Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of DES
2.2. Electrochemical Synthesis of Ag Nanoparticles and Decoration of MWCNTs
2.3. Zeta Potential Analysis
2.4. Physicochemical Characterization
2.4.1. Density and Dynamic Viscosity
2.4.2. Refractive Index
2.4.3. Surface Tension
2.4.4. Ionic Conductivity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characterization
3.2. Zeta Potential Analysis
3.3. Physicochemical Properties of MWCNTs and AgMWCNTs
3.3.1. Density
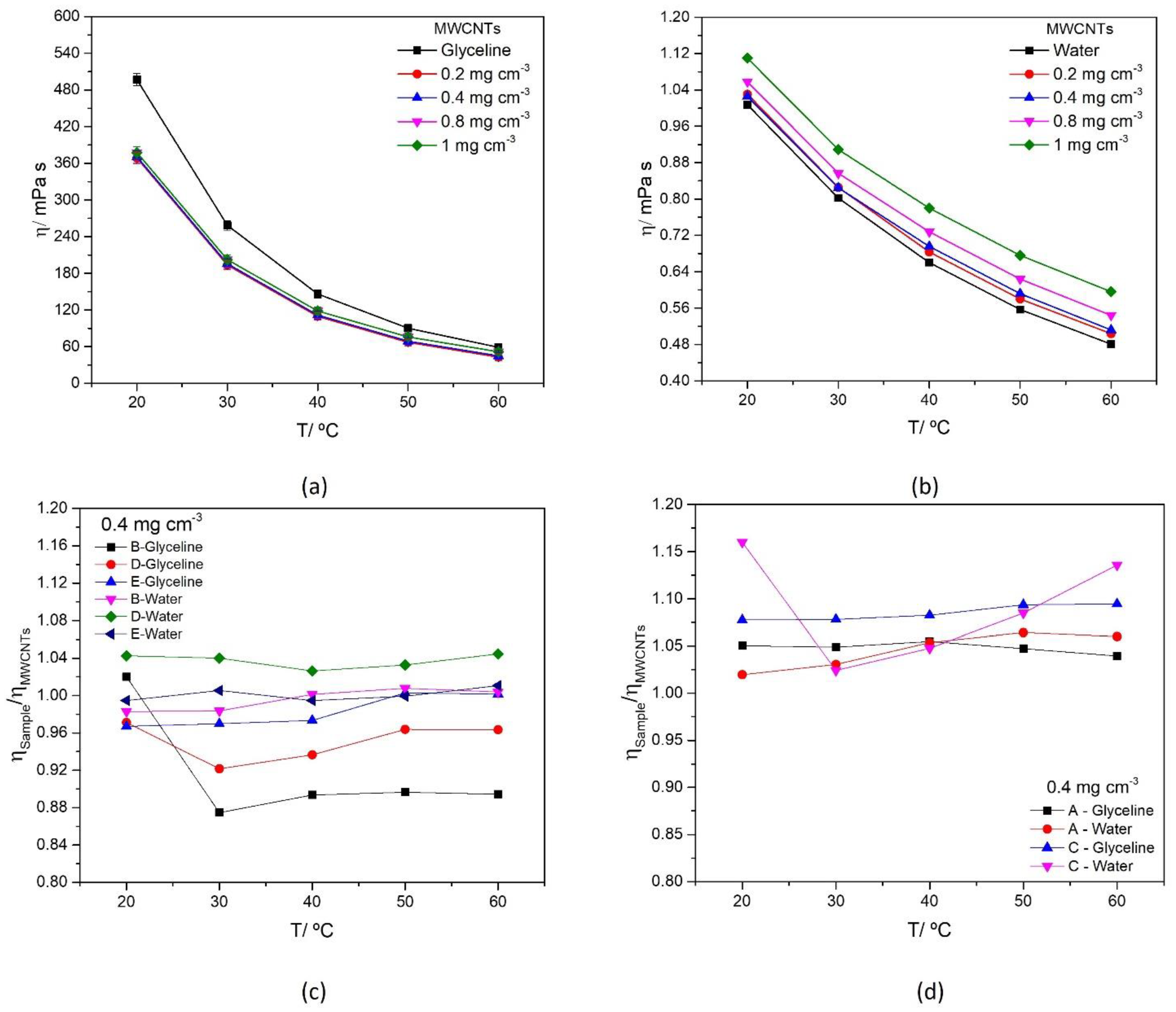
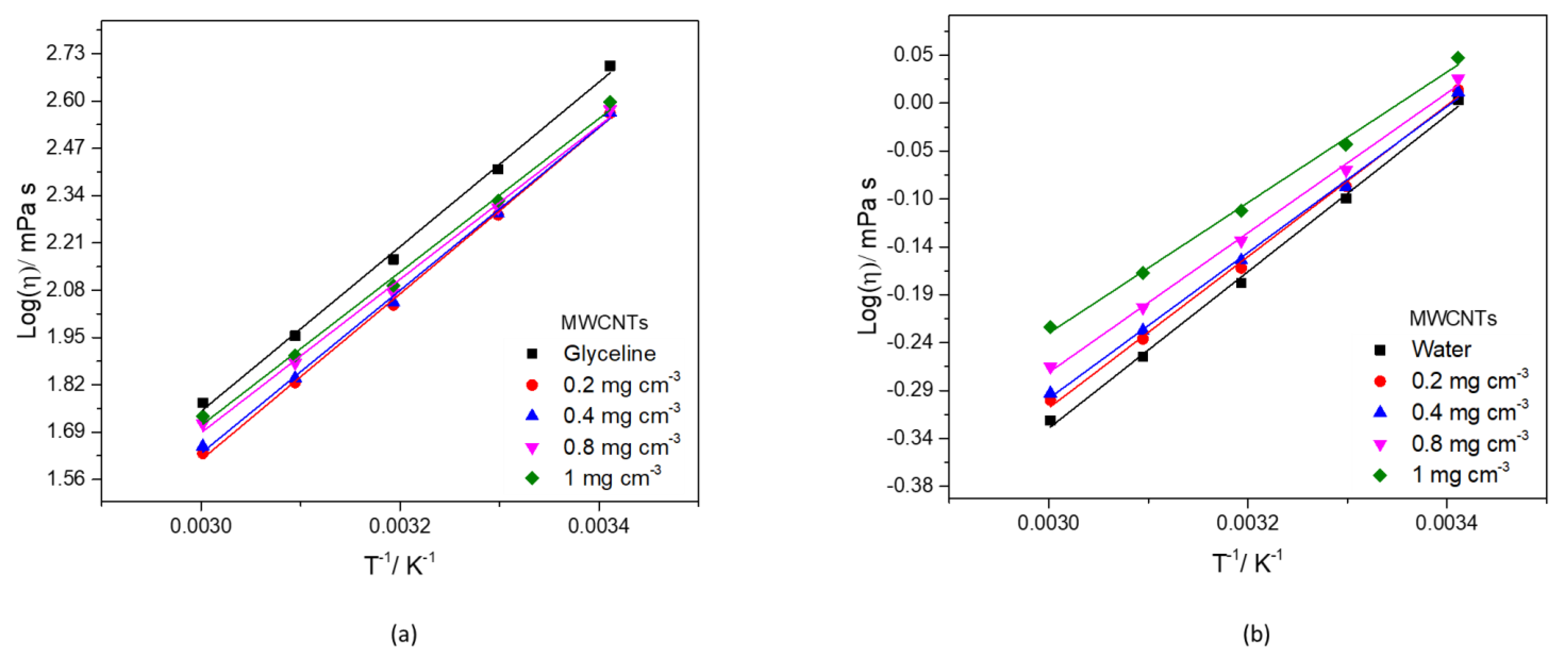
3.3.2. Dynamic Viscosity
3.3.3. Surface Tension
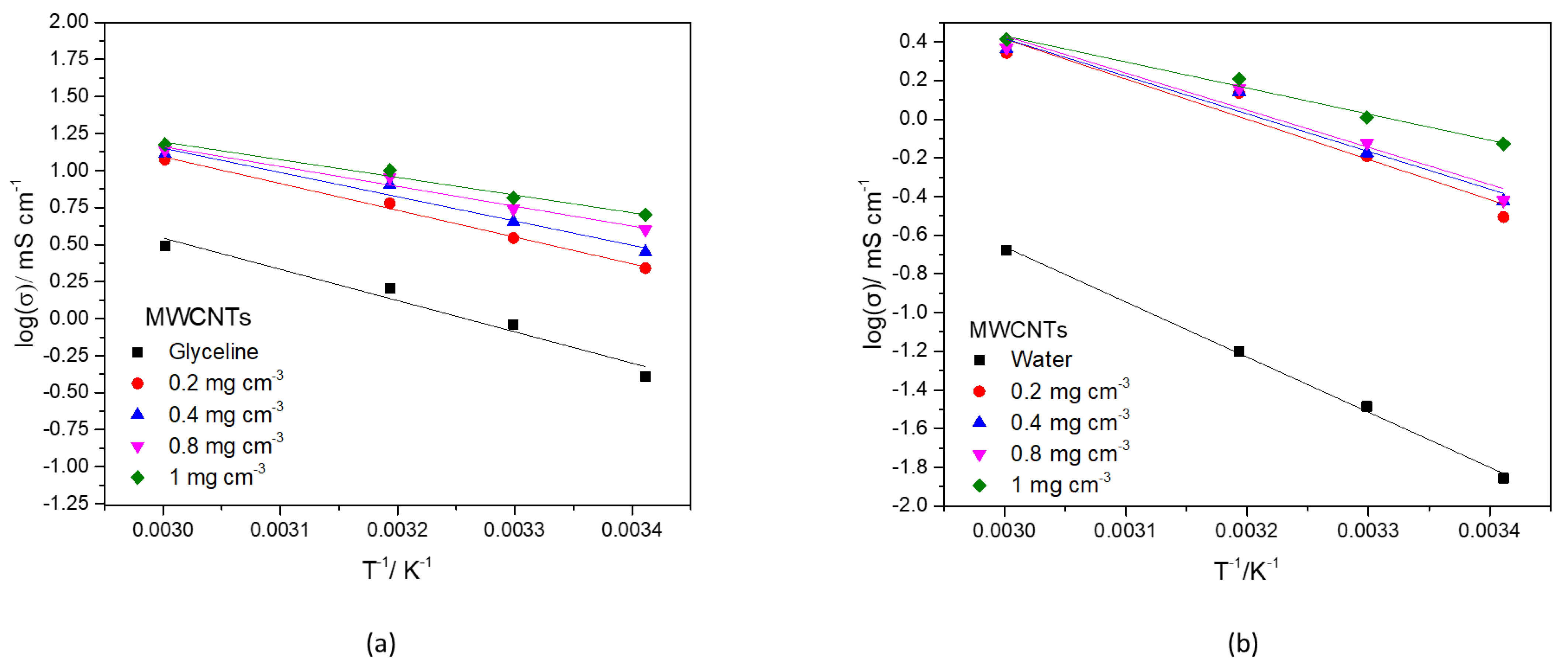
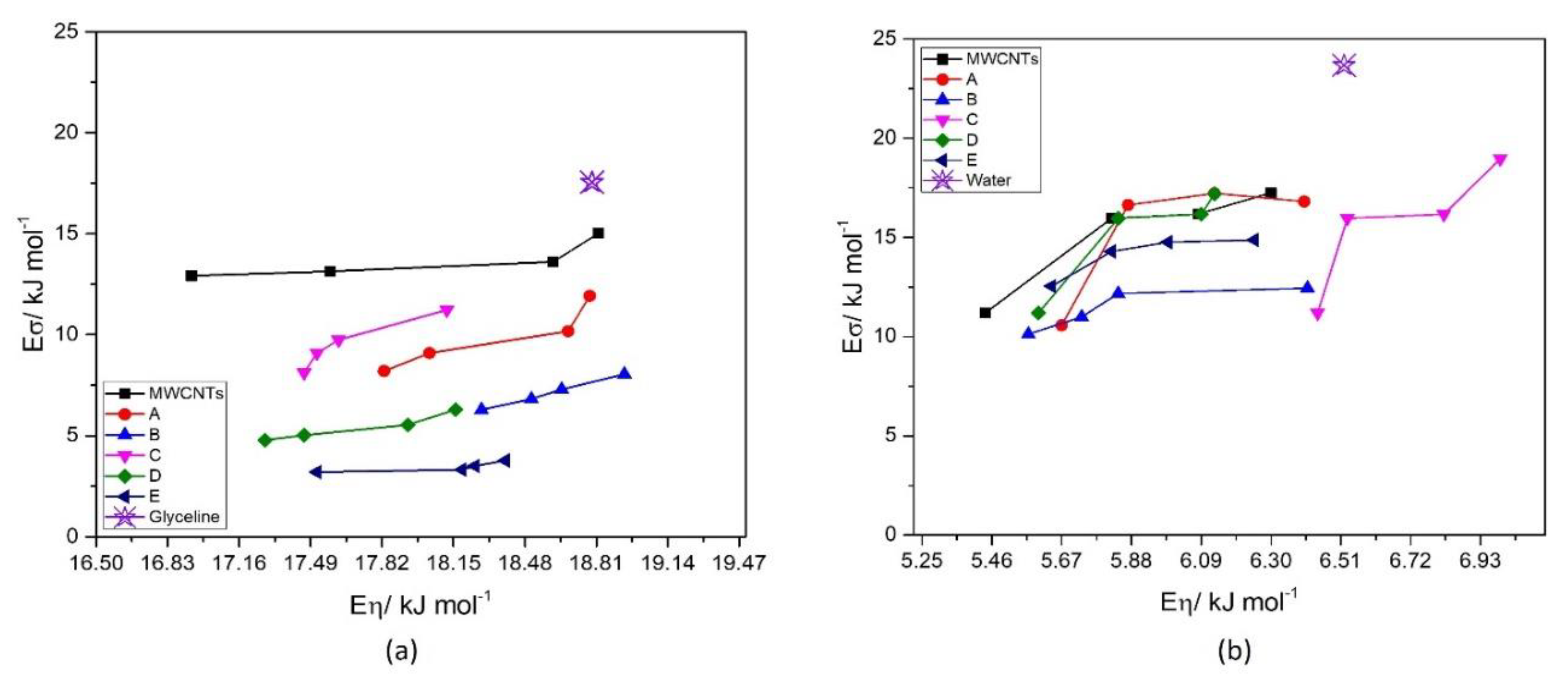
3.3.4. Ionic Conductivity
3.3.5. Refractive Index
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Wu, T.-M.; Lin, Y.-W. Doped polyaniline/multi-walled carbon nanotube composites: Preparation, characterization and properties. Polymer (Guildf.) 2006, 47, 3576–3582. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Zebarjad, S.M.; Janghorban, K. Decoration of multi-walled carbon nanotubes with silver nanoparticles and investigation on its colloid stability. Mater. Chem. Phys. 2013, 139, 113–117. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; Cuesta Castro, D.P.; Ortiz-Trujillo, I.C.; Botero Palacio, L.E.; Galeano Upegui, B.J.; Escobar Mora, N.J.; Carlos Cornelio, J.A. Compounds of carbon nanotubes decorated with silver nanoparticles via in-situ by chemical vapor deposition (CVD). J. Mater. Res. Technol. 2019, 8, 5893–5898. [Google Scholar] [CrossRef]
- Georgakilas, V.; Gournis, D.; Tzitzios, V.; Pasquato, L.; Guldi, D.M.; Prato, M. Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater. Chem. 2007, 17, 2679–2694. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Salavati-Niasari, M. A simple microwave approach for synthesis and characterization of Ag2S–AgInS2 nanocomposites. Compos. Part B Eng. 2014, 56, 490–496. [Google Scholar] [CrossRef]
- Cozzarini, L.; Bertolini, G.; Šuran-Brunelli, S.T.; Radivo, A.; Bracamonte, M.V.; Tavagnacco, C.; Goldoni, A. Metal decorated carbon nanotubes for electrocatalytic water splitting. Int. J. Hydrogen Energy 2017, 42, 18763–18773. [Google Scholar] [CrossRef]
- Duc Chinh, V.; Speranza, G.; Migliaresi, C.; Van Chuc, N.; Minh Tan, V.; Phuong, N.-T. Synthesis of Gold Nanoparticles Decorated with Multiwalled Carbon Nanotubes (Au-MWCNTs) via Cysteaminium Chloride Functionalization. Sci. Rep. 2019, 9, 5667. [Google Scholar] [CrossRef] [Green Version]
- Guzsvány, V.; Vajdle, O.; Gurdeljević, M.; Kónya, Z. Ag or Au Nanoparticles Decorated Multiwalled Carbon Nanotubes Coated Carbon Paste Electrodes for Amperometric Determination of H2O2. Top. Catal. 2018, 61, 1350–1361. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Eshghi, H.; Heris, S.Z.; Baniadam, M. Highly Dispersed Multiwalled Carbon Nanotubes Decorated with Ag Nanoparticles in Water and Experimental Investigation of the Thermophysical Properties. J. Phys. Chem. C 2012, 116, 3369–3375. [Google Scholar] [CrossRef]
- Tang, L.; Duan, F.; Chen, M. Silver nanoparticle decorated polyaniline/multiwalled super-short carbon nanotube nanocomposites for supercapacitor applications. RSC Adv. 2016, 6, 65012–65019. [Google Scholar] [CrossRef]
- Patole, A.; Lubineau, G. Carbon nanotubes with silver nanoparticle decoration and conductive polymer coating for improving the electrical conductivity of polycarbonate composites. Carbon N. Y. 2015, 81, 720–730. [Google Scholar] [CrossRef]
- Dinh, N.X.; Van Quy, N.; Huy, T.Q.; Le, A.T. Decoration of silver nanoparticles on multiwalled carbon nanotubes: Antibacterial mechanism and ultrastructural analysis. J. Nanomater. 2015, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- Markoulidis, F.; Todorova, N.; Grilli, R.; Lekakou, C. Trapalis Composite Electrodes of Activated Carbon and Multiwall Carbon Nanotubes Decorated with Silver Nanoparticles for High Power Energy Storage. J. Compos. Sci. 2019, 3, 97. [Google Scholar] [CrossRef] [Green Version]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz Méndez, U.; De La Fuente, I.G. Decoration of Carbon Nanotubes with Metal Nanoparticles: Recent Trends. Synth. React. Inorg. Met. Nano-Met. Chem. 2015, 46, 55–76. [Google Scholar] [CrossRef]
- Travessa, D.N.; Da Silva, F.S.; Cristovan, F.H.; Jorge, A.M.; Cardoso, K.R. Ag ion decoration for surface modifications of multi-walled carbon nanotubes. Mater. Res. 2014, 17, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Xin, F.; Li, L. Decoration of carbon nanotubes with silver nanoparticles for advanced CNT/polymer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 961–967. [Google Scholar] [CrossRef]
- Cojocaru, A.; Brincoveanu, O.; Pantazi, A.; Balan, D.; Enachescu, M.; Visan, T.; Anicai, L. Electrochemical preparation of Ag nanoparticles involving choline chloride-glycerol deep eutectic solvents. Bulg. Chem. Commun. 2017, 49, 194–204. [Google Scholar]
- Kataria, J.; Devi, P.; Rani, P. Importance of structures and interactions in ionic liquid-nanomaterial composite systems as a novel approach for their utilization in safe lithium metal batteries: A review. J. Mol. Liq. 2021, 339, 116736. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Zaib, Q.; Adeyemi, I.; Warsinger, D.M.; AlNashef, I.M. Deep Eutectic Solvent Assisted Dispersion of Carbon Nanotubes in Water. Front. Chem. 2020, 8, 808. [Google Scholar] [CrossRef]
- Izutsu, K. Fundamentals of Chemistry in Nonaqueous Solutions: Electrochemical Aspects. In Electrochemistry in Nonaqueous Solutions; Wiley: New York, NY, USA, 2009; ISBN 978-3-527-62916-9. [Google Scholar]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Lazar, O.A.; Silva, A.F.; Anicai, L.; Pereira, C.M.; Enachescu, M. Characterization and electrochemical studies of MWCNTs decorated with Ag nanoparticles through pulse reversed current electrodeposition using a deep eutectic solvent for energy storage applications. J. Mater. Res. Technol. 2021, 15, 342–359. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical characterization of ionic liquids able to dissolve biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Sabri, M.A.; Jabbar, N.A.; Nancarrow, P.; Mjalli, F.S.; AlNashef, I. Thermal Conductivities of Choline Chloride-Based Deep Eutectic Solvents and Their Mixtures with Water: Measurement and Estimation. Molecules 2020, 25, 3816. [Google Scholar] [CrossRef]
- Crespo, E.A.; Costa, J.M.L.; Palma, A.M.; Soares, B.; Martín, M.C.; Segovia, J.J.; Carvalho, P.J.; Coutinho, J.A.P. Thermodynamic characterization of deep eutectic solvents at high pressures. Fluid Phase Equilib. 2019, 500, 112249. [Google Scholar] [CrossRef]
- Xu, X.; Range, J.; Gygli, G.; Pleiss, J. Analysis of Thermophysical Properties of Deep Eutectic Solvents by Data Integration. J. Chem. Eng. Data 2020, 65, 1172–1179. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Batista, M.L.S.; Cláudio, A.F.M.; Santos, L.M.N.B.F.; Marrucho, I.M.; Freire, M.G.; Coutinho, J.A.P. Thermophysical properties and water saturation of [PF6]-based ionic liquids. J. Chem. Eng. Data 2010, 55, 5065–5073. [Google Scholar] [CrossRef]
- Tariq, M.; Forte, P.A.S.; Gomes, M.F.C.; Lopes, J.N.C.; Rebelo, L.P.N. Densities and refractive indices of imidazolium- and phosphonium-based ionic liquids: Effect of temperature, alkyl chain length, and anion. J. Chem. Thermodyn. 2009, 41, 790–798. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Passos, H.; Lopes-Da-Silva, J.A.; Fernandes, A.M.; Freire, M.G.; Coutinho, J.A.P. Thermophysical properties of five acetate-based ionic liquids. J. Chem. Eng. Data 2012, 57, 3005–3013. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Lu, W.; Yao, K.; Xu, H. Effect of alkyl chain length in anion on dissolution of cellulose in 1-butyl-3-methylimidazolium carboxylate ionic liquids. J. Mol. Liq. 2014, 197, 211–214. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Carvalho, P.J.; Oliveira, N.M.C. Predictive methods for the estimation of thermophysical properties of ionic liquids. RSC Adv. 2012, 2, 7322–7346. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, N.; Church, J.S.; Salim, N.V.; Hanley, T.L.; Amini, A.; Fox, B.L. Dispersing single-walled carbon nanotubes in ionic liquids: A quantitative analysis. RSC Adv. 2013, 3, 20034–20039. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chu, H.; Li, Y. Why Single-Walled Carbon Nanotubes Can Be Dispersed in Imidazolium-Based Ionic Liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Polo-Luque, M.L.; Simonet, B.M.; Valcárcel, M. Functionalization and dispersion of carbon nanotubes in ionic liquids. TrAC Trends Anal. Chem. 2013, 47, 99–110. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Walvekar, R.; Khalid, M.; Shahbaz, K.; Gupta, T.C.S.M. Stability and thermophysical studies on deep eutectic solvent based carbon nanotube nanofluid. Mater. Res. Express 2017, 4, 075028. [Google Scholar] [CrossRef]
- Chen, L.; Deng, J.; Song, Y.; Hong, S.; Lian, H. Highly Stable Dispersion of Carbon Nanotubes in Deep Eutectic Solvent for the Preparation of CNT-Embedded Carbon Xerogels for Supercapacitors. ChemElectroChem 2019, 6, 5750–5758. [Google Scholar] [CrossRef]
- Walvekar, R.; Singh, A.; Khalid, M.; Gupta, T.; Yin, W.W. Thermophysical properties of deep eutectic solvent-carbon nanotubes (DES-CNT) based nanolubricant. J. Therm. Eng. 2020, 6, 53–64. [Google Scholar] [CrossRef]
- Vakili-Nezhaad, G.; Dorany, A. Effect of single-walled carbon nanotube on the viscosity of lubricants. Energy Procedia 2012, 14, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Vakili-Nezhaad, G.R.; Dorany, A. Investigation of the effect of multiwalled carbon nanotubes on the viscosity index of lube oil cuts. Chem. Eng. Commun. 2009, 196, 997–1007. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Luo, R.; Li, Q.; Du, B.; Zhou, S.; Chen, Y. Preparation and Characterization of Solid Electrolyte Doped with Carbon Nanotubes and its Preliminary Application in NO2 Gas Sensors. Front. Mater. 2019, 6, 113. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, K.C.; Dong, R.X.; Te Liu, C.; Wang, C.C.; Ho, K.C.; Lin, J.J. Polymer-dispersed MWCNT gel electrolytes for high performance of dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 6982–6989. [Google Scholar] [CrossRef]
- Tamate, R.; Saruwatari, A.; Nakanishi, A.; Matsumae, Y.; Ueno, K.; Dokko, K.; Watanabe, M. Excellent dispersibility of single-walled carbon nanotubes in highly concentrated electrolytes and application to gel electrode for Li-S batteries. Electrochem. Commun. 2019, 109, 106598. [Google Scholar] [CrossRef]
- Azizighannad, S.; Wang, Z.; Siddiqui, Z.; Kumar, V.; Mitra, S. Nano carbon doped polyacrylamide gel electrolytes for high performance supercapacitors. Molecules 2021, 26, 2631. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Gerhardt, R.A. Fabrication and Supercapacitor Applications of Multiwall Carbon Nanotube Thin Films. C 2021, 7, 70. [Google Scholar] [CrossRef]
- Deshiikan, S.R.; Papadopoulos, K.D. Modified Booth equation for the calculation of zeta potential. Colloid Polym. Sci. 1998, 276, 117–124. [Google Scholar] [CrossRef]
- David, R. Lide Concentrative properties of aqueous solutions: Density, refractive index, freezing point depression, and viscosity. In CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 1997; ISBN B008VROK8M. [Google Scholar]
- Daerr, A.; Mogne, A. Pendent_Drop: An ImageJ Plugin to Measure the Surface Tension from an Image of a Pendent Drop. J. Open Res. Softw. 2016, 4, 2–6. [Google Scholar]
- Sabri, F.N.A.M.; Zakaria, M.R.; Akil, H.M. Dispersion and stability of multiwalled carbon nanotubes (MWCNTs) in different solvents. AIP Conf. Proc. 2020, 2267, 20043. [Google Scholar]
- Hanzel, O.; Sedláček, J.; Šajgalík, P. New approach for distribution of carbon nanotubes in alumina matrix. J. Eur. Ceram. Soc. 2014, 34, 1845–1851. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Naser, J.; Jibril, B.; Alizadeh, V.; Gano, Z. Tetrabutylammonium Chloride Based Ionic Liquid Analogues and Their Physical Properties. J. Chem. Eng. Data 2014, 59, 2242–2251. [Google Scholar] [CrossRef]
- Nowosielski, B.; Jamrógiewicz, M.; Łuczak, J.; Śmiechowski, M.; Warmińska, D. Experimental and predicted physicochemical properties of monopropanolamine-based deep eutectic solvents. J. Mol. Liq. 2020, 309, 113110. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent developments in deep eutectic solvents in chemical sciences. Monatshefte Chem. Chem. Mon. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Liu, C.; Lu, X.; Feng, X.; Ji, X. Thermodynamic Study of Choline Chloride-Based Deep Eutectic Solvents with Water and Methanol. J. Chem. Eng. Data 2020, 65, 2446–2457. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 102nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2004. [Google Scholar]
- Al-Murshedi, A.Y.M.; Alesary, H.F.; Al-Hadrawi, R. Thermophysical properties in deep eutectic solvents with/without water. J. Phys. Conf. Ser. 2019, 1294, 052041. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Fan, X.; Lian, Z. Experimental study on an inverter heat pump with HFC125 operating near the refrigerant critical point. Appl. Therm. Eng. 2012, 39, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Rheological and Tribological Properties of Ionic Liquid-Based Nanofluids Containing Functionalized Multi-Walled Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 8749–8754. [Google Scholar] [CrossRef]
- Alizadeh, J.; Keshavarz Moraveji, M. An experimental evaluation on thermophysical properties of functionalized graphene nanoplatelets ionanofluids. Int. Commun. Heat Mass Transf. 2018, 98, 31–40. [Google Scholar] [CrossRef]
- Awais, M.; Ullah, N.; Ahmad, J.; Sikandar, F.; Ehsan, M.M.; Salehin, S.; Bhuiyan, A.A. Heat transfer and pressure drop performance of Nanofluid: A state-of- the-art review. Int. J. Thermofluids 2021, 9, 100065. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.A.; Danilov, F.I. Effect of water content on physicochemical properties and electrochemical behavior of ionic liquids containing choline chloride, ethylene glycol and hydrated nickel chloride. J. Mol. Liq. 2015, 212, 716–722. [Google Scholar] [CrossRef]
- Ghosh, S.; Ryder, K.; Roy, S. Electrochemical and transport properties of ethaline containing copper and tin chloride. Trans. IMF 2014, 92, 41–46. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Fu, L.; Yang, Y.; Wang, Y.; Hu, X.; Wang, F.; Mu, T. Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Ind. Eng. Chem. Res. 2019, 58, 12741–12750. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Sa, V.; Kornev, K.G. Analysis of Stability of Nanotube Dispersions Using Surface Tension Isotherms. Langmuir 2011, 27, 13451–13460. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Abbott, A.P. Application of Hole Theory to the Viscosity of Ionic and Molecular Liquids. ChemPhysChem 2004, 5, 1242–1246. [Google Scholar] [CrossRef]
- Fürth, R. On the theory of the liquid state: I. The statistical treatment of the thermodynamics of liquids by the theory of holes. Math. Proc. Cambridge Philos. Soc. 1941, 37, 252–275. [Google Scholar] [CrossRef]
- Bockris, J.O.; Richards, N.E. The Compressibilities, Free Volumes and Equation of State for Molten Electrolytes: Some Alkali Halides and Nitrates. Proc. R. Soc. Lond. A. Math. Phys. Sci. 1957, 241, 44–66. [Google Scholar]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem. A Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Rashidi, M.M.; Akhiani, A.R.; Tahan Latibari, S.; Mehrali, M.; Metselaar, H.S.C. Experimental and numerical investigation of the effective electrical conductivity of nitrogen-doped graphene nanofluids. J. Nanoparticle Res. 2015, 17, 267. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Nabati Shoghl, S.; Jamali, J.; Keshavarz Moraveji, M. Electrical conductivity, viscosity, and density of different nanofluids: An experimental study. Exp. Therm. Fluid Sci. 2016, 74, 339–346. [Google Scholar] [CrossRef]
- Kausar, A.; Siddiq, M. Carbon nanotubes/silver nanoparticles/poly(azo-thiourea) hybrids: Morphological, tensile and conductivity profile. J. Compos. Mater. 2014, 48, 3271–3280. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Shariff, A.M.; Bustam, M.A.; Murshid, G. Physicochemical Properties of Aqueous Solutions of Sodium l-Prolinate as an Absorbent for CO2 Removal. J. Chem. Eng. Data 2014, 59, 362–368. [Google Scholar] [CrossRef]
- Navarro, S.S.; Leron, R.B.; Soriano, A.N.; Li, M.-H. Thermophysical property characterization of aqueous amino acid salt solution containing serine. J. Chem. Thermodyn. 2014, 78, 23–31. [Google Scholar] [CrossRef]
- Murshid, G.; Shariff, A.M.; Keong, L.K.; Bustam, M.A. Physical Properties of Aqueous Solutions of Piperazine and (2-Amino-2-methyl-1-propanol + Piperazine) from (298.15 to 333.15) K. J. Chem. Eng. Data 2011, 56, 2660–2663. [Google Scholar] [CrossRef]
- Leron, R.B.; Soriano, A.N.; Li, M.-H. Densities and refractive indices of the deep eutectic solvents (choline chloride+ethylene glycol or glycerol) and their aqueous mixtures at the temperature ranging from 298.15 to 333.15K. J. Taiwan Inst. Chem. Eng. 2012, 43, 551–557. [Google Scholar] [CrossRef]
- Shahriari, E.; Moradi, M.; Varnamkhasti, M.G. Investigation of nonlinear optical properties of Ag nanoparticles. Int. J. Opt. Photonics 2015, 9, 107–114. [Google Scholar]
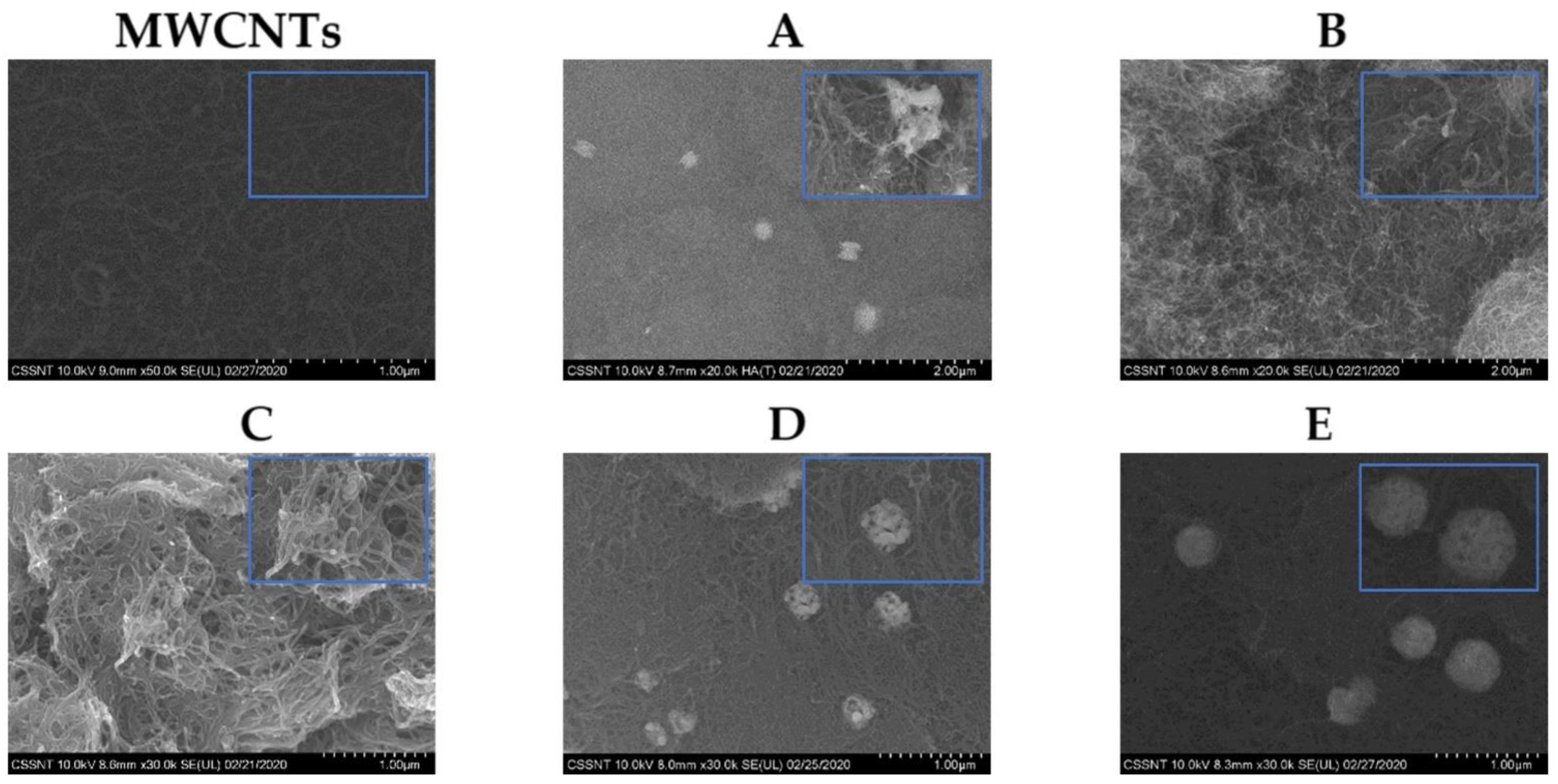





| Samples | Ag NPs Amount/wt. % (from EDX Analysis) | Ag NPs Size/nm (from SEM Image) | Specific Capacitance /F g−1 (using 3 Electrode Cell) |
|---|---|---|---|
| A | 3 ± 1 | 12 ± 5 | 5.2 ± 0.3 |
| B | 11 ± 3 | 16 ± 4 | 14.6 ± 1.0 |
| C | 1.0 ± 0.2 | 18 ± 3 | 7.1 ± 0.8 |
| D | 28 ± 8 | 33 ± 6 | 16.3 ± 1.2 |
| E | 24 ± 9 | 46 ± 7 | 28.5 ± 1.6 |
| Solvent | ρ/g cm−3 | T/K | Reference | Solvent | ρ/g cm−3 | T/K | Reference |
|---|---|---|---|---|---|---|---|
| glyceline | 1.198 | 293 | [27] | water | 0.99829 | 293 | This work |
| 1.190 | This work | 0.99821 | [58] | ||||
| 1.180 | 298 | [59] | 0.99232 | 313 | This work | ||
| 1.190 | [60] | 0.99222 | [58] | ||||
| 1.130 | 363 | [60] | 0.98329 | 333 | This work | ||
| 1.190 | [27] | 0.98320 | [58] |
| Solvent | η/mPa s | T/K | Reference | Solvent | η/mPa s | T/K | Reference |
|---|---|---|---|---|---|---|---|
| glyceline | 497 | 293 | This work | Water | 1.0016 | 293 | [58] |
| 1.0074 | This work | ||||||
| 325 | 298 | [59] | |||||
| 423 | [60] | 0.7972 | 303 | [58] | |||
| 0.8023 | This work | ||||||
| 259 | 303 | This work | |||||
| 0.6527 | 313 | [58] | |||||
| 146 | 313 | This work | 0.6604 | This work |
| Glyceline | MWCNTs Concentration/mg cm−3 | ||||
|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.8 | 1.0 | ||
| ln (η0) | −5.0 ± 0.2 | −5.2 ± 0.2 | −5.1 ± 0.2 | −4.6 ± 0.2 | −4.6 ± 0.2 |
| (Eη/R)/K | 2261 ± 63 | 2281 ± 48 | 2239 ± 54 | 2109 ± 72 | 2119 ± 75 |
| R2 | 0.997 | 0.998 | 0.998 | 0.996 | 0.996 |
| Water | MWCNTs Concentration/mg cm−3 | ||||
|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.8 | 1.0 | ||
| ln (η0) | −2.68 ± 0.07 | −2.58 ± 0.07 | −2.49 ± 0.06 | −2.37 ± 0.06 | −2.19 ±0.06 |
| (Eη/R)/K | 784 ± 21 | 758 ± 22 | 732 ± 18 | 700 ± 19 | 654 ± 20 |
| R2 | 0.997 | 0.997 | 0.998 | 0.997 | 0.996 |
| Solvent | γ/mN m−1 | T/K | Reference | Solvent | γ/mN m−1 | T/K | Reference |
|---|---|---|---|---|---|---|---|
| glyceline | 57.80 | 293 | [67] | water | - | - | |
| 56.33 | This work | - | |||||
| 68.10 | 298 | [59] | 71.19 | 303 | [58] | ||
| 59.01 | [60] | 71.54 | This work | ||||
| 54.11 | 313 | This work | 69.60 | 313 | [58] | ||
| 69.88 | This work | ||||||
| 53.77 | 323 | This work | 67.94 | 323 | [58] | ||
| 68.19 | This work | ||||||
| 53.31 | 333 | This work | 64.48 | 333 | [58] | ||
| 65.43 | This work |
| Glyceline | MWCNTs Concentration/mg cm−3 | ||||
|---|---|---|---|---|---|
| T/°C | 0.2 | 0.4 | 0.8 | 1.0 | |
| 20 | 4.216 | 4.160 | 4.110 | 4.035 | 3.964 |
| 30 | 4.300 | 4.244 | 4.188 | 4.114 | 4.042 |
| 40 | 4.446 | 4.326 | 4.265 | 4.193 | 4.119 |
| 50 | 4.531 | 4.405 | 4.342 | 4.269 | 4.197 |
| 60 | 4.634 | 4.483 | 4.415 | 4.347 | 4.274 |
| Solvent | σ/mS cm−1 | T/K | Reference | Solvent | σ/µS cm−1 | T/K | Reference |
|---|---|---|---|---|---|---|---|
| glyceline | 0.413 | 293 | This work | water | 0.045 | 293 | [58] |
| 0.050 | This work | ||||||
| 2.039 | 298 | [59] | 0.056 | Milli-Q® water * | |||
| 0.981 | [60] | ||||||
| 0.850 | [76] | 0.061 | 303 | [58] | |||
| 0.055 | This work | ||||||
| 0.433 | 303 | This work | |||||
| 0.070 | 313 | [58] | |||||
| 1.501 | 313 | This work | 0.061 | This work | |||
| 1.914 | 323 | This work | 0.075 | 323 | [58] | ||
| 0.067 | This work | ||||||
| 3.099 | 333 | This work | |||||
| 3.441 | [76] | 0.083 | 333 | [58] | |||
| 0.071 | This work | ||||||
| 7.652 | 343 | [59] | |||||
| 4.520 | [60] |
| MWCNTs Concentration/mg cm−3 | |||||
|---|---|---|---|---|---|
| Glyceline | 0.2 | 0.4 | 0.8 | 1.0 | |
| ln (σ0) | 6.9 ± 0.9 | 6.5 ± 0.3 | 6.1 ± 0.6 | 5.2 ± 0.5 | 4.8 ± 0.4 |
| (Eσ/R)/K | −2111 ± 274 | −1806 ± 85 | −1638 ±193 | −1340 ± 144 | −1194 ± 110 |
| R2 | 0.989 | 0.991 | 0.979 | 0.977 | 0.972 |
| MWCNTs Concentration/mg cm−3 | |||||
|---|---|---|---|---|---|
| Water | 0.2 | 0.4 | 0.8 | 1.0 | |
| ln (σ0) | 7.9 ± 0.3 | 7 ± 1 | 6.3 ± 0.9 | 6 ± 1 | 4.5 ± 0.3 |
| (Eσ/R)/K | −2848 ± 95 | −2076 ± 366 | −1945 ± 286 | −1921 ± 308 | −1347 ± 104 |
| R2 | 0.997 | 0.981 | 0.979 | 0.982 | 0.991 |
| Solvent | RI | T/K | Reference | Solvent | RI | T/K | Reference |
|---|---|---|---|---|---|---|---|
| glyceline | 1.3331 | 293 | This work | water | 1.3321 | 293 | [58] |
| 1.3325 | 298 | [84] | 1.3310 | 298 | |||
| 1.3231 | 303 | This work | 1.3242 | 303 | |||
| 1.3319 | [84] | ||||||
| 1.3201 | 313 | This work | 1.3229 | 313 | |||
| 1.3305 | [84] | ||||||
| 1.3131 | 323 | This work | 1.3214 | 323 | |||
| 1.3289 | [84] | ||||||
| 1.3099 | 333 | This work | 1.3197 | 333 | |||
| 1.3269 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Silva, A.F.; Anicai, L.; Enachescu, M.; Pereira, C.M. Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties? Nanomaterials 2022, 12, 99. https://doi.org/10.3390/nano12010099
Brandão ATSC, Rosoiu S, Costa R, Silva AF, Anicai L, Enachescu M, Pereira CM. Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties? Nanomaterials. 2022; 12(1):99. https://doi.org/10.3390/nano12010099
Chicago/Turabian StyleBrandão, Ana T. S. C., Sabrina Rosoiu, Renata Costa, A. Fernando Silva, Liana Anicai, Marius Enachescu, and Carlos M. Pereira. 2022. "Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties?" Nanomaterials 12, no. 1: 99. https://doi.org/10.3390/nano12010099








