Silver Nanoparticle-Intercalated Cotton Fiber for Catalytic Degradation of Aqueous Organic Dyes for Water Pollution Mitigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Ag NP–Cotton Catalyst
2.3. Catalytic Degradation Studies and Turnover Determinations
3. Results and Discussion
3.1. Characterization of Ag NP–Cotton Catalyst
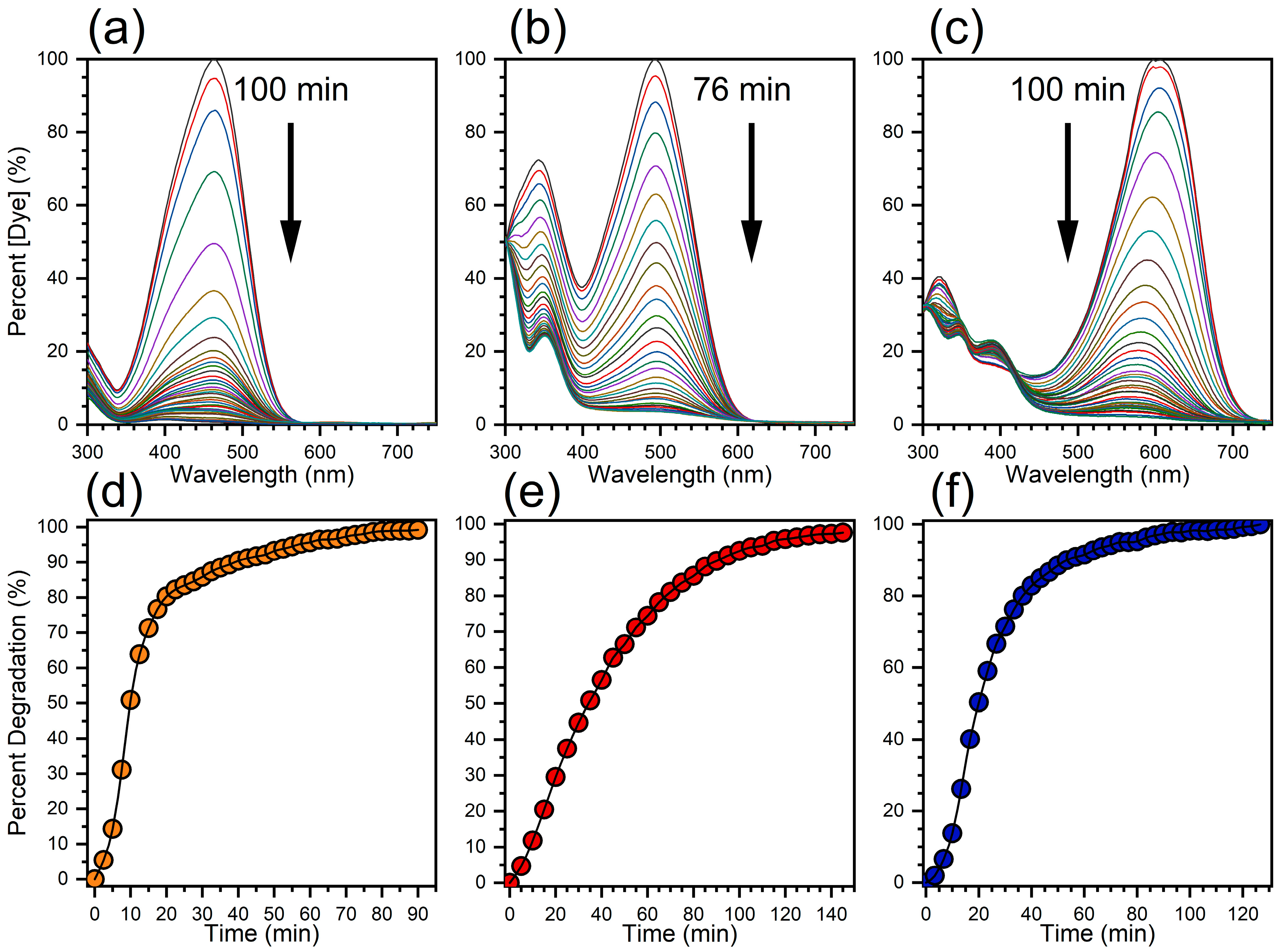
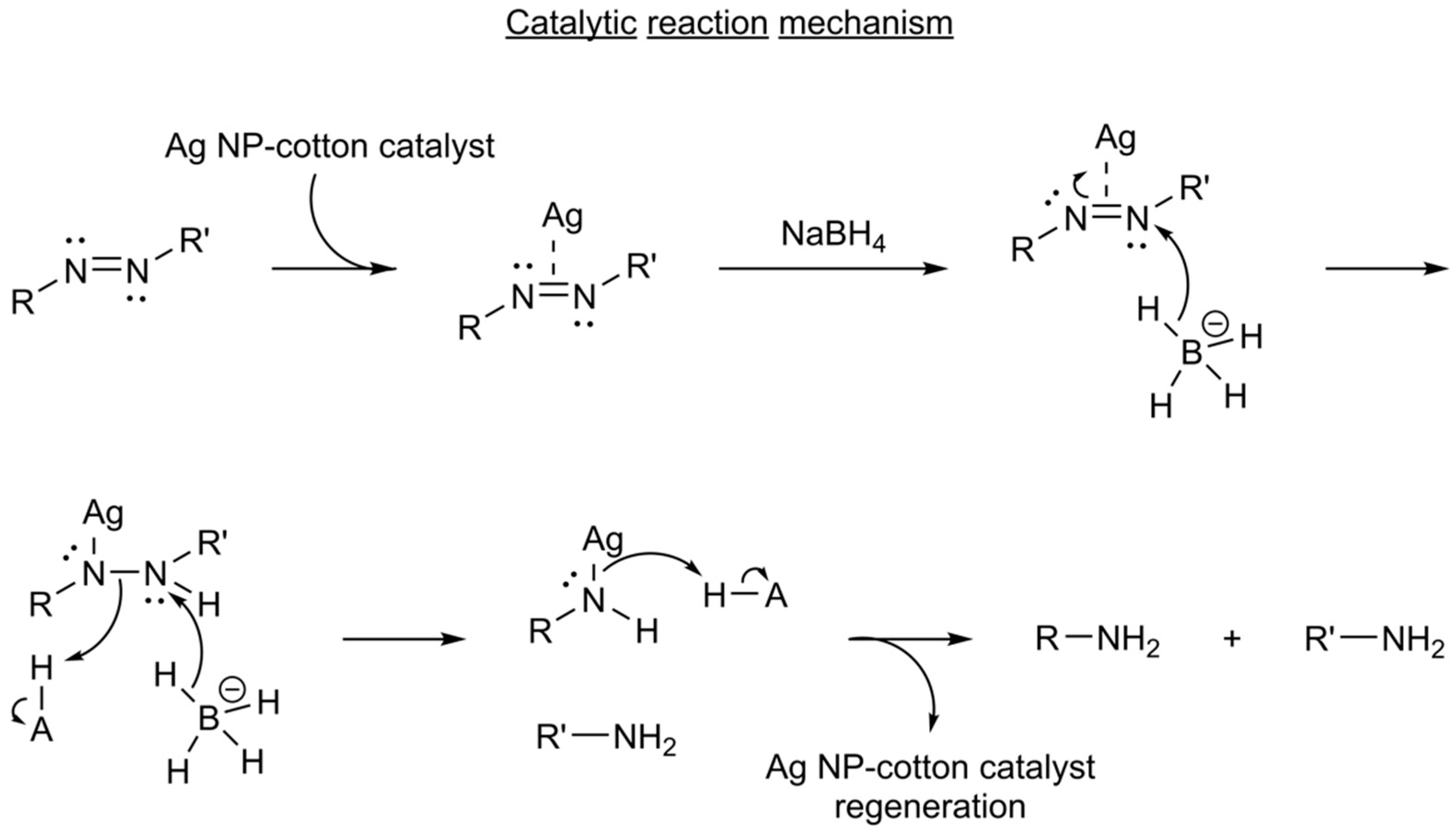
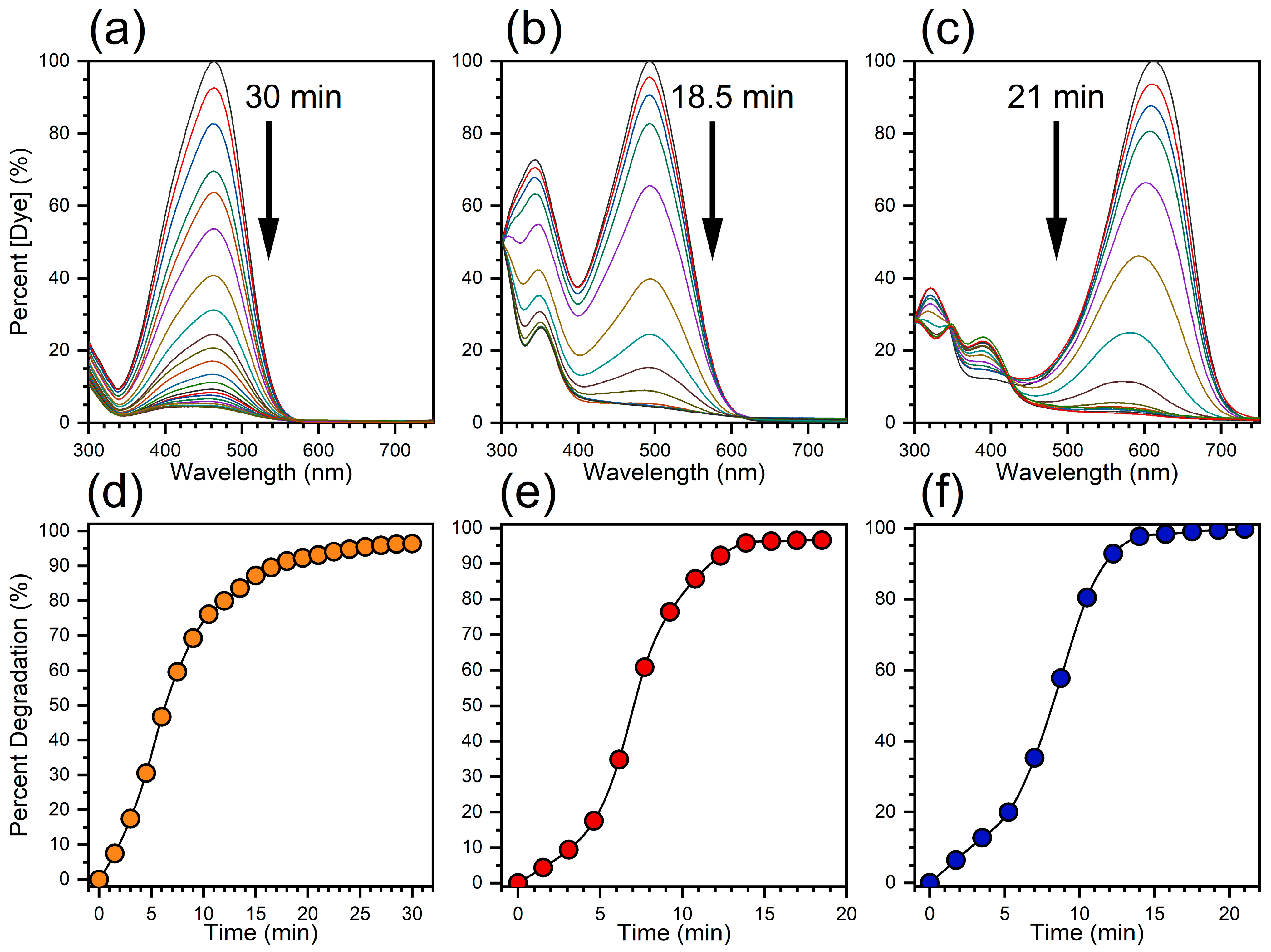
3.2. Catalytic Activity Performance of Ag NP–Cotton
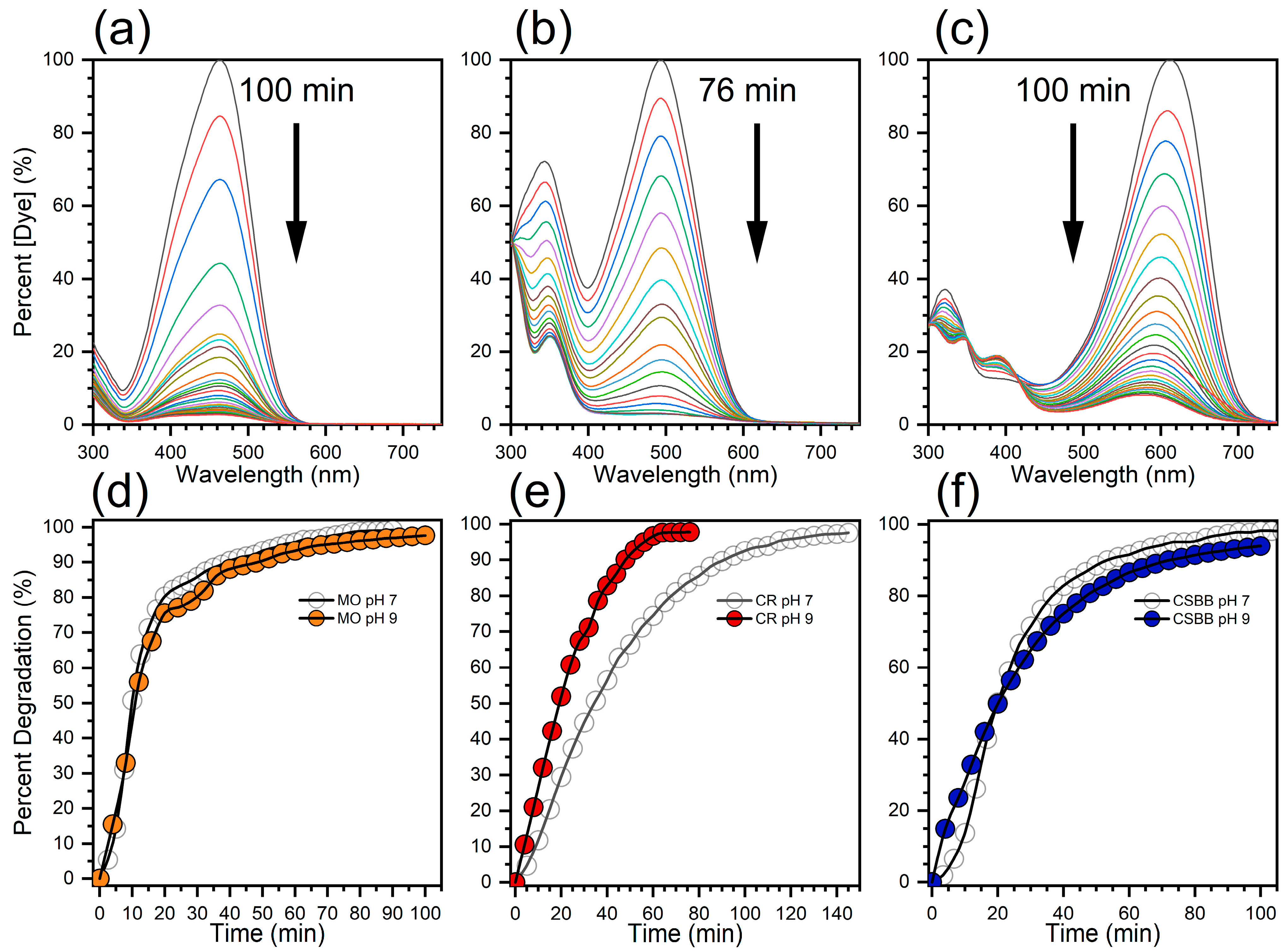
3.3. Effect of pH on Catalytic Performance
3.4. Recyclability of Ag NP–Cotton Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef] [PubMed]
- Pirkarami, A.; Olya, M.E. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J. Saudi Chem. Soc. 2017, 21, S179–S186. [Google Scholar] [CrossRef] [Green Version]
- van Loosdrecht, M.C.; Brdjanovic, D. Water treatment. Anticipating the next century of wastewater treatment. Science 2014, 344, 1452–1453. [Google Scholar] [CrossRef] [PubMed]
- Kuenemann, M.A.; Szymczyk, M.; Chen, Y.; Sultana, N.; Hinks, D.; Freeman, H.S.; Williams, A.J.; Fourches, D.; Vinueza, N.R. Weaver′s historic accessible collection of synthetic dyes: A cheminformatics analysis. Chem. Sci. 2017, 8, 4334–4339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, G.; Qu, J.; Liu, H. Degradation of azo dye Acid Orange 7 in water by Fe0/granular activated carbon system in the presence of ultrasound. J. Hazard. Mater. 2007, 144, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.; Pech, N.; de Aragao Umbuzeiro, G.; Moreau, X. Multi-scale biomarker evaluation of the toxicity of a commercial azo dye (Disperse Red 1) in an animal model, the freshwater cnidarian Hydra attenuata. Water Res. 2016, 96, 62–73. [Google Scholar] [CrossRef]
- Hernandez-Zamora, M.; Martinez-Jeronimo, F. Congo red dye diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans, and zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2019, 26, 11743–11755. [Google Scholar] [CrossRef]
- Abe, F.R.; Soares, A.; Oliveira, D.P.; Gravato, C. Toxicity of dyes to zebrafish at the biochemical level: Cellular energy allocation and neurotoxicity. Environ. Pollut. 2018, 235, 255–262. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Martyniuk, C.J.; de Solla, S.R.; Balakrishnan, V.K.; Langlois, V.S. Sediment contaminated with the Azo Dye disperse yellow 7 alters cellular stress- and androgen-related transcription in Silurana tropicalis larvae. Environ. Sci. Technol. 2014, 48, 2952–2961. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, V.; Dalmas, F.; Sebille, B.; Amiel, C. Novel phosphorus-containing cyclodextrin polymers and their affinity for calcium cations and hydroxyapatite. Carbohydr. Polym. 2013, 98, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Hanis, K.K.A.; Muhammad Nasri, A.R.; Wan Farahiyah, W.K.; Mohd Rabani, M.Y. Bacterial Degradation of Azo Dye Congo Red by Bacillus sp. J. Phys. Conf. Ser. 2020, 1529, 022048. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their Impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Umbuzeiro, G.A. Effects of a textile azo dye on mortality, regeneration, and reproductive performance of the planarian, Girardia tigrina. Environ. Sci. Eur. 2014, 26, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz, E.R.; Umbuzeiro, G.A.; de-Almeida, G.; Caloto-Oliveira, A.; Chequer, F.M.; Zanoni, M.V.; Dorta, D.J.; Oliveira, D.P. Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames test, HepG2 cytotoxicity assay, and Daphnia acute toxicity test. Environ. Toxicol. 2011, 26, 489–497. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Su, J.C.; Horton, J.J. Allergic contact dermatitis from azo dyes. Australas. J. Dermatol. 1998, 39, 48–49. [Google Scholar] [CrossRef]
- Brown, T.; Slack, R.; Rushton, L.; British Occupational Cancer Burden Study, G. Occupational cancer in Britain. Urinary tract cancers: Bladder and kidney. Br. J. Cancer 2012, 107 (Suppl. 1), S76–S84. [Google Scholar] [CrossRef] [Green Version]
- Diacu, E. Colors: Properties and Determination of Synthetic Pigments. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 284–290. [Google Scholar] [CrossRef]
- Didier de Vasconcelos, G.M.; Mulinari, J.; de Arruda Guelli Ulson de Souza, S.M.; Ulson de Souza, A.A.; de Oliveira, D.; de Andrade, C.J. Biodegradation of azo dye-containing wastewater by activated sludge: A critical review. World J. Microbiol. Biotechnol. 2021, 37, 101. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2018, 17, 145–155. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vanaamudan, A.; Sadhu, M.; Pamidimukkala, P. Chitosan-Guar gum blend silver nanoparticle bionanocomposite with potential for catalytic degradation of dyes and catalytic reduction of nitrophenol. J. Mol. Liq. 2018, 271, 202–208. [Google Scholar] [CrossRef]
- Kolya, H.; Maiti, P.; Pandey, A.; Tripathy, T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Technol. 2015, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Aryan; Ruby; Mehata, M.S. Green synthesis of silver nanoparticles using Kalanchoe pinnata leaves (life plant) and their antibacterial and photocatalytic activities. Chem. Phys. Lett. 2021, 778, 138760. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D. Internally dispersed synthesis of uniform silver nanoparticles via in situ reduction of [Ag(NH3)2]+ along natural microfibrillar substructures of cotton fiber. Cellulose 2014, 21, 2963–2972. [Google Scholar] [CrossRef]
- Boylston, E.K.; Hinojosa, O.; Hebert, J.J. A quick embedding method for light and electron microscopy of textile fibers. Biotech. Histochem. 1991, 66, 122–124. [Google Scholar] [CrossRef]
- Thibodeaux, D.P.; Evans, J.P. Cotton Fiber Maturity by Image Analysis. Text. Res. J. 2016, 56, 130–139. [Google Scholar] [CrossRef]
- Sreedhala, S.; Sudheeshkumar, V.; Vinod, C.P. Structure sensitive chemical reactivity by palladium concave nanocubes and nanoflowers synthesised by a seed mediated procedure in aqueous medium. Nanoscale 2014, 6, 7496–7502. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Easson, M.; Jordan, J.H.; Bland, J.M.; Hinchliffe, D.J.; Condon, B.D. Application of Brown Cotton-Supported Palladium Nanoparticles in Suzuki-Miyaura Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 6304–6309. [Google Scholar] [CrossRef]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.F.; Ávalos-Borja, M.; Castillón-Barraza, F.F.; Posada-Amarillas, A. Assessment of growth of silver nanoparticles synthesized from an ethylene glycol–silver nitrate–polyvinylpyrrolidone solution. Phys. E Low-Dimens. Syst. Nanostruct. 2005, 25, 438–448. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, F.; Guang, S.; Cai, Z. Self-assembly of Ag nanoparticles on the woven cotton fabrics as mechanical flexible substrates for surface enhanced Raman scattering. J. Alloys Compd. 2017, 726, 484–489. [Google Scholar] [CrossRef]
- Hillyer, M.B.; Nam, S.; Condon, B.D. Quantification and spatial resolution of silver nanoparticles in cotton textiles by surface-enhanced Raman spectroscopy (SERS). J. Nanopart. Res. 2020, 22, 42. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Lin, X.; Huang, T.; Huang, F.; Wang, W.; Shi, J. Photocatalytic activity of a Bi-based oxychloride Bi4NbO8Cl. J. Mater. Chem. 2007, 17, 138–146. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Sundaram, N.G. Efficient visible light photocatalysis of Bi4TaO8Cl nanoparticles synthesized by solution combustion technique. RSC Adv. 2013, 3, 14371–14378. [Google Scholar] [CrossRef]
- Green, F.J. The Sigma-Aldrich Handbook of Stains, Dyes, and Indicators; Aldrich Chemical Co.: Milwaukee, WI, USA, 1990; p. 1000. [Google Scholar]
- Advincula, R.C.; Fells, E.; Park, M.-k. Molecularly Ordered Low Molecular Weight Azobenzene Dyes and Polycation Alternate Multilayer Films: Aggregation, Layer Order, and Photoalignment. Chem. Mater. 2001, 13, 2870–2878. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, G.; Kumar, D.D.; Kumar, M.R.; Venkatesh, N.; Sakthivel, P. Silver decorated CeO2 nanoparticles for rapid photocatalytic degradation of textile rose bengal dye. Sci. Rep. 2021, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Nadhe, S.; Singh, R.; Chopade, B.A. Decolorization of textile dyes by combination of gold nanocatalysts obtained from Acinetobacter sp. SW30 andNaBH4. Environ. Technol. Innov. 2018, 9, 186–197. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon 2019, 5, e01356. [Google Scholar] [CrossRef] [Green Version]
- Bo, Z.J.; Zhang, L.J.; Yan, L.; Tian, H.S.; Wei, H. Oxidation of Methyl Orange Solution with Potassium Peroxydisulfate. Iran. J. Chem. Chem. Eng. 2012, 31, 21–24. [Google Scholar] [CrossRef]
- Nam, S.; Hillyer, M.B.; Condon, B.D.; Lum, J.S.; Richards, M.N.; Zhang, Q. Silver Nanoparticle-Infused Cotton Fiber: Durability and Aqueous Release of Silver in Laundry Water. J. Agric. Food Chem. 2020, 68, 13231–13240. [Google Scholar] [CrossRef]
- Nam, S.; Ernst, N.; Chavez, S.E.; Hillyer, M.B.; Condon, B.D.; Gibb, B.C.; Sun, L.; Guo, H.; He, L. Practical SERS method for assessment of the washing durability of textiles containing silver nanoparticles. Anal. Methods 2020, 12, 1186–1196. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Kumar, H.A.K.; Mandal, B.K. Biofabricated silver nanoparticles as green catalyst in the degradation of different textile dyes. J. Environ. Chem. Eng. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- Albeladi, S.S.R.; Malik, M.A.; Al-Thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]




| Dye | Molecular Weight (g/mol) | UV/Vis λmax (nm) | Structure |
|---|---|---|---|
| Methyl Orange (MO) | 327.33 | 464 | 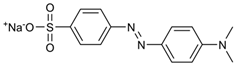 |
| Congo Red (CR) | 696.67 | 496 | 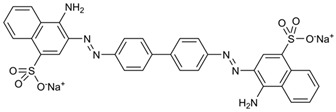 |
| Chicago Sky Blue 6B (CSBB) | 992.80 | 618 | 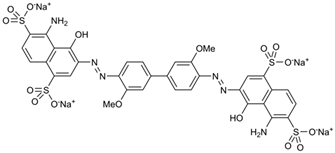 |
| Dye | Ag NP–Cotton Catalyst Loading | Time (min) a | TON a | TOF (min−1) a |
|---|---|---|---|---|
| MO | 200 mg | 57.5 | 2.4 | 0.041 |
| CR | 200 mg | 115 | 2.2 | 0.019 |
| CSBB | 200 mg | 73.3 | 1.6 | 0.021 |
| MO | 400 mg | 25.5 | 1.2 | 0.046 |
| CR | 400 mg | 13.9 | 1.1 | 0.082 |
| CSBB | 400 mg | 14.0 | 0.8 | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillyer, M.B.; Jordan, J.H.; Nam, S.; Easson, M.W.; Condon, B.D. Silver Nanoparticle-Intercalated Cotton Fiber for Catalytic Degradation of Aqueous Organic Dyes for Water Pollution Mitigation. Nanomaterials 2022, 12, 1621. https://doi.org/10.3390/nano12101621
Hillyer MB, Jordan JH, Nam S, Easson MW, Condon BD. Silver Nanoparticle-Intercalated Cotton Fiber for Catalytic Degradation of Aqueous Organic Dyes for Water Pollution Mitigation. Nanomaterials. 2022; 12(10):1621. https://doi.org/10.3390/nano12101621
Chicago/Turabian StyleHillyer, Matthew Blake, Jacobs H. Jordan, Sunghyun Nam, Michael W. Easson, and Brian D. Condon. 2022. "Silver Nanoparticle-Intercalated Cotton Fiber for Catalytic Degradation of Aqueous Organic Dyes for Water Pollution Mitigation" Nanomaterials 12, no. 10: 1621. https://doi.org/10.3390/nano12101621
APA StyleHillyer, M. B., Jordan, J. H., Nam, S., Easson, M. W., & Condon, B. D. (2022). Silver Nanoparticle-Intercalated Cotton Fiber for Catalytic Degradation of Aqueous Organic Dyes for Water Pollution Mitigation. Nanomaterials, 12(10), 1621. https://doi.org/10.3390/nano12101621







