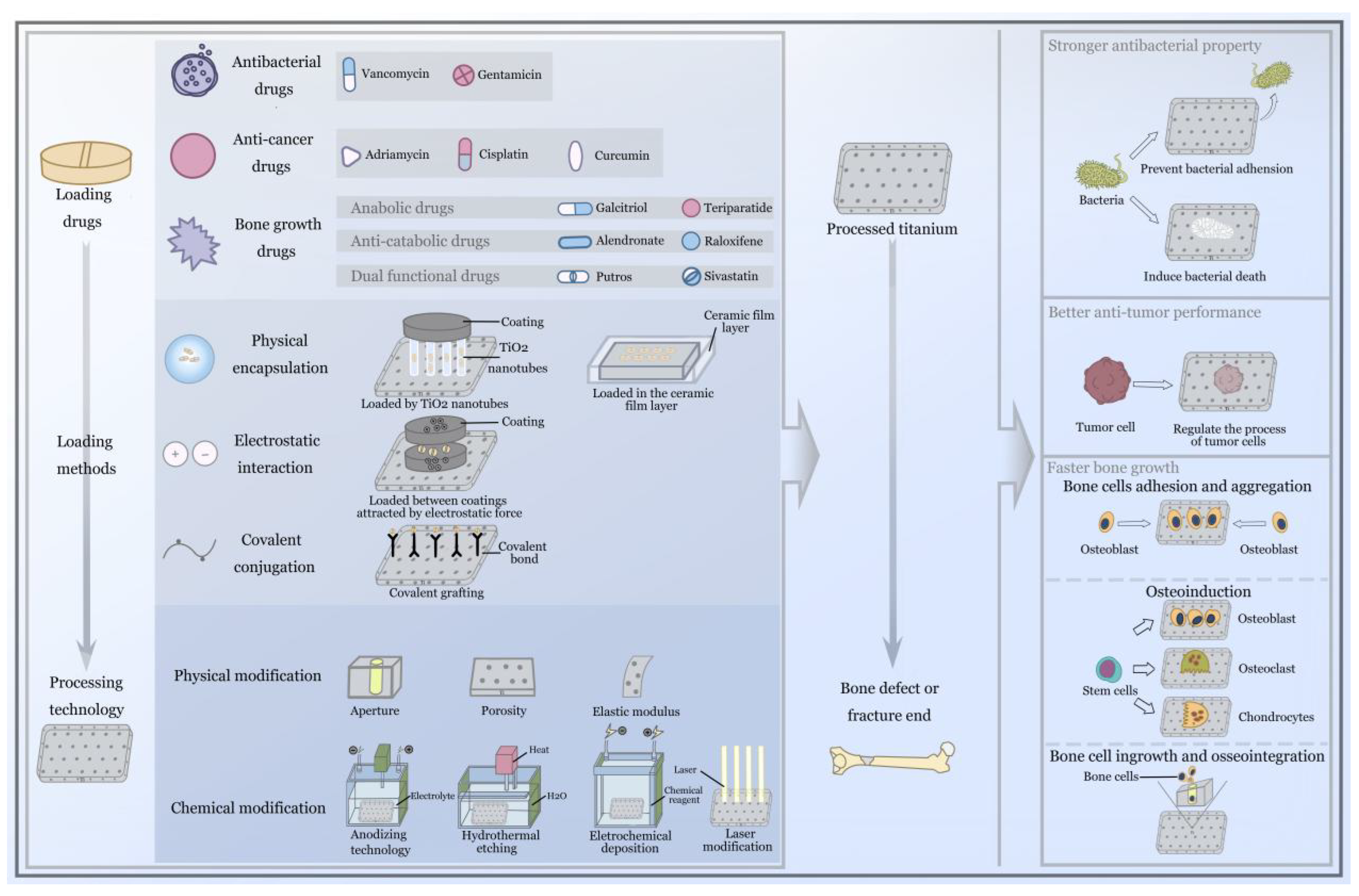
5.1. Loaded Drugs and Their Pharmacological Properties
5.2. Molecular Biological Mechanisms of Bone Cells and the Surrounding Tissues
5.3. Processing Technology of Titanium Implants
5.4. Clinical Application of Titanium Implants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buck, D.W.; Dumanian, G.A. Bone Biology and Physiology. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-P.; Zhang, X.-F.; Sun, L.; Chen, E.-M. Current and future uses of skeletal stem cells for bone regeneration. World J. Stem Cells 2020, 12, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yao, Y.; Tang, W.; Han, D.; Zhang, L.; Zhao, K.; Wang, S.; Meng, Y. Design of dental implants at materials level: An overview. J. Biomed. Mater. Res. Part A 2020, 108, 1634–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2020, 6, 346–360. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2016, 73, 22–40. [Google Scholar] [CrossRef]
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants—issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef]
- Plekhova, N.G.; Lyapun, I.N.; Drobot, E.I.; Shevchuk, D.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Functional State of Mesenchymal Stem Cells upon Exposure to Bioactive Coatings on Titanium Alloys. Bull. Exp. Biol. Med. 2020, 169, 147–156. [Google Scholar] [CrossRef]
- Han, C.-H.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T. Quantitative and qualitative investigations of surface enlarged titanium and titanium alloy implants. Clin. Oral Implant. Res. 1998, 9, 1–10. [Google Scholar] [CrossRef]
- Le Clerc, N.; Baudouin, R.; Carlevan, M.; Khoueir, N.; Verillaud, B.; Herman, P. 3D titanium implant for orbital reconstruction after maxillectomy. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 732–739. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2017, 53, 1–11. [Google Scholar] [CrossRef]
- Fraser, D.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Biomechanical analysis of the osseointegration of porous tantalum implants. J. Prosthet. Dent. 2019, 123, 811–820. [Google Scholar] [CrossRef]
- Barik, A.; Chakravorty, N. Targeted Drug Delivery from Titanium Implants: A Review of Challenges and Approaches. Trends Biomed. Res. 2019, 1–17. [Google Scholar] [CrossRef]
- Maher, S.; Mazinani, A.; Barati, M.R.; Losic, D. Engineered titanium implants for localized drug delivery: Recent advances and perspectives of Titania nanotubes arrays. Expert Opin. Drug Deliv. 2018, 15, 1021–1037. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Zhu, X.; Jiangab, L.; Shic, W.; Wangab, Y.; Xuab, N.; Gangabd, F.; Wangab, X.; Zhaoab, L.; et al. Dual directions to address the problem of aseptic loosening via electrospun PLGA @ aspirin nanofiber coatings on titanium. Biomaterials 2020, 257, 120237. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Yuan, Z.; Shen, M.; Song, Y.; Liu, H.; Deng, J.; Zhong, X.; Zhang, X. Establishing an osteoimmunomodulatory coating loaded with aspirin on the surface of titanium primed with phase-transited lysozyme. Int. J. Nanomed. 2019, 14, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Sumathra, M.; Rajan, M.; Praphakar, R.A.; Marraiki, N.; Elgorban, A.M. In Vivo Assessment of a Hydroxyapatite/κ-Carrageenan–Maleic Anhydride–Casein/Doxorubicin Composite-Coated Titanium Bone Implant. ACS Biomater. Sci. Eng. 2020, 6, 1650–1662. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef]
- Rossi, M.; Bezerra, F.J.B.; da Silva, R.A.; Crulhas, B.P.; Fernandes, C.J.C.; Nascimento, A.S.; Pedrosa, V.; Padilha, P.; Zambuzzi, W.F. Titanium-released from dental implant enhances pre-osteoblast adhesion by ROS modulating crucial intracellular pathways. J. Biomed. Mater. Res. Part A 2017, 105, 2968–2976. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Park, Y.-S.; Cho, J.-Y.; Lee, S.-J.; Hwang, C.I. Modified Titanium Implant as a Gateway to the Human Body: The Implant Mediated Drug Delivery System. BioMed Res. Int. 2014, 2014, 801358. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Li, H.; Zhou, D.; Chen, Z.; Gu, Z. Local and Targeted Delivery of Immune Checkpoint Blockade Therapeutics. Accounts Chem. Res. 2020, 53, 2521–2533. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.O.; Crisan, B.; Crisan, L.; Baciut, M.; Onisor, F.; Baciut, G.; Câmpian, R.S.; Bran, S. Systemic drugs that influence titanium implant osseointegration. Drug Metab. Rev. 2016, 49, 92–104. [Google Scholar] [CrossRef]
- Rahman, S.; Gulati, K.; Kogawa, M.; Atkins, G.J.; Pivonka, P.; Findlay, D.M.; Losic, D. Drug diffusion, integration, and stability of nanoengineered drug-releasing implants in bone ex-vivo. J. Biomed. Mater. Res. Part A 2015, 104, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Losic, D.; Gulati, K.; Aw, M.S. Nanoengineered drug-releasing Ti wires as an alternative for local delivery of chemotherapeutics in the brain. Int. J. Nanomed. 2012, 7, 2069–2076. [Google Scholar] [CrossRef] [Green Version]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Lai, Y.-K.; Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Zhou, W.; Fan, D.; Lin, X.; Jing, Z.; Cai, H.; Cheng, Y.; Liu, X.; et al. Sustainable release of vancomycin from micro-arc oxidised 3D-printed porous Ti6Al4V for treating methicillin-resistant Staphylococcus aureus bone infection and enhancing osteogenesis in a rabbit tibia osteomyelitis model. Biomater. Sci. 2020, 8, 3106–3115. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of Micro- to Nanostructured 3D-Printed Drug-Releasing Titanium Implants for Enhanced Osseointegration and Localized Delivery of Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef] [PubMed]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, R.; Huo, X.; Xiong, W.; Kang, L.; Xue, Y. Incidence of Surgical Site Infection After Spine Surgery. Spine 2020, 45, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2018, 83, 37–54. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Arweiler-Harbeck, D.; Arnolds, J.; Hussain, T.; Hansen, S.; Bertram, R.; Buer, J.; Lang, S.; Steinmann, J.; Höing, B. Imaging studies of bacterial biofilms on cochlear implants—Bioactive glass (BAG) inhibits mature biofilm. PLOS ONE 2020, 15, e0229198. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontology 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Boger, D.L. Maxamycins: Durable Antibiotics Derived by Rational Redesign of Vancomycin. Accounts Chem. Res. 2020, 53, 2587–2599. [Google Scholar] [CrossRef]
- Katarincic, J.A.; Fantry, A.; DePasse, J.M.; Feller, R. Local Modalities for Preventing Surgical Site Infections. J. Am. Acad. Orthop. Surg. 2018, 26, 14–25. [Google Scholar] [CrossRef]
- Fathi, M.; Akbari, B.; Taheriazam, A. Antibiotics drug release controlling and osteoblast adhesion from Titania nanotubes arrays using silk fibroin coating. Mater. Sci. Eng. C 2019, 103, 109743. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, S.; Wang, Y.; Sun, T.; Li, Z.; Cai, L.; Wang, X.; Zhou, L.; Lai, R. Study of a new bone-targeting titanium implant–bone interface. Int. J. Nanomed. 2016, 11, 6307–6324. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Liu, X.; Mao, C.; Cui, Z.; Yang, X.; Yeung, K.; Zheng, Y.; Wu, S. Infection-prevention on Ti implants by controlled drug release from folic acid/ZnO quantum dots sealed titania nanotubes. Mater. Sci. Eng. C 2018, 85, 214–224. [Google Scholar] [CrossRef]
- Xu, N.; Cheng, H.; Xu, J.; Li, F.; Gao, B.; Li, Z.; Gao, C.; Huo, K.; Fu, J.; Xiong, W. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. Int. J. Nanomed. 2017, 12, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Hesselvig, A.B.; Arpi, M.; Madsen, F.; Bjarnsholt, T.; Odgaard, A.; the ICON Study Group. Does an Antimicrobial Incision Drape Prevent Intraoperative Contamination? A Randomized Controlled Trial of 1187 Patients. Clin. Orthop. Relat. Res. 2020, 478, 1007–1015. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.; Lietaert, K.; van Kessel, K.; Pouran, B.; van der Wal, B.; Vogely, H.; Van Hecke, W.; Fluit, A.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Auñón, Á; Esteban, J.; Doadrio, A.L.; Boiza-Sánchez, M.; Mediero, A.; Eguibar-Blázquez, D.; Cordero-Ampuero, J.; Conde, A.; Arenas, M.; De-Damborenea, J.; et al. Staphylococcus aureus Prosthetic Joint Infection Is Prevented by a Fluorine- and Phosphorus-Doped Nanostructured Ti–6Al–4V Alloy Loaded with Gentamicin and Vancomycin. J. Orthop. Res. 2019, 38, 588–597. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Haider, W.; Pompa, L.; Deen, K.M. Electrochemical & osteoblast adhesion study of engineered TiO 2 nanotubular surfaces on titanium alloys. Mater. Sci. Eng. C 2016, 58, 160–168. [Google Scholar] [CrossRef]
- Bezuidenhout, M.B.; Van Staden, A.D.; Oosthuizen, G.A.; Dimitrov, D.M.; Dicks, L.M.T. Delivery of Antibiotics from Cementless Titanium-Alloy Cubes May Be a Novel Way to Control Postoperative Infections. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xue, Y.; Zhang, L.; Han, Y. A superparamagnetic Fe3O4–TiO2 composite coating on titanium by micro-arc oxidation for percutaneous implants. J. Mater. Chem. B 2019, 7, 5265–5276. [Google Scholar] [CrossRef]
- Chernozem, R.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Prymak, O.; Loza, K.; Epple, M.; Ennen-Roth, F.; Wittmar, A.; et al. Functionalization of titania nanotubes with electrophoretically deposited silver and calcium phosphate nanoparticles: Structure, composition and antibacterial assay. Mater. Sci. Eng. C 2019, 97, 420–430. [Google Scholar] [CrossRef]
- Stein, S.; Kruck, L.; Warnecke, D.; Seitz, A.; Dürselen, L.; Ignatius, A. Osseointegration of titanium implants with a novel silver coating under dynamic loading. Eur. Cells Mater. 2020, 39, 249–259. [Google Scholar] [CrossRef]
- Pradhan, D.; Wren, A.; Misture, S.; Mellott, N. Investigating the structure and biocompatibility of niobium and titanium oxides as coatings for orthopedic metallic implants. Mater. Sci. Eng. C 2016, 58, 918–926. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2000 2019, 79, 178–189. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Nancy, D.; Rajendran, N. Vancomycin incorporated chitosan/gelatin coatings coupled with TiO2–SrHAP surface modified cp-titanium for osteomyelitis treatment. Int. J. Biol. Macromol. 2018, 110, 197–205. [Google Scholar] [CrossRef]
- Boix-Lemonche, G.; Guillem-Marti, J.; D’Este, F.; Manero, J.M.; Skerlavaj, B. Covalent grafting of titanium with a cathelicidin peptide produces an osteoblast compatible surface with antistaphylococcal activity. Colloids Surfaces B Biointerfaces 2019, 185, 110586. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, L.; Carrié, H.; Nguyen, M.N.; Plawinski, L.; Durrieu, M.-C.; Héroguez, V. Vancomycin Functionalized Nanoparticles for Bactericidal Biomaterial Surfaces. Biomacromolecules 2016, 17, 1339–1346. [Google Scholar] [CrossRef]
- Swanson, T.E.; Cheng, X.; Friedrich, C. Development of chitosan-vancomycin antimicrobial coatings on titanium implants. J. Biomed. Mater. Res. Part A 2011, 97A, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Dehghani, M.; Simchi, A. Antibiotic-loaded chitosan–Laponite films for local drug delivery by titanium implants: Cell proliferation and drug release studies. J. Mater. Sci. Mater. Med. 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaee, S.Y.; Bagheri, R.; Heidarpour, H.; Vossoughi, M.; Golizadeh, M.; Samadikuchaksaraei, A. Nanofibrillated chitosan coated highly ordered titania nanotubes array/graphene nanocomposite with improved biological characters. Carbohydr. Polym. 2020, 254, 117465. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Liu, X.; Yeung, K.; Wu, S. Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Colloids Surfaces B Biointerfaces 2017, 151, 165–177. [Google Scholar] [CrossRef]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO 2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Prevention the formation of biofilm on orthopedic implants by melittin thin layer on chitosan/bioactive glass/vancomycin coatings. J. Mater. Sci. Mater. Med. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Soldatos, T.; Chalian, M.; Attar, S.; McCarthy, E.F.; Carrino, J.A.; Fayad, L.M. Imaging differentiation of pathologic fractures caused by primary and secondary bone tumors. Eur. J. Radiol. 2013, 82, e36–e42. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Onishi, E.; Fujibayashi, S.; Takemoto, M.; Neo, M.; Maruyama, T.; Kokubo, T.; Nakamura, T. Enhancement of bone-bonding ability of bioactive titanium by prostaglandin E2 receptor selective agonist. Biomaterials 2008, 29, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhang, X.; Zuscik, M.; Drissi, M.H.; Schwarz, E.M.; O’Keefe, R.J. Fibroblasts Express RANKL and Support Osteoclastogenesis in a COX-2-Dependent Manner After Stimulation with Titanium Particles. J. Bone Miner. Res. 2005, 20, 1136–1148. [Google Scholar] [CrossRef]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jedrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, J.; Chen, J.; Zeng, Y.; Zhu, Z.; Wan, Y. Fabrication of Curcumin-Modified TiO2 Nanoarrays via Cyclodextrin Based Polymer Functional Coatings for Osteosarcoma Therapy. Adv. Health Mater. 2019, 8, e1901031. [Google Scholar] [CrossRef]
- Kaur, G.; Willsmore, T.; Gulati, K.; Zinonos, I.; Wang, Y.; Kurian, M.; Hay, S.; Losic, D.; Evdokiou, A. Titanium wire implants with nanotube arrays: A study model for localized cancer treatment. Biomaterials 2016, 101, 176–188. [Google Scholar] [CrossRef]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Controlled Delivery of Curcumin and Vitamin K2 from Hydroxyapatite-Coated Titanium Implant for Enhanced in Vitro Chemoprevention, Osteogenesis, and in Vivo Osseointegration. ACS Appl. Mater. Interfaces 2020, 12, 13644–13656. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [Google Scholar] [CrossRef]
- Kurzweg, H.; Heimann, R.; Troczynski, T.; Wayman, M. Development of plasma-sprayed bioceramic coatings with bond coats based on titania and zirconia. Biomaterials 1998, 19, 1507–1511. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, S.; Wikesjö, U.M.E.; Johansson, P.H.; Johansson, C.B.; Sul, Y.-T. Bone tissue response following local drug delivery of bisphosphonate through titanium oxide nanotube implants in a rabbit model. J. Clin. Periodontol. 2017, 44, 941–949. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Li, Y.; Ren, M.; Xiang, J.; Zhang, Z.; Ji, P.; Yang, S. 1α,25-Dihydroxyvitamin D3-loaded hierarchical titanium scaffold enhanced early osseointegration. Mater. Sci. Eng. C 2019, 109, 110551. [Google Scholar] [CrossRef]
- Gulati, K.; Prideaux, M.; Kogawa, M.; Lima-Marques, L.; Atkins, G.; Findlay, D.M.; Losic, D. Anodized 3D-printed titanium implants with dual micro- and nano-scale topography promote interaction with human osteoblasts and osteocyte-like cells. J. Tissue Eng. Regen. Med. 2016, 11, 3313–3325. [Google Scholar] [CrossRef]
- Lai, M.; Yan, X.; Jin, Z. The response of bone cells to titanium surfaces modified by simvastatin-loaded multilayered films. J. Biomater. Sci. Polym. Ed. 2018, 29, 1895–1908. [Google Scholar] [CrossRef]
- Jakobsen, T.; Bechtold, J.E.; Søballe, K.; Jensen, T.; Vestermark, M.T.; Baas, J. Local delivery of zoledronate from a poly (D,L-lactide)-coating increases fixation of hydroxy-coated implants. J. Orthop. Res. 2016, 35, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. North Am. 2019, 52, 231–242. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Wolf-Brandstetter, C.; Lode, A.; Hanke, T.; Scharnweber, D.; Worch, H. Influence of modified extracellular matrices on TI6AL4V implants on binding and release of VEGF. J. Biomed. Mater. Res. Part A 2006, 79, 882–894. [Google Scholar] [CrossRef]
- Oates, M.; Chen, R.; Duncan, M.; Hunt, J. The angiogenic potential of three-dimensional open porous synthetic matrix materials. Biomaterials 2007, 28, 3679–3686. [Google Scholar] [CrossRef]
- Murphy, W.; Simmons, C.; Kaigler, D.; Mooney, D. Bone Regeneration via a Mineral Substrate and Induced Angiogenesis. J. Dent. Res. 2004, 83, 204–210. [Google Scholar] [CrossRef]
- Dai, J.; Rabie, A.B.M. VEGF: An Essential Mediator of Both Angiogenesis and Endochondral Ossification. J. Dent. Res. 2007, 86, 937–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, H.; Bundgaard, K.G.; Christensen, K.S.; Lind, M.; Hansen, E.S.; Hvid, I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J. Orthop. Res. 2003, 21, 335–340. [Google Scholar] [CrossRef]
- Deckers, M.M.L.; Karperien, M.; Van Der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Löwik, C.W.G.M. Expression of Vascular Endothelial Growth Factors and Their Receptors during Osteoblast Differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef]
- Geiger, F.; Bertram, H.; Berger, I.; Lorenz, H.; Wall, O.; Eckhardt, C.; Simank, H.-G.; Richter, W. Vascular Endothelial Growth Factor Gene-Activated Matrix (VEGF165-GAM) Enhances Osteogenesis and Angiogenesis in Large Segmental Bone Defects. J. Bone Miner. Res. 2005, 20, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017, 2017, CD003188. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Kwon, D.H.; Kang, B.-S.; Oh, S.-J.; Johansson, C. Experimental evidence for interfacial biochemical bonding in osseointegrated titanium implants. Clin. Oral Implant. Res. 2011, 24, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Ruan, F.; Zheng, Q.; Wang, J. Mechanisms of bone anabolism regulated by statins. Biosci. Rep. 2012, 32, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Stein, D.; Lee, Y.; Schmid, M.J.; Killpack, B.; Genrich, M.A.; Narayana, N.; Marx, D.B.; Cullen, D.M.; Reinhardt, R.A. Local Simvastatin Effects on Mandibular Bone Growth and Inflammation. J. Periodontol. 2005, 76, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Pathak, J.L.; Hu, X.; Jin, Y.; Wu, Z.; Al-Baadani, M.A.; Wu, S.; Zhang, H.; Farkasdi, S.; Liu, Y.; et al. Sustained Release of Zoledronic Acid from Mesoporous TiO2-Layered Implant Enhances Implant Osseointegration in Osteoporotic Condition. J. Biomed. Nanotechnol. 2018, 14, 1965–1978. [Google Scholar] [CrossRef]
- Peter, B.; Pioletti, D.; Laïb, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.-Y.; Bouler, J.-M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Raines, A.L.; Berger, M.; Patel, N.; Hyzy, S.L.; Boyan, B.D.; Schwartz, Z. VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface. J. Biomed. Mater. Res. Part A 2018, 107, 423–433. [Google Scholar] [CrossRef]
- Leedy, M.R.; Jennings, J.A.; Haggard, W.O.; Bumgardner, J.D. Effects of VEGF-loaded chitosan coatings. J. Biomed. Mater. Res. Part A 2013, 102, 752–759. [Google Scholar] [CrossRef]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E.; et al. Atomic Layer Deposition of a Silver Nanolayer on Advanced Titanium Orthopedic Implants Inhibits Bacterial Colonization and Supports Vascularized de Novo Bone Ingrowth. Adv. Health Mater. 2017, 6, 1700033. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Gheban, D.; Benea, H.R.C. Tibolone, alendronate, and simvastatin enhance implant osseointegration in a preclinical in vivo model. Clin. Oral Implant. Res. 2020, 31, 655–668. [Google Scholar] [CrossRef]
- Mu, C.; Hu, Y.; Huang, L.; Shen, X.; Li, M.; Li, L.; Gu, H.; Yu, Y.; Xia, Z.; Cai, K. Sustained raloxifene release from hyaluronan-alendronate-functionalized titanium nanotube arrays capable of enhancing osseointegration in osteoporotic rabbits. Mater. Sci. Eng. C 2018, 82, 345–353. [Google Scholar] [CrossRef]
- Chikazu, D.; Tomizuka, K.; Ogasawara, T.; Saijo, H.; Koizumi, T.; Mori, Y.; Yonehara, Y.; Susami, T.; Takato, T. Cyclooxygenase-2 activity is essential for the osseointegration of dental implants. Int. J. Oral Maxillofac. Surg. 2007, 36, 441–446. [Google Scholar] [CrossRef]
- Gomes, F.I.F.; Aragão, M.G.B.; Pinto, V.D.P.T.; Gondim, D.V.; Barroso, F.C.; E Silva, A.A.R.; Bezerra, M.M.; Chaves, H.V. Effects of Nonsteroidal Anti-inflammatory Drugs on Osseointegration: A Review. J. Oral Implant. 2015, 41, 219–230. [Google Scholar] [CrossRef]
- Goodman, S.; Ma, T.; Trindade, M.; Ikenoue, T.; Matsuura, I.; Wong, N.; Fox, N.; Genovese, M.; Regula, D.; Smith, R.L. COX-2 selective NSAID decreases bone ingrowth in vivo. J. Orthop. Res. 2002, 20, 1164–1169. [Google Scholar] [CrossRef]
- Almagro, M.I.; Roman-Blas, J.A.; Bellido, M.; Castañeda, S.; Cortez, R.; Herrero-Beaumont, G. PTH [1-34] enhances bone response around titanium implants in a rabbit model of osteoporosis. Clin. Oral Implant. Res. 2012, 24, 1027–1034. [Google Scholar] [CrossRef]
- Sim, I.-W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Hjelle, C.P.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qi, M.; Wang, Y.; Feng, X.; Liu, H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, W.; Li, Q.; Zhao, D.; Qu, J.; Yuan, Z.; Cheng, Z.; Zhu, X.; Zhuang, X.; Zhang, Z. 3D-printing magnesium–polycaprolactone loaded with melatonin inhibits the development of osteosarcoma by regulating cell-in-cell structures. J. Nanobiotechnology 2021, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.; van Blitterswijk, C.; Barralet, J. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Human alveolar bone cell adhesion and growth on ion-implanted titanium. J. Biomed. Mater. Res. 2004, 69A, 651–657. [Google Scholar] [CrossRef]
- Zhao, X.; You, L.; Wang, T.; Zhang, X.; Li, Z.; Ding, L.; Li, J.; Xiao, C.; Han, F.; Li, B. Enhanced Osseointegration of Titanium Implants by Surface Modification with Silicon-doped Titania Nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Rodil, S.E.; Hyzy, S.L.; Dunn, G.R.; Almaguer-Flores, A.; Schwartz, Z.; Boyan, B.D. Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces. Biomaterials 2015, 51, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, T.; Ayukawa, Y.; Shibata, Y.; Takeshita, T.; Atsuta, I.; Ogino, Y.; Yasunami, N.; Yamashita, Y.; Koyano, K. Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. J. Clin. Med. 2020, 9, 2627. [Google Scholar] [CrossRef]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, E.; Dhandapani, V.S.; Dharmalingam, K.; Marimuthu, M.; Veerapandian, M.; Arumugam, M.K.; Kim, S.; Kim, B.; Ramasundaram, S.; Kim, D.-E. RF magnetron sputtering mediated NiTi/Ag coating on Ti-alloy substrate with enhanced biocompatibility and durability. Mater. Sci. Eng. C 2019, 99, 304–314. [Google Scholar] [CrossRef]
- Kurt, M.Ş.; Arslan, M.E.; Yazici, A.; Mudu, I.; Arslan, E. Tribological, biocompatibility, and antibiofilm properties of tungsten–germanium coating using magnetron sputtering. J. Mater. Sci. Mater. Med. 2021, 32, 1–12. [Google Scholar] [CrossRef]
- Wolke, J.G.C. In vivo dissolution behavior of various RF magnetron-sputtered Ca-P coatings on roughened titanium implants. Biomaterials 2003, 24, 2623–2629. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Choi, J.-M.; Kim, H.-E.; Lee, I.-S. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 2000, 21, 469–473. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2022, 12, 47. https://doi.org/10.3390/nano12010047
Ma X, Gao Y, Zhao D, Zhang W, Zhao W, Wu M, Cui Y, Li Q, Zhang Z, Ma C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials. 2022; 12(1):47. https://doi.org/10.3390/nano12010047
Chicago/Turabian StyleMa, Xiao, Yun Gao, Duoyi Zhao, Weilin Zhang, Wei Zhao, Meng Wu, Yan Cui, Qin Li, Zhiyu Zhang, and Chengbin Ma. 2022. "Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics" Nanomaterials 12, no. 1: 47. https://doi.org/10.3390/nano12010047
APA StyleMa, X., Gao, Y., Zhao, D., Zhang, W., Zhao, W., Wu, M., Cui, Y., Li, Q., Zhang, Z., & Ma, C. (2022). Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials, 12(1), 47. https://doi.org/10.3390/nano12010047





