Metallic Phase Transition Metal Dichalcogenide Quantum Dots as Promising Bio-Imaging Materials
Abstract
:1. Introduction
2. Materials and Methods
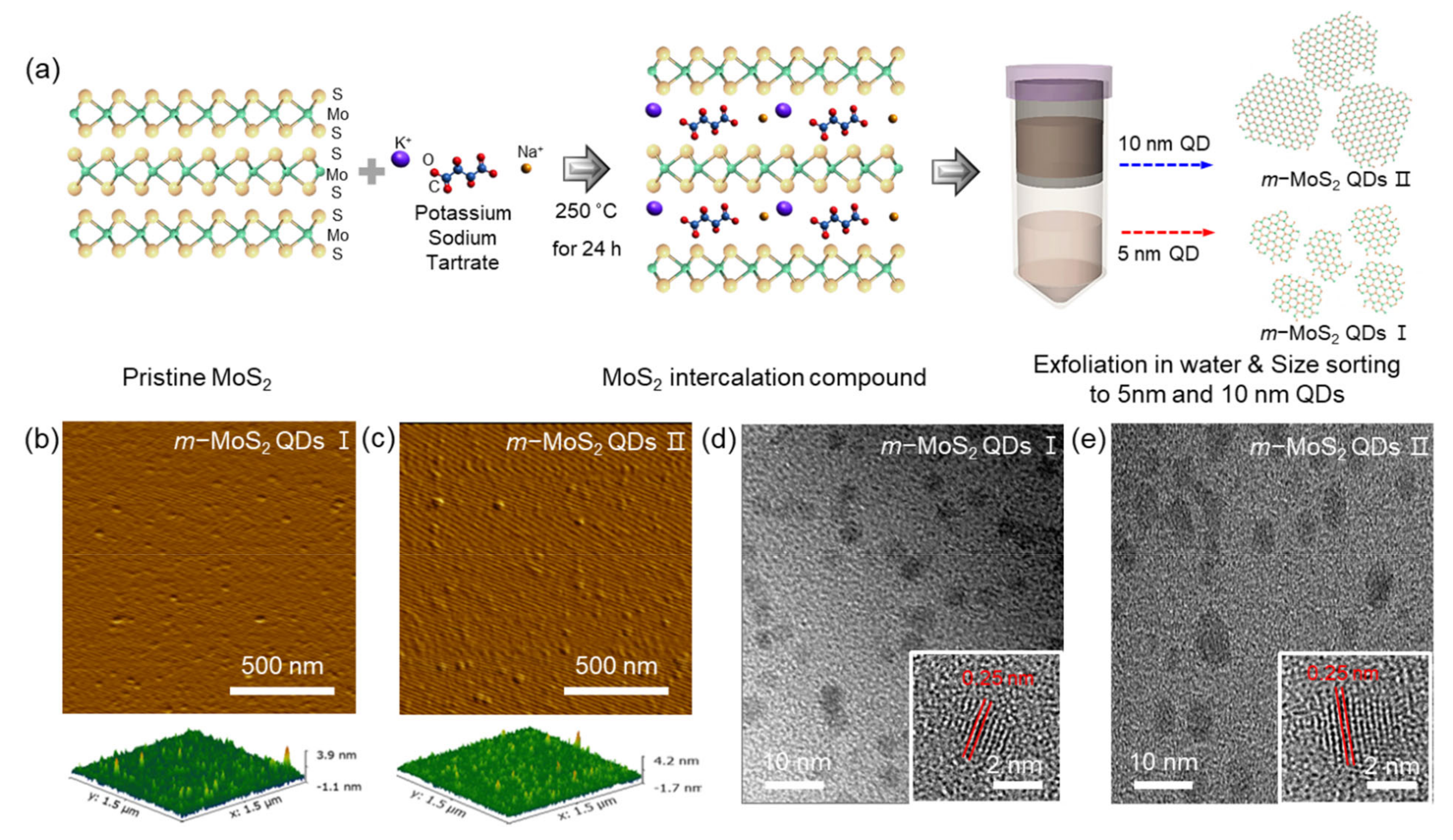
2.1. Preparation of m-MoS2-QDs
2.2. Characterization
2.3. Density Functional Calculations
2.4. Cytotoxicity Evaluation
2.5. Cell Imaging
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, C.; Pan, A.; Yu, R.; Duan, X. Two-dimensional transition metal dichalcogenides as atomically thin semiconductors: Opportunities and challenges. Chem. Soc. Rev. 2015, 44, 8859–8876. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Wang, S.; Robertson, A.; Warner, J.H. Atomic structure of defects and dopants in 2D layered transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 6764–6794. [Google Scholar] [CrossRef]
- Tan, S.J.; Abdelwahab, I.; Ding, Z.; Zhao, X.; Yang, T.; Loke, G.Z.; Lin, H.; Verzhbitskiy, I.; Poh, S.M.; Xu, H. Chemical stabilization of 1T′ phase transition metal dichalcogenides with giant optical Kerr nonlinearity. J. Am. Chem. Soc. 2017, 139, 2504–2511. [Google Scholar] [CrossRef]
- Srivastava, A.; Sidler, M.; Allain, A.V.; Lembke, D.S.; Kis, A.; Imamoğlu, A. Optically active quantum dots in monolayer WSe 2. Nat. Nanotechnol. 2015, 10, 491–496. [Google Scholar] [CrossRef]
- Luan, C.-Y.; Xie, S.; Ma, C.; Wang, S.; Kong, Y.; Xu, M. Elucidation of luminescent mechanisms of size-controllable MoSe2 quantum dots. Appl. Phys. Lett. 2017, 111, 073105. [Google Scholar] [CrossRef]
- García-Santamaría, F.; Chen, Y.; Vela, J.; Schaller, R.D.; Hollingsworth, J.A.; Klimov, V.I. Suppressed auger recombination in “giant” nanocrystals boosts optical gain performance. Nano Lett. 2009, 9, 3482–3488. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.-S.; Fukumura, D.; Jain, R.K. Compact high-quality CdSe–CdS core–shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-H.; Jang, M.-H.; Yoon, H.; Kim, H.J.; Cho, Y.-H.; Jeon, S.; Song, S.-H. Metallic phase transition metal dichalcogenide quantum dots showing different optical charge excitation and decay pathways. NPG Asia Mater. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Yu, J.; Luo, M.; Lv, Z.; Huang, S.; Hsu, H.-H.; Kuo, C.-C.; Han, S.-T.; Zhou, Y. Recent advances in optical and optoelectronic data storage based on luminescent nanomaterials. Nanoscale 2020, 12, 23391–23423. [Google Scholar] [PubMed]
- Dhanabalan, S.C.; Dhanabalan, B.; Ponraj, J.S.; Bao, Q.; Zhang, H. 2D–Materials-Based Quantum Dots: Gateway Towards Next-Generation Optical Devices. Adv. Opt. Mater. 2017, 5, 1700257. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, L.; Li, X.; Xu, H. Fabrication of transition metal dichalcogenides quantum dots based on femtosecond laser ablation. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of few-layer MoS2 nanosheets: Preparation of luminescent quantum dots via a transition of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef]
- Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S.F. One-step hydrothermal synthesis of monolayer MoS2 quantum dots for highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 10693–10697. [Google Scholar] [CrossRef]
- Ha, H.D.; Han, D.J.; Choi, J.S.; Park, M.; Seo, T.S. Dual role of blue luminescent MoS2 quantum dots in fluorescence resonance energy transfer phenomenon. Small 2014, 10, 3858–3862. [Google Scholar] [CrossRef]
- Park, K.H.; Jung, S.; Kim, J.; Ko, B.-M.; Shim, W.-G.; Hong, S.-J.; Song, S.H. Boosting Photovoltaic Performance in Organic Solar Cells by Manipulating the Size of MoS2 Quantum Dots as a Hole-Transport Material. Nanomaterials 2021, 11, 1464. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phy. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phy. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phy. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Jang, M.-H.; Jeong, J.-M.; Yoon, H.; Cho, Y.-H.; Jeong, W.-I.; Kim, B.-H.; Jeon, S. Primary hepatocyte imaging by multiphoton luminescent graphene quantum dots. Chem. Commun. 2015, 51, 8041–8043. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Cao, Z.; Lin, X.; Li, X.; Fang, Y.; An, X.; Fu, Y.; Jin, J.; Li, R. MoS2 quantum dot decorated RGO: A designed electrocatalyst with high active site density for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 21772–21778. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Wu, L.; Ye, M.; Liu, X.; Wang, Q.; Hou, S.; Lu, P.; Sun, L.; Zheng, J. Phase-selective synthesis of 1T′ MoS2 monolayers and heterophase bilayers. Nat. Mater. 2018, 17, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of intercalated metals on the electrocatalytic activity of 1T-MoS2 for the hydrogen evolution reaction. ACS Energy Lett. 2017, 3, 7–13. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, L.; Zhang, W.; Pan, H.; Sun, Y.; Ge, C.; Du, Y.; Tang, N. Ultrasmall and monolayered tungsten dichalcogenide quantum dots with giant spin–valley coupling and purple luminescence. ACS Omega 2018, 3, 12188–12194. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Sofer, Z.K.; Pumera, M. 1T-phase transition metal dichalcogenides (MoS2, MoSe2, WS2, and WSe2) with fast heterogeneous electron transfer: Application on second-generation enzyme-based biosensor. ACS Appl. Mater. Interfaces 2017, 9, 40697–40706. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Yao, Y.; Ao, K.; Lv, P.; Wei, Q. MoS2 coexisting in 1T and 2H phases synthesized by common hydrothermal method for hydrogen evolution reaction. Nanomaterials 2019, 9, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of monolayer MoS2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS2 targets in water. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dolinska, J.; Chidambaram, A.; Adamkiewicz, W.; Estili, M.; Lisowski, W.; Iwan, M.; Palys, B.; Sudholter, E.J.; Marken, F.; Opallo, M. Synthesis and characterization of porous carbon–MoS2 nanohybrid materials: Electrocatalytic performance towards selected biomolecules. J. Mater. Chem. B 2016, 4, 1448–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Yang, J.Y.; Jung, S.; Ko, B.M.; Song, G.; Hong, S.-J.; Kim, N.C.; Lee, D.; Song, S.H. Metallic Phase Transition Metal Dichalcogenide Quantum Dots as Promising Bio-Imaging Materials. Nanomaterials 2022, 12, 1645. https://doi.org/10.3390/nano12101645
Park KH, Yang JY, Jung S, Ko BM, Song G, Hong S-J, Kim NC, Lee D, Song SH. Metallic Phase Transition Metal Dichalcogenide Quantum Dots as Promising Bio-Imaging Materials. Nanomaterials. 2022; 12(10):1645. https://doi.org/10.3390/nano12101645
Chicago/Turabian StylePark, Kwang Hyun, Jun Yong Yang, Sunggyeong Jung, Byoung Min Ko, Gian Song, Soon-Jik Hong, Nam Chul Kim, Dongju Lee, and Sung Ho Song. 2022. "Metallic Phase Transition Metal Dichalcogenide Quantum Dots as Promising Bio-Imaging Materials" Nanomaterials 12, no. 10: 1645. https://doi.org/10.3390/nano12101645
APA StylePark, K. H., Yang, J. Y., Jung, S., Ko, B. M., Song, G., Hong, S.-J., Kim, N. C., Lee, D., & Song, S. H. (2022). Metallic Phase Transition Metal Dichalcogenide Quantum Dots as Promising Bio-Imaging Materials. Nanomaterials, 12(10), 1645. https://doi.org/10.3390/nano12101645




