Nanoarchitectonics of Carbon Nanostructures: Sodium Dodecyl Sulfonate @ Sodium Chloride System
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of SDSN Solution
2.2. Preparation of SDSN @ NaCl System
2.3. Preparation of Carbon Nanostructures
2.4. After-Treatment
2.5. Characterization
3. Results and Discussion
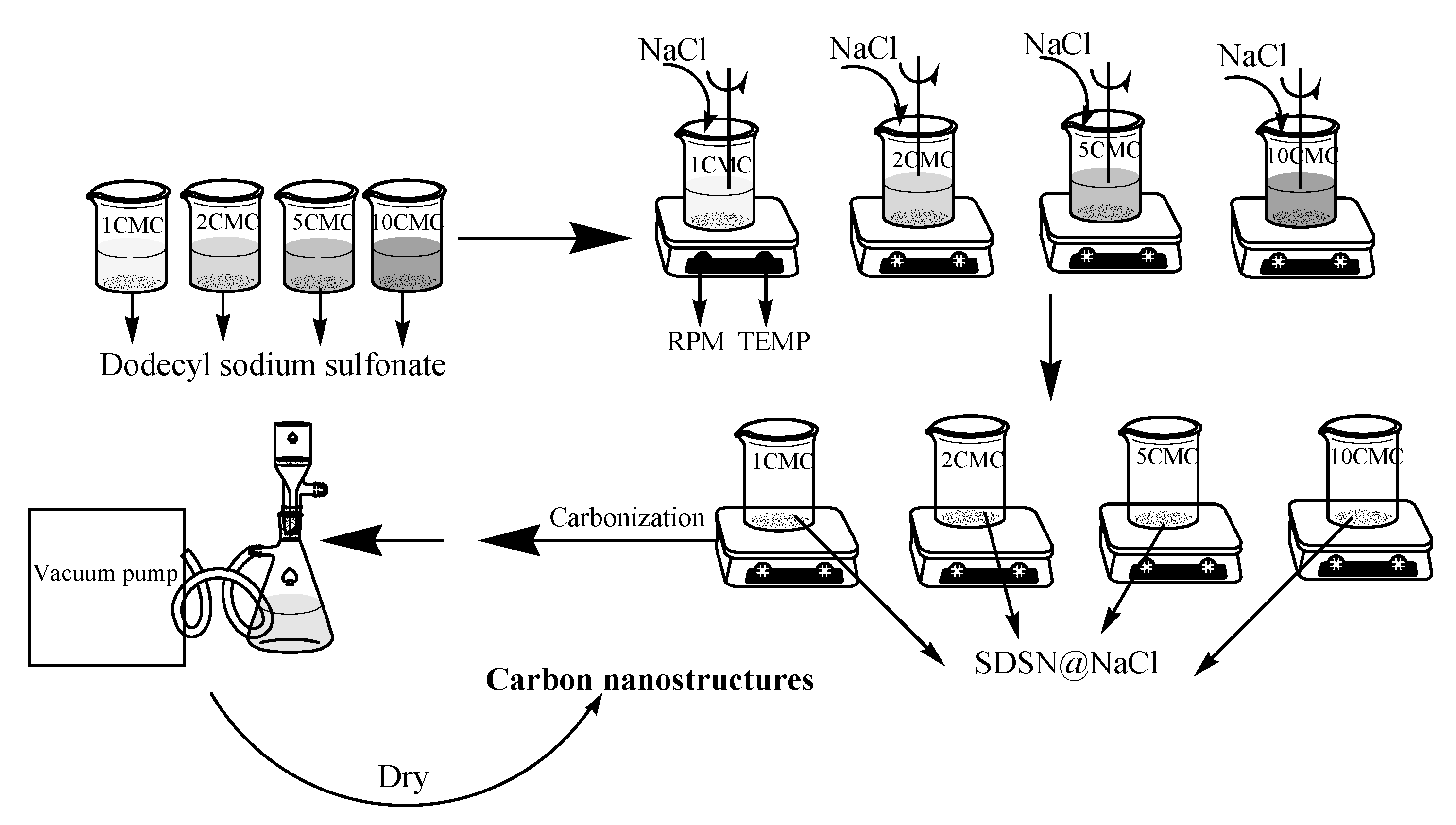
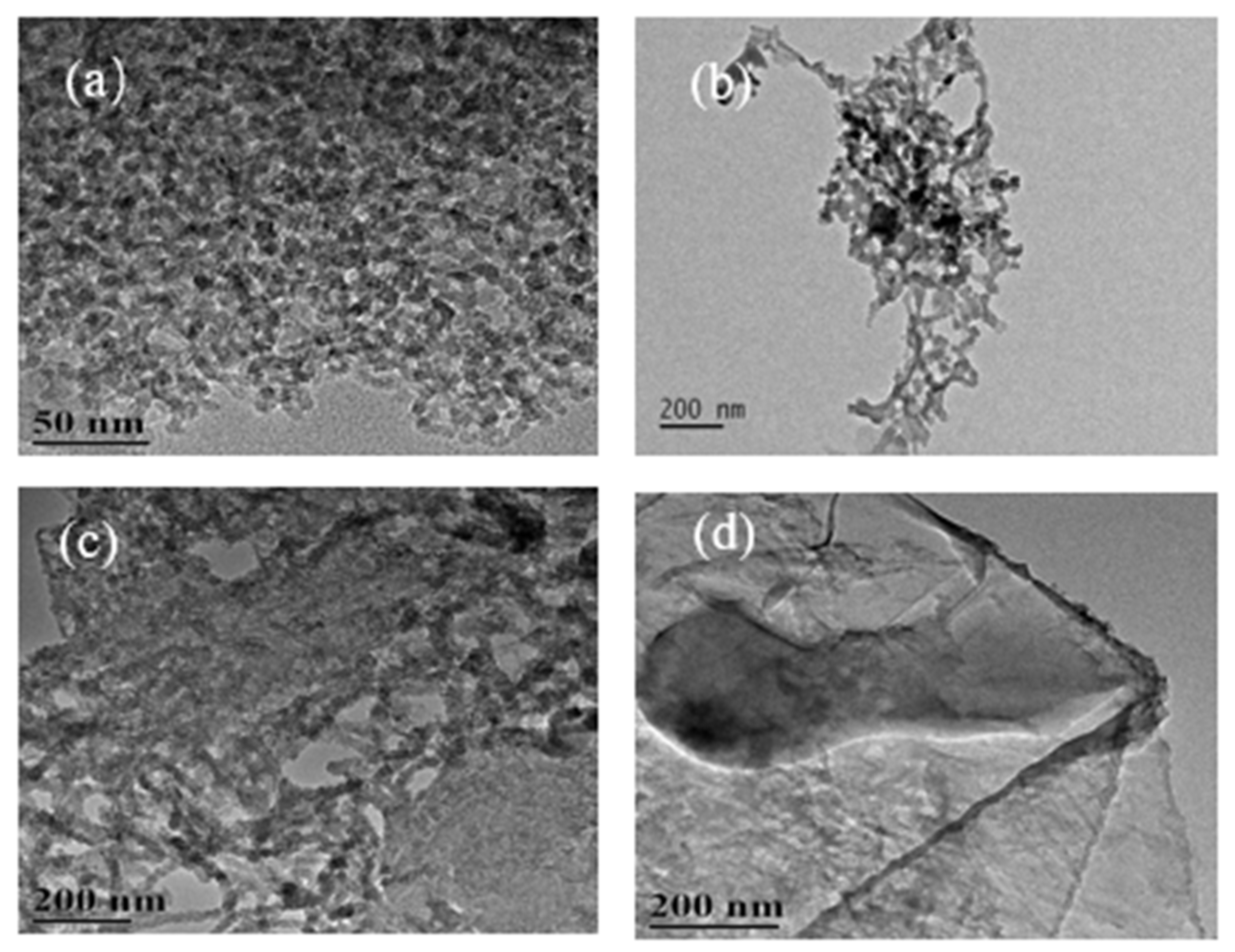
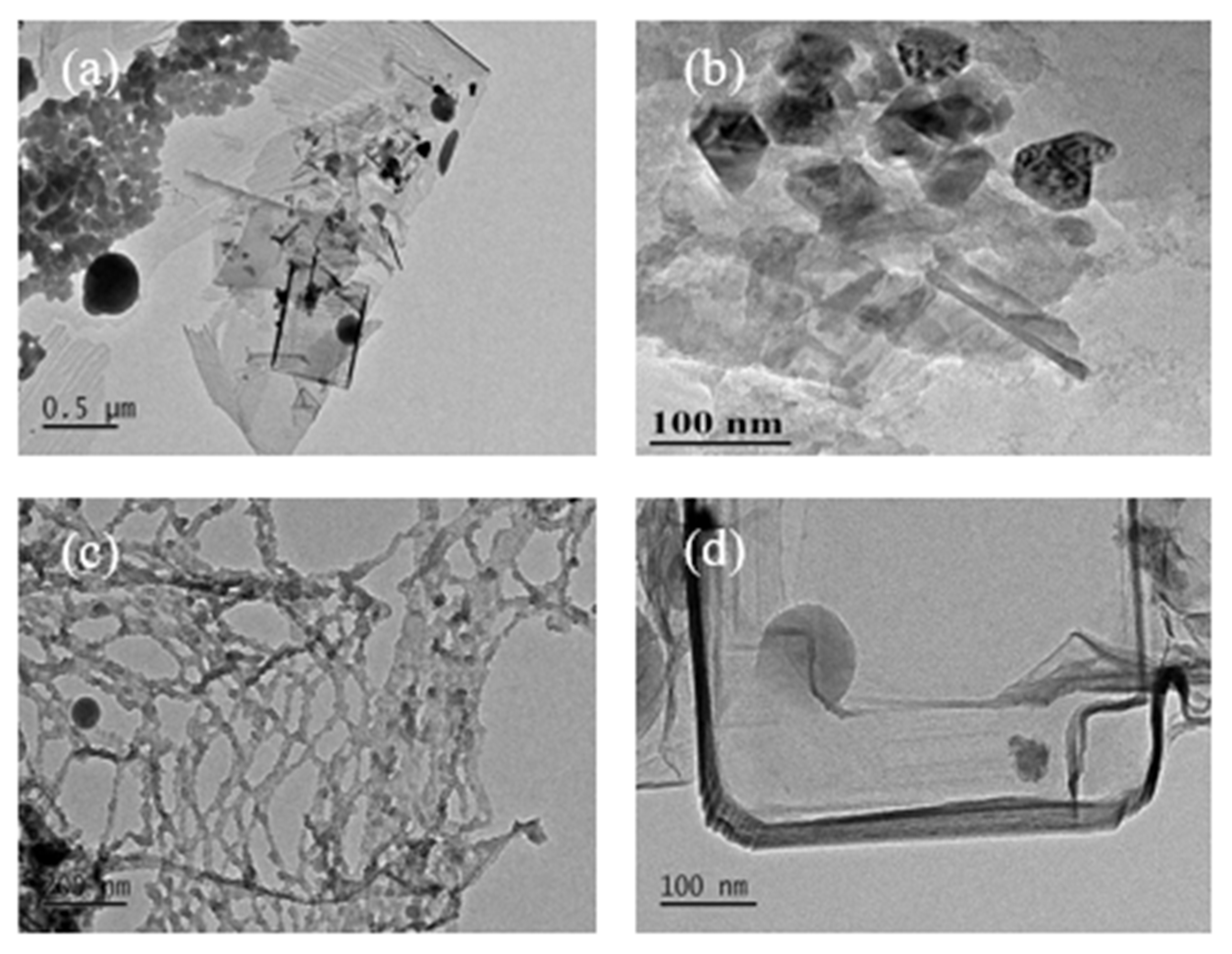
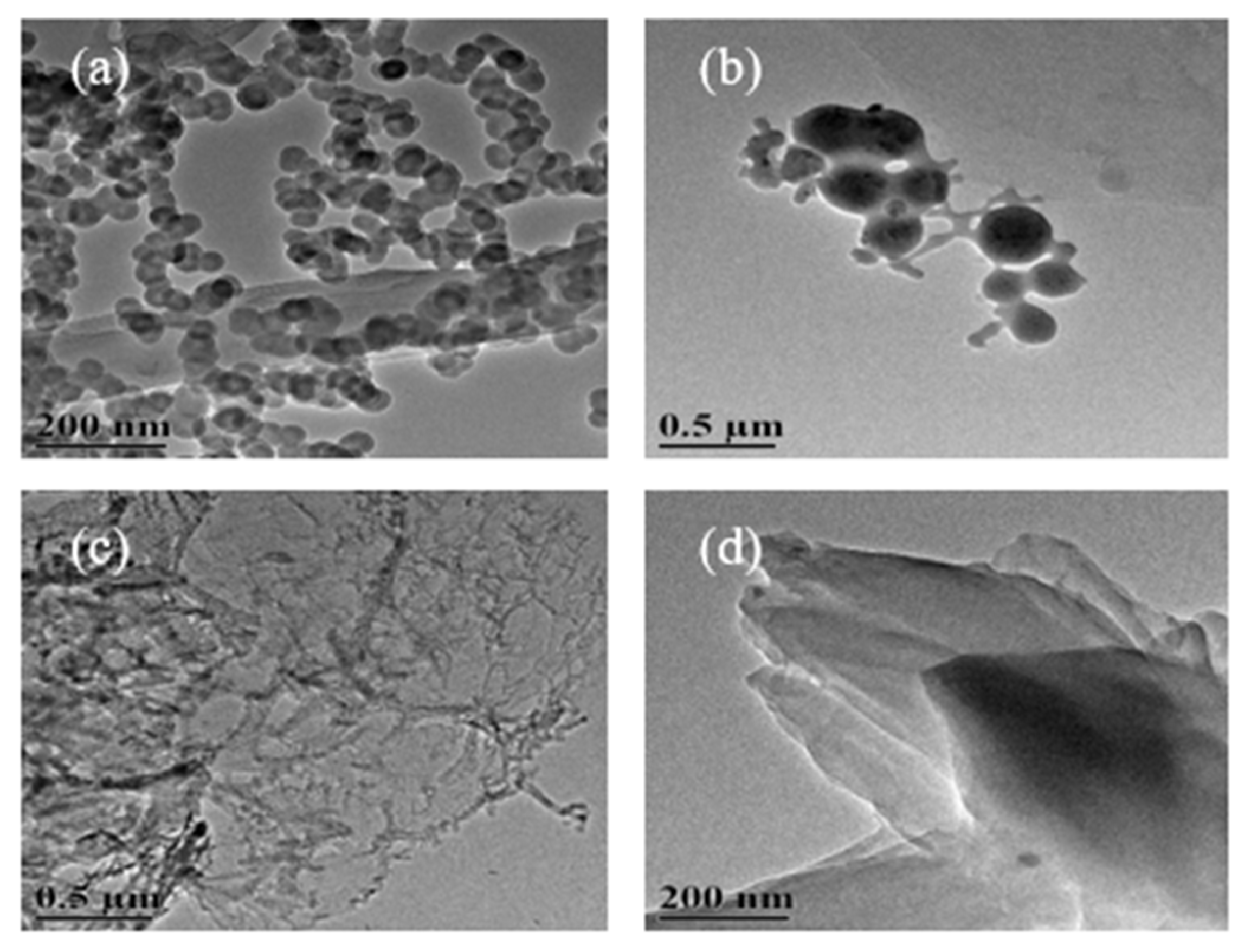
3.1. TEM Image Analysis of Carbon Nanostructures
3.1.1. TEM Images of Carbon Nanostructures at 500 °C
3.1.2. TEM Images of Carbon Nanostructures at 600 °C
3.1.3. TEM Images of Carbon Nanostructures at 800 °C
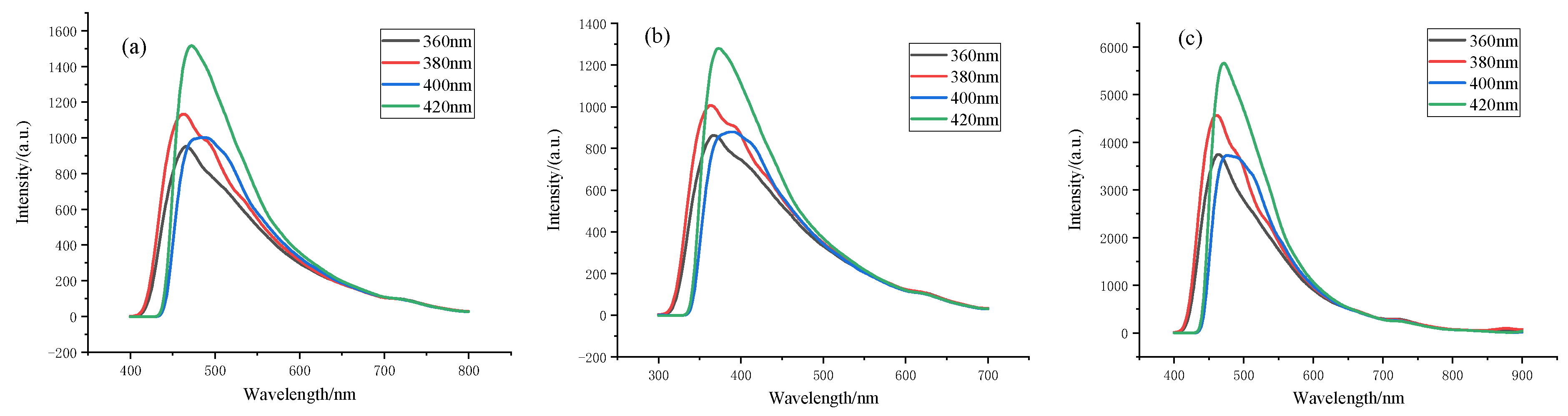
3.2. PL Spectrum Analysis of Spherical Carbon Nanostructures
3.3. Analysis of Graphitization Degree
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubles of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Jiang, J.; He, Y.; Li, S.; Cui, H. Amino acids as the source for producing carbon nanodots: Microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem. Commun. 2012, 48, 9634–9636. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Bai, Y.; Algarni, M. Study on the strengthening mechanisms of Cu/CNT nano-composites. Mater. Sci. Eng. A 2015, 645, 347–356. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, P.V.; Gueorguiev, G.; Stafström, S. Bonding, charge rearrangement and interface dipoles of benzene, graphene, and PAH molecules on Au (1 1 1) and Cu (1 1 1). Carbon 2015, 81, 620–628. [Google Scholar] [CrossRef]
- Xu, K.; Liu, H.; Shi, Y.-C.; You, J.-Y.; Ma, X.-Y.; Cui, H.-J.; Yan, Q.-B.; Chen, G.-C.; Su, G. Preparation of T-carbon by plasma enhanced chemical vapor deposition. Carbon 2020, 157, 270–276. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Hartley, G.S. Aqueous Solutions of Parrifin-Chain Salts; Hermann: Paris, France, 1936. [Google Scholar]
- Marchesan, S. Growth, Properties, and Applications of Branched Carbon Nanostructures. Nanomaterials 2021, 11, 2728. [Google Scholar]
- Monalisa, G.; Mohan Rao, G. Synthesis of vertically aligned and tree-like carbon nanostructures. Carbon 2018, 133, 239–248. [Google Scholar]
- Ramanan, V.; Siddaiah, B.; Raji, K.; Ramamurthy, P. Green synthesis of multifunctionalized, nitrogen-doped, highly fluorescent carbon dots from waste expanded polystyrene and its application in the fluorimetric detection of Au3+ ions in aqueous media. ACS Sustain. Chem. Eng. 2018, 6, 1627–1638. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Pan, J.; Chow, T.P.; Aboalsaud, M.A.; Lai, Z.P.; Sheng, P. Peierls-type metal-insulator transition in carbon nanostructures. Carbon 2021, 172, 106–111. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Sahu, S.K.; Kumer, S. Synthesis of green fluorescent nano carbon spheres using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent nano carbon spheres from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain Specificity in Antimic-robial Activity of Silver and Copper Nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Wang, S.; Wang, X.; Yi, Z.; Yang, H. Photocatalytic activity tuning in a novel Ag2S/ CQDs/CuBi2O 4 composite: Synthesis and photocatalytic mechanism. Mater. Res. Bull. 2019, 115, 140–149. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; Dos Santos, R.B. Self-healing in carbon nitride evidenced as material inflation and superlubric behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–16243. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Wang, M.; Lai, Y.; Fang, J.; Li, J.; Qin, F.; Zhang, K.; Lu, H. N-doped porous carbon derived from biomass as an advanced electrocatalyst for aqueous aluminium/air battery. Int. J. Hydrog. Energy 2015, 40, 16230–16237. [Google Scholar] [CrossRef]
- Andrews, R.J.; Smith, C.F.; Alexander, A.J. Mechanism of carbon nanotube growth from camphor and camphor analogs by chemical vapor deposition. Carbon 2006, 44, 341–347. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D. Raman and X-ray studies of Ce1− x RE x O2− y, where RE = La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, H. Nanoarchitectonics of Carbon Nanostructures: Sodium Dodecyl Sulfonate @ Sodium Chloride System. Nanomaterials 2022, 12, 1652. https://doi.org/10.3390/nano12101652
Chen Q, Li H. Nanoarchitectonics of Carbon Nanostructures: Sodium Dodecyl Sulfonate @ Sodium Chloride System. Nanomaterials. 2022; 12(10):1652. https://doi.org/10.3390/nano12101652
Chicago/Turabian StyleChen, Qi, and Haichao Li. 2022. "Nanoarchitectonics of Carbon Nanostructures: Sodium Dodecyl Sulfonate @ Sodium Chloride System" Nanomaterials 12, no. 10: 1652. https://doi.org/10.3390/nano12101652




