Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
2.3. Measurement and Characterization
3. Results
3.1. Elemental and Structural Properties of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Powders
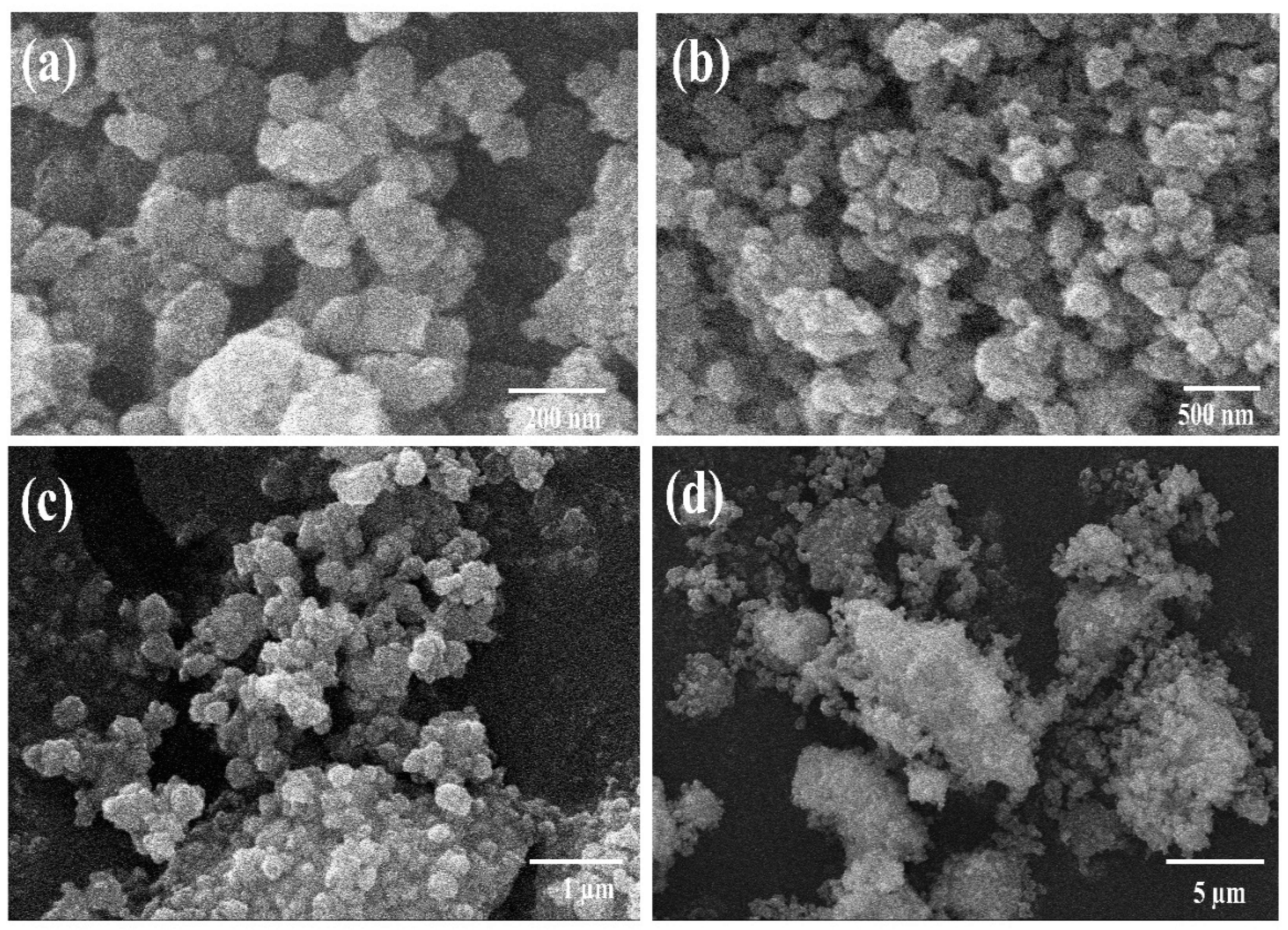
3.2. Surface Morphological Characteristics of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
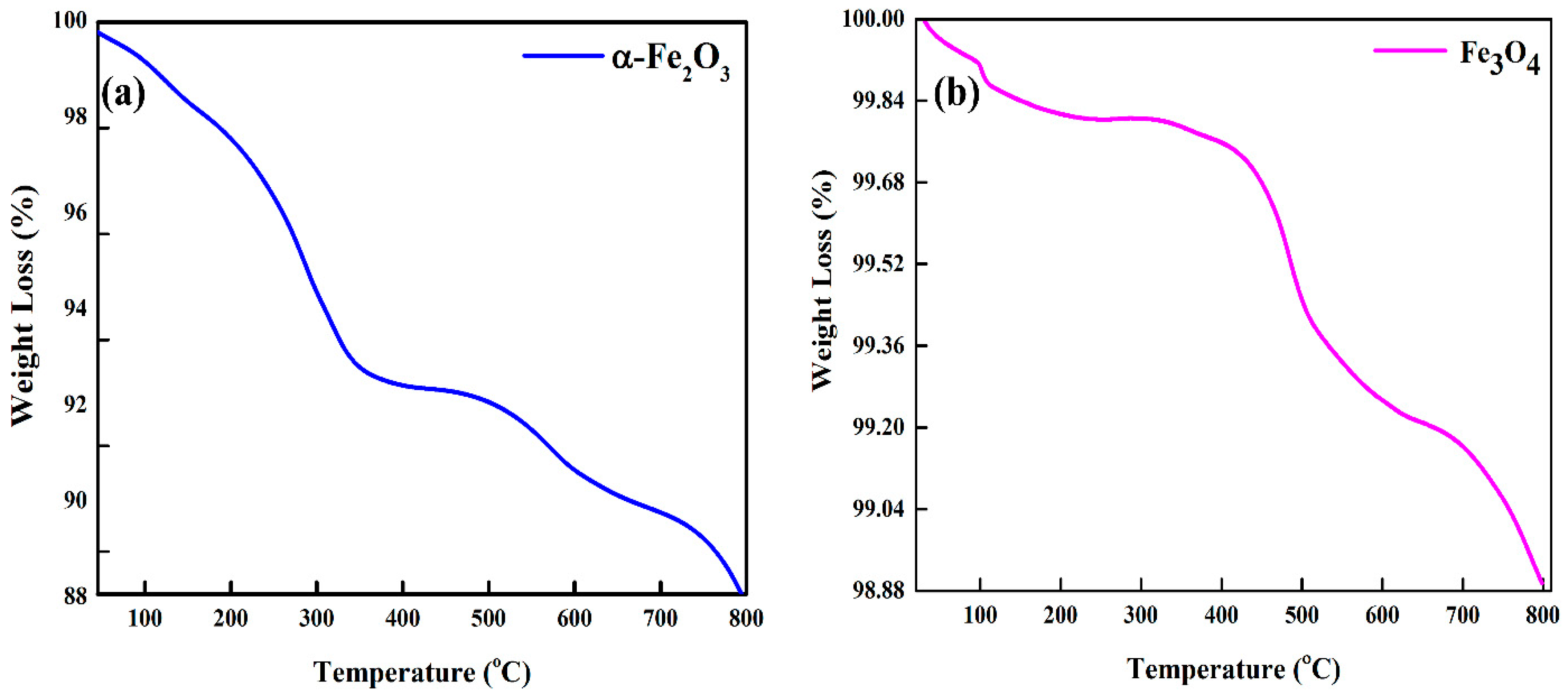
3.3. Thermal Stability of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
3.4. Absorption Characteristics of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
3.5. Electrochemical Measurements of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
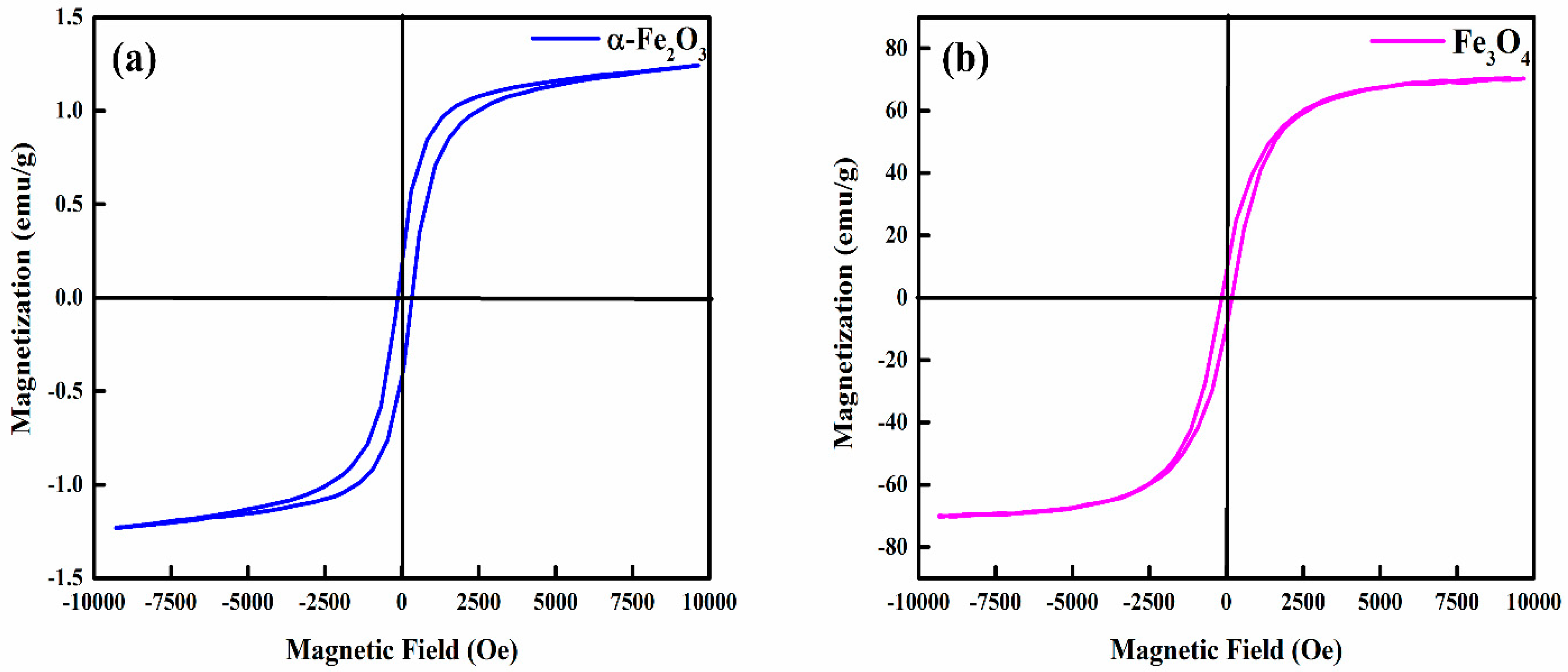
3.6. Magnetic Properties of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LED | Light-Emitting Diode |
| Li-ion | Lithium ion |
| BPR | Ball to powder ratio |
| UV–Vis | Ultraviolet–visible |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| PL | Photoluminescence |
| KOH | Potassium hydroxide |
| EDS | Energy dispersive spectroscopy |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| Epp | Peak-to-peak gap |
| GCE | Glassy carbon electrode |
| VSM | Vibrating sample magnetometer |
| Ms | Saturation magnetization |
| Hc | Coercivity |
| Mr | Remanent magnetization |
References
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7, 7672. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Mai, W.; Tu, J.; Fan, H.J. Integration of Energy Harvesting and Electrochemical Storage Devices. Adv. Mater. Technol. 2017, 2, 1700182. [Google Scholar] [CrossRef]
- Lai, S.Y.; Cavallo, C.; Abdelhamid, M.; Lou, F.; Koposov, A. Advanced and Emerging Negative Electrodes for Li-Ion Capacitors: Pragmatism vs. Performance. Energies 2021, 14, 3010. [Google Scholar] [CrossRef]
- Taeño, M.; Maestre, D.; Cremades, A. An approach to emerging optical and optoelectronic applications based on NiO micro- and nanostructures. Nanophotonics 2021, 10, 1785–1799. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.-H.; Ma, Y.-R. One-Dimensional Metal-Oxide Nanostructures: Recent Developments in Synthesis, Characterization, and Applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef] [Green Version]
- Grilli, M.L. Metal Oxides. Metals 2020, 10, 820. [Google Scholar] [CrossRef]
- Fouad, D.E.; Zhang, C.; El-Didamony, H.; Yingnan, L.; Mekuria, T.D.; Shah, A.H. Improved size, morphology and crystallinity of hematite (α-Fe2O3) nanoparticles synthesized via the precipitation route using ferric sulfate precursor. Results Phys. 2019, 12, 1253–1261. [Google Scholar] [CrossRef]
- Rauf, A.; Adil, M.; Mian, S.A.; Rahman, G.; Ahmed, E.; Din, Z.M.U.; Qun, W. Tuning the optoelectronic properties of hematite with rhodium doping for photoelectrochemical water splitting using density functional theory approach. Sci. Rep. 2021, 11, 41. [Google Scholar] [CrossRef]
- Yao, J.; Wang, W.; Zuo, X.; Li, G. Enhanced photoelectric conversion performance by morphology-controlled growth of Fe3O4 in dye-sensitized solar cells. Mater. Lett. 2018, 220, 16–19. [Google Scholar] [CrossRef]
- Nikolaeva, N.V.; Aleksandrova, T.N.; Chanturiya, E.L.; Afanasova, A. Mineral and Technological Features of Magnetite–Hematite Ores and Their Influence on the Choice of Processing Technology. ACS Omega 2021, 6, 9077–9085. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.M.S.N.; Mantilaka, M.M.M.G.P.G.; Palihawadana, T.C.; Chandrakumara, G.T.D.; De Silva, R.T.; Pitawala, H.M.T.G.A.; de Silva, K.M.N.; Amaratunga, G.A.J. Facile and low-cost synthesis of pure hematite (α-Fe2O3) nanoparticles from naturally occurring laterites and their superior adsorption capability towards acid-dyes. RSC Adv. 2019, 9, 21249–21257. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Prozorov, R.; Koltypin, Y.; Kataby, G.; Felner, I.; Gedanken, A. Synthesis of pure amorphous Fe2O3. J. Mater. Res. 2011, 12, 402–406. [Google Scholar] [CrossRef]
- Rafi, M.M.; Ahmed, K.S.Z.; Nazeer, K.P.; Kumar, D.S.; Thamilselvan, M. Synthesis, characterization and magnetic properties of hematite (α-Fe2O3) nanoparticles on polysaccharide templates and their antibacterial activity. Appl. Nanosci. 2015, 5, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Luan, P.; Xie, M.; Liu, D.; Fu, X.; Jing, L. Effective charge separation in the rutile TiO2 nanorod-coupled α-Fe2O3 with exceptionally high visible activities. Sci. Rep. 2014, 4, 6180. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Kumar, P. Zn Doped α-Fe2O3: An Efficient Material for UV Driven Photocatalysis and Electrical Conductivity. Crystals 2020, 10, 273. [Google Scholar]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Lukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Fonin, M.; Pentcheva, R.; Dedkov, Y.S.; Sperlich, M.; Vyalikh, D.V.; Scheffler, M.; Rüdiger, U.; Güntherodt, G. Surface electronic structure of the Fe3O4(100): Evidence of a half-metal to metal transition. Phys. Rev. B 2005, 72, 104436. [Google Scholar] [CrossRef] [Green Version]
- Orel, V.E.; Tselepi, M.; Mitrelias, T.; Zabolotny, M.; Shevchenko, A.; Rykhalskyi, A.; Romanov, A.; Orel, V.B.; Burlaka, A.; Lukin, S.; et al. The comparison between superparamagnetic and ferromagnetic iron oxide nanoparticles for cancer nanotherapy in the magnetic resonance system. Nanotechnology 2019, 30, 415701. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Sawadh, P.; Palei, P.K.; Kokate, K.K. Effect of synthesis route on the structural, optical and magnetic properties of Fe3O4 nanoparticles. Ceram. Int. 2014, 40, 1945–1949. [Google Scholar] [CrossRef]
- Leshuk, T.; Krishnakumar, H.; Gu, F. Size-Tunable Fe3O4 Spherical Nanoclusters Through a One-Pot Hydrothermal Synthesis. J. Nanosci. Nanotechnol. 2015, 15, 5378–5383. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Jeong, J.R.; Kim, C. A facile route to sonochemical synthesis of magnetic iron oxide (Fe3O4) nanoparticles. Thin Solid Film. 2011, 519, 8277–8279. [Google Scholar] [CrossRef]
- Liu, X.-D.; Chen, H.; Liu, S.-S.; Ye, L.; Li, Y.-P. Hydrothermal synthesis of superparamagnetic Fe3O4 nanoparticles with ionic liquids as stabilizer. Mater. Res. Bull. 2015, 62, 217–221. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Related magnetic properties of CoFe2O4 cobalt ferrite particles synthesised by the polyol method with NaBH4 and heat treatment: New micro and nanoscale structures. RSC Adv. 2015, 5, 56560–56569. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (α − Fe2O3) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Basith, D.M.; Islam, M.A.; Ahmmad, B.; Hossain, M.S.; Mølhave, K. Preparation of high crystalline nanoparticles of rare-earth based complex pervoskites and comparison of their structural and magnetic properties with bulk counterparts. Mater. Res. Express 2017, 4, 075012. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshana, G.; Kottegoda, N.; Senaratne, A.; De Alwis, A.; Karunaratne, V. Synthesis of Magnetite Nanoparticles by Top-Down Approach from a High Purity Ore. J. Nanomater. 2015, 2015, 317312. [Google Scholar] [CrossRef] [Green Version]
- Kebede, G.; del Rosario, A.V.; Ali, A.Y.; Femi, O.E. Production of magnetite nanoparticles from Ethiopian iron ore using solvent extraction and studying parameters that affect crystallite size. Mater. Res. Express 2020, 7, 105016. [Google Scholar]
- Piras, C.C.; Fernández-Prieto, S.; de Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Malkani, M.S.; Malik, Z. Mineral Resources of Pakistan: A Review. Geol. Surv. Pak. Rec. 2016, 128, 1–90. [Google Scholar]
- Basith, M.A.; Ngo, D.-T.; Quader, A.; Rahman, M.A.; Sinha, B.L.; Ahmmad, B.; Hirose, F.; Mølhave, K. Simple top-down preparation of magnetic Bi0.9Gd0.1Fe1−xTixO3 nanoparticles by ultrasonication of multiferroic bulk material. Nanoscale 2014, 6, 14336–14342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, M.A.; Imam, N.; Ahmed, M.; El Sherbiny, D.H. Spectrophotometric analysis of hematite/magnetite nanocomposites in comparison with EDX and XRF techniques. J. Mol. Liq. 2017, 231, 288–295. [Google Scholar] [CrossRef]
- Ding, D.; Yan, X.; Zhang, X.; He, Q.; Qiu, B.; Jiang, D.; Wei, H.; Guo, J.; Umar, A.; Sun, L.; et al. Preparation and Enhanced Properties of Fe3O4 Nanoparticles Reinforced Polyimide Nanocomposites. Superlattices Microstruct. 2015, 85, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, C. Raman Spectroscopy of Iron Oxide of Nanoparticles (Fe3O4). J. Mater. Sci. Eng. 2015, 5, 1000217. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Divya, J.; Pramothkumar, A.; Hilary, H.J.L.; Jayanthi, P.J.; Prabakar, P.C.J. Impact of copper (Cu) and iron (Fe) co-doping on structural, optical, magnetic and electrical properties of tin oxide (SnO2) nanoparticles for optoelectronics applications. J. Mater. Sci. Mater. Electron. 2021, 32, 16775–16785. [Google Scholar] [CrossRef]
- Sun, S.; Li, D.; Yang, C.; Fu, L.; Kong, D.; Lu, Y.; Guo, Y.; Liu, D.; Guan, P.; Zhang, Z.; et al. Direct Atomic-Scale Observation of Ultrasmall Ag Nanowires that Exhibit fcc, bcc, and hcp Structures under Bending. Phys. Rev. Lett. 2022, 128, 015701. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zeng, Z.; Zhou, H.; He, J.; Liu, P.; Chen, M.; Han, J.; Srolovitz, D.J.; Teng, J.; et al. Tracking the sliding of grain boundaries at the atomic scale. Science 2022, 375, 1261–1265. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic Characterization of Iron Oxide Nanoparticles for Biomedical Applications. In Biomedical Nanotechnology; Humana Press: New York, NY, USA, 2017; Volume 1570, pp. 47–71. [Google Scholar]
- Rufus, D.; Sreeju, N.; Philip, D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016, 6, 94206–94217. [Google Scholar] [CrossRef]
- Razik, A.A.; Ahmed, I.; Elfiky, E. Auto-combustion Synthesis and Characterization of Iron Oxide Nanoparticles (α-Fe2O3) for Removal of Lead Ions from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2021, 31, 384–396. [Google Scholar]
- Sathya, A.; Gnanaprakash, G.; Panneerselvam, G.; Antony, M.P.; Philip, J. Effect of Surfactant Monolayer on Reduction of Fe3O4 Nanoparticles under Vacuum. J. Phys. Chem. C 2008, 112, 18376–18383. [Google Scholar]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.; Ismail, M.I.M. Synthesis and Some Physical Properties of Magnetite (Fe3O4) Nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Xu, Y.; Schoonen, M.A. The Absolute Energy Position of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Rada, S.; Culea, E.; Rada, M. Novel ZrO2 based ceramics stabilized by Fe2O3, SiO2 and Y2O3. Chem. Phys. Lett. 2018, 696, 92–99. [Google Scholar] [CrossRef]
- Pal, M.; Rakshit, R.; Mandal, K.J.A.P.L. Facile functionalization of Fe2O3 nanoparticles to induce inherent photoluminescence and excellent photocatalytic activity. Appl. Phys. Lett. 2014, 104, 233110. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Kim, M.-S.; Bae, T.-S.; Yu, J.-S. Hematite (α-Fe2O3) nanoparticles on vulcan carbon as an ultrahigh capacity anode material in lithium ion battery. Electrochim. Acta 2013, 114, 60–67. [Google Scholar] [CrossRef]
- Ramasami, A.; Ravishankar, T.N.; Sureshkumar, K.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishnappa, T.; Balakrishna, G.R. Synthesis, Exploration of Energy Storage and Electrochemical Sensing Properties of Hematite Nanoparticles. J. Alloy Compd. 2016, 671, 552–559. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Noh, M.F.M.; Ab Halim, A.; Rahim, M.A.F.A.; Ita, N.S.H.M.; Mohamed, N.A.; Nasir, S.N.F.M.; Ismail, A.F.; Teridi, M.A.M. Cyclic voltammetry—A promising approach towards improving photoelectrochemical activity of hematite. J. Alloy Compd. 2021, 852, 156757. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Torres-Torres, D.; Mojica-Gomez, J.; Estrada-Arteaga, C.; Antaño-López, R. Characterization by electrochemical impedance spectroscopy of magnetite nanoparticles supported on carbon paste electrode. Electrochim. Acta 2011, 56, 8078–8084. [Google Scholar] [CrossRef]
- Kamali, K.Z.; Alagarsamy, P.; Huang, N.M.; Ong, B.H.; Lim, H.N. Hematite Nanoparticles-Modified Electrode Based Electrochemical Sensing Platform for Dopamine. Sci. World J. 2014, 2014, 396135. [Google Scholar]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Jacob, J.; Khadar, M.A. VSM and Mössbauer study of nanostructured hematite. J. Magn. Magn. Mater. 2010, 322, 614–621. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Q.; Chen, Q. One-step hydrothermal process to prepare highly crystalline Fe3O4 nanoparticles with improved magnetic properties. Mater. Res. Bull. 2003, 38, 1113–1118. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, A.A.; Javed, S.; Javed, H.M.A.; Jamshaid, M.; Ali, U.; Akram, M.A. Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics. Nanomaterials 2022, 12, 1635. https://doi.org/10.3390/nano12101635
Qureshi AA, Javed S, Javed HMA, Jamshaid M, Ali U, Akram MA. Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics. Nanomaterials. 2022; 12(10):1635. https://doi.org/10.3390/nano12101635
Chicago/Turabian StyleQureshi, Akbar Ali, Sofia Javed, Hafiz Muhammad Asif Javed, Muhammad Jamshaid, Usman Ali, and Muhammad Aftab Akram. 2022. "Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics" Nanomaterials 12, no. 10: 1635. https://doi.org/10.3390/nano12101635





