Fabrication of Ultrafine, Highly Ordered Nanostructures Using Carbohydrate-Inorganic Hybrid Block Copolymers
Abstract
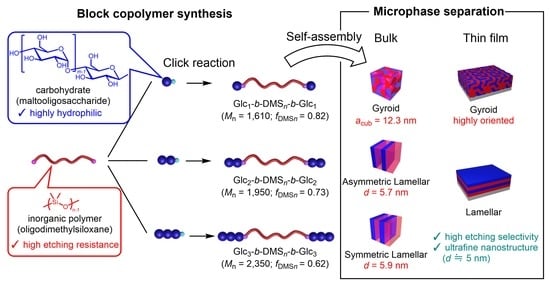
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Thermal Properties
2.3. Microphase Separation Behavior in the Bulk State
2.4. Microphase Separation Behavior in the Thin Film State
2.5. Preparation of GYR Structure Nanoporous Materials
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manabe, K.; Tsai, S.-Y.; Kuretani, S.; Kometani, S.; Ando, K.; Agata, Y.; Ohta, N.; Chiang, Y.-W.; Lin, I.-M.; Fujii, S.; et al. Chiral Silica with Preferred-Handed Helical Structure via Chiral Transfer. JACS Au 2021, 1, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High χ–Low N Block Polymers: How Far Can We Go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Correia, C.G.; Pound-lana, G.; Bézard, P.; Petit-etienne, C.; Gay, G.; Cunge, G.; Cabannes-boué, B.; Navarro, C.; Cayrefourcq, I.; et al. Multifunctional Top-Coats Strategy for DSA of High-χ Block Copolymers. J. Photopolym. Sci. Technol. 2021, 34, 11–16. [Google Scholar] [CrossRef]
- Sun, J.; Lee, C.; Osuji, C.O.; Gopalan, P. Synthesis of High Etch Contrast Poly(3-hydroxystyrene)-Based Triblock Copolymers and Self-Assembly of Sub-5 nm Features. Macromolecules 2021, 54, 9542–9550. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Luo, L.; Li, P.; Liu, Y.; Shen, Z.; Lei, T.; Yang, H.; Fan, X.; Zhou, Q.-F. Sub-5 nm Homeotropically Aligned Columnar Structures of Hybrids Constructed by Porphyrin and Oligo(Dimethylsiloxane). Chem. Commun. 2021, 58, 108–111. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Chevalier, X.; Gomes Correia, C.; Pound-Lana, G.; Bézard, P.; Sérégé, M.; Petit-Etienne, C.; Gay, G.; Cunge, G.; Cabannes-Boué, B.; Nicolet, C.; et al. Lithographically Defined Cross-Linkable Top Coats for Nanomanufacturing with High-χBlock Copolymers. ACS Appl. Mater. Interfaces 2021, 13, 11224–11236. [Google Scholar] [CrossRef]
- Pound-Lana, G.; Bézard, P.; Petit-Etienne, C.; Cavalaglio, S.; Cunge, G.; Cabannes-Boué, B.; Fleury, G.; Chevalier, X.; Zelsmann, M. Dry-Etching Processes for High-Aspect-Ratio Features with Sub-10 nm Resolution High-χ Block Copolymers. ACS Appl. Mater. Interfaces 2021, 13, 49184–49193. [Google Scholar] [CrossRef]
- Cummins, C.; Pino, G.; Mantione, D.; Fleury, G. Engineering block copolymer materials for patterning ultra-low dimensions. Mol. Syst. Des. Eng. 2020, 5, 1642–1657. [Google Scholar] [CrossRef]
- Hirai, T.; Leolukman, M.; Liu, C.C.; Han, E.; Kim, Y.J.; Ishida, Y.; Hayakawa, T.; Kakimoto, M.-A.; Nealey, P.F.; Gopalan, P. One-Step Direct-Patterning Template Utilizing Self-Assembly of POSS-Containing Block Copolymers. Adv. Mater. 2009, 21, 4334–4338. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.-Y.; Yan, X.-Y.; Zhang, W.; Li, X.-H.; Xu, Y.; Dai, S.; Liu, Y.; Zhang, B.-X.; Feng, X.; Yin, J.; et al. Ordered Mesoporous Silica Pyrolyzed from Single-Source Self-Assembled Organic–Inorganic Giant Surfactants. J. Am. Chem. Soc. 2021, 143, 12935–12942. [Google Scholar] [CrossRef] [PubMed]
- Lamers, B.A.G.; Van Der Tol, J.J.B.; Vonk, K.M.; De Waal, B.F.M.; Palmans, A.R.A.; Meijer, E.W.; Vantomme, G. Consequences of Molecular Architecture on the Supramolecular Assembly of Discrete Block Co-oligomers. Macromolecules 2020, 53, 10289–10298. [Google Scholar] [CrossRef]
- Lo, T.-Y.; Krishnan, M.; Lu, K.-Y.; Ho, R.-M. Silicon-containing block copolymers for lithographic applications. Prog. Polym. Sci. 2018, 77, 19–68. [Google Scholar] [CrossRef]
- Rodwogin, M.D.; Spanjers, C.S.; Leighton, C.; Hillmyer, M.A. Polylactide–Poly(dimethylsiloxane)–Polylactide Triblock Copolymers as Multifunctional Materials for Nanolithographic Applications. ACS Nano 2010, 4, 725–732. [Google Scholar] [CrossRef]
- Cushen, J.D.; Bates, C.M.; Rausch, E.L.; Dean, L.M.; Zhou, S.X.; Willson, C.G.; Ellison, C.J. Thin Film Self-Assembly of Poly(Trimethylsilylstyrene-b-D,L-lactide) with Sub-10 nm Domains. Macromolecules 2012, 45, 8722–8728. [Google Scholar] [CrossRef]
- Maher, M.J.; Rettner, C.T.; Bates, C.M.; Blachut, G.; Carlson, M.C.; Durand, W.J.; Ellison, C.J.; Sanders, D.P.; Cheng, J.Y.; Willson, C.G. Directed Self-Assembly of Silicon-Containing Block Copolymer Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 3323–3328. [Google Scholar] [CrossRef]
- Otsuka, I.; Zhang, Y.; Isono, T.; Rochas, C.; Kakuchi, T.; Satoh, T.; Borsali, R. Sub-10 nm Scale Nanostructures in Self-Organized Linear Di- and Triblock Copolymers and Miktoarm Star Copolymers Consisting of Maltoheptaose and Polystyrene. Macromolecules 2015, 48, 1509–1517. [Google Scholar] [CrossRef]
- Luo, Y.; Montarnal, D.; Kim, S.; Shi, W.; Barteau, K.P.; Pester, C.W.; Hustad, P.D.; Christianson, M.D.; Fredrickson, G.H.; Kramer, E.J.; et al. Poly(dimethylsiloxane-b-methyl methacrylate): A Promising Candidate for Sub-10 nm Patterning. Macromolecules 2015, 48, 3422–3430. [Google Scholar] [CrossRef]
- Van Genabeek, B.; de Waal, B.F.M.; Gosens, M.M.J.; Pitet, L.M.; Palmans, A.R.A.; Meijer, E.W. Synthesis and Self-Assembly of Discrete Dimethylsiloxane–Lactic Acid Diblock Co-oligomers: The Dononacontamer and Its Shorter Homologues. J. Am. Chem. Soc. 2016, 138, 4210–4218. [Google Scholar] [CrossRef]
- Van Genabeek, B.; Lamers, B.A.G.; de Waal, B.F.M.; van Son, M.H.C.; Palmans, A.R.A.; Meijer, E.W. Amplifying (Im)perfection: The Impact of Crystallinity in Discrete and Disperse Block Co-oligomers. J. Am. Chem. Soc. 2017, 139, 14869–14872. [Google Scholar] [CrossRef] [PubMed]
- Van Genabeek, B.B.; De Waal, B.F.M.; Ligt, B.; Palmans, A.R.A.; Meijer, E.B. Dispersity under Scrutiny: Phase Behavior Differences between Disperse and Discrete Low Molecular Weight Block Co-Oligomers. ACS Macro Lett. 2017, 6, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Lamers, B.A.G.; de Waal, B.F.M.; Meijer, E.W. The iterative synthesis of discrete dimethylsiloxane oligomers: A practical guide. J. Polym. Sci. 2020, 59, 1142–1150. [Google Scholar] [CrossRef]
- Berrocal, J.A.; Zha, R.; De Waal, B.F.M.; Lugger, J.A.M.; Lutz, M.; Meijer, E.B. Unraveling the Driving Forces in the Self-Assembly of Monodisperse Naphthalenediimide-Oligodimethylsiloxane Block Molecules. ACS Nano 2017, 11, 3733–3741. [Google Scholar] [CrossRef]
- Berrocal, J.A.; Heideman, G.H.; de Waal, B.F.M.; Enache, M.; Havenith, R.W.A.; Stöhr, M.; Meijer, E.W.; Feringa, B.L. Engineering Long-Range Order in Supramolecular Assemblies on Surfaces: The Paramount Role of Internal Double Bonds in Discrete Long-Chain Naphthalenediimides. J. Am. Chem. Soc. 2020, 142, 4070–4078. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Sun, J.; Choo, Y.; Rokhlenko, Y.; Dwyer, J.H.; Schweitzer, B.; Hayakawa, T.; Osuji, C.O.; Gopalan, P. Self-Assembly of an Ultrahigh-χ Block Copolymer with Versatile Etch Selectivity. Macromolecules 2018, 51, 6460–6467. [Google Scholar] [CrossRef]
- Yu, X.; Yue, K.; Hsieh, I.-F.; Li, Y.; Dong, X.-H.; Liu, C.; Xin, Y.; Wang, H.-F.; Shi, A.-C.; Newkome, G.R.; et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Natl. Acad. Sci. USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ishida, Y.; Maeda, R.; Tokita, M.; Hayakawa, T. Alkylated cage silsesquioxanes: A comprehensive study of thermal properties and self-assembled structure. RSC Adv. 2014, 4, 34981–34986. [Google Scholar] [CrossRef]
- Wang, L.; Ishida, Y.; Maeda, R.; Tokita, M.; Horiuchi, S.; Hayakawa, T. Alkylated Cage Silsesquioxane Forming a Long-Range Straight Ordered Hierarchical Lamellar Nanostructure. Langmuir 2014, 30, 9797–9803. [Google Scholar] [CrossRef]
- Young, W.; Epps, T.H. Salt Doping in PEO-Containing Block Copolymers: Counterion and Concentration Effects. Macromolecules 2009, 42, 2672–2678. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Xu, J.; Kim, B.; Hong, S.W.; Jeong, U.; Xu, T.; Russell, T.P. Macroscopic 10-Terabit–per–Square-Inch Arrays from Block Copolymers with Lateral Order. Science 2009, 323, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, Z.; Zhang, W.; Choi, J.; Huang, C.; Jeong, G.; Coughlin, E.B.; Hsu, Y.; Yang, X.; Lee, K.Y.; et al. Directed Self-Assembly of Poly(2-vinylpyridine)-b-polystyrene-b-poly(2-vinylpyridine) Triblock Copolymer with Sub-15 nm Spacing Line Patterns Using a Nanoimprinted Photoresist Template. Adv. Mater. 2015, 27, 4364–4370. [Google Scholar] [CrossRef]
- Isono, T.; Kawakami, N.; Watanabe, K.; Yoshida, K.; Otsuka, I.; Mamiya, H.; Ito, H.; Yamamoto, T.; Tajima, K.; Borsali, R.; et al. Microphase separation of carbohydrate-based star-block copolymers with sub-10 nm periodicity. Polym. Chem. 2019, 10, 1119–1129. [Google Scholar] [CrossRef]
- Isono, T.; Otsuka, I.; Kondo, Y.; Halila, S.; Fort, S.; Rochas, C.; Satoh, T.; Borsali, R.; Kakuchi, T. Sub-10 nm Nano-Organization in AB2- and AB3-Type Miktoarm Star Copolymers Consisting of Maltoheptaose and Polycaprolactone. Macromolecules 2013, 46, 1461–1469. [Google Scholar] [CrossRef]
- Nowak, S.R.; Lachmayr, K.K.; Yager, K.G.; Sita, L.R. Stable Thermotropic 3D and 2D Double Gyroid Nanostructures with Sub-2-nm Feature Size from Scalable Sugar–Polyolefin Conjugates. Angew. Chem. Int. Ed. 2021, 60, 8710–8716. [Google Scholar] [CrossRef] [PubMed]
- Isono, T.; Komaki, R.; Lee, C.; Kawakami, N.; Ree, B.J.; Watanabe, K.; Yoshida, K.; Mamiya, H.; Yamamoto, T.; Borsali, R.; et al. Rapid access to discrete and monodisperse block co-oligomers from sugar and terpenoid toward ultrasmall periodic nanostructures. Commun. Chem. 2020, 3, 135. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, S.; Yamamoto, T.; Tajima, K.; Borsali, R.; Isono, T.; Satoh, T. Chain-End Functionalization with a Saccharide for 10 nm Microphase Separation: “Classical” PS-b-PMMA versus PS-b-PMMA-Saccharide. Macromolecules 2018, 51, 8870–8877. [Google Scholar] [CrossRef]
- Otsuka, I.; Isono, T.; Rochas, C.; Halila, S.; Fort, S.; Satoh, T.; Kakuchi, T.; Borsali, R. 10 nm Scale Cylinder–Cubic Phase Transition Induced by Caramelization in Sugar-Based Block Copolymers. ACS Macro Lett. 2012, 1, 1379–1382. [Google Scholar] [CrossRef]
- Isono, T.; Nakahira, S.; Hsieh, H.-C.; Katsuhara, S.; Mamiya, H.; Yamamoto, T.; Chen, W.-C.; Borsali, R.; Tajima, K.; Satoh, T. Carbohydrates as Hard Segments for Sustainable Elastomers: Carbohydrates Direct the Self-Assembly and Mechanical Properties of Fully Bio-Based Block Copolymers. Macromolecules 2020, 53, 5408–5417. [Google Scholar] [CrossRef]
- Katsuhara, S.; Mamiya, H.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. Metallopolymer-block-oligosaccharide for sub-10 nm microphase separation. Polym. Chem. 2020, 11, 2995–3002. [Google Scholar] [CrossRef]
- Seo, M.; Kim, H.; Lee, E.; Li, S. Ordered Microdomain Structures in Saccharide–Polystyrene–Saccharide Hybrid Conjugates. Biomacromolecules 2021, 22, 4659–4668. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.R.; Hwang, W.; Sita, L.R. Dynamic Sub-10-nm Nanostructured Ultrathin Films of Sugar–Polyolefin Conjugates Thermoresponsive at Physiological Temperatures. J. Am. Chem. Soc. 2017, 139, 5281–5284. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.S.; Hwang, W.; Sita, L.R. End-Group-Functionalized Poly(α-olefinates) as Non-Polar Building Blocks: Self-Assembly of Sugar–Polyolefin Hybrid Conjugates. Angew. Chem. Int. Ed. 2016, 55, 4683–4687. [Google Scholar] [CrossRef] [PubMed]
- Togashi, D.; Otsuka, I.; Borsali, R.; Takeda, K.; Enomoto, K.; Kawaguchi, S.; Narumi, A. Maltopentaose-Conjugated CTA for RAFT Polymerization Generating Nanostructured Bioresource-Block Copolymer. Biomacromolecules 2014, 15, 4509–4519. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, W.-C.; Borsali, R. Carbohydrate-Based Block Copolymer Thin Films: Ultrafast Nano-Organization with 7 nm Resolution Using Microwave Energy. Adv. Mater. 2017, 29, 1701645. [Google Scholar] [CrossRef]
- Otsuka, I.; Fuchise, K.; Halila, S.; Fort, S.; Aissou, K.; Pignot-Paintrand, I.; Chen, Y.; Narumi, A.; Kakuchi, T.; Borsali, R. Thermoresponsive Vesicular Morphologies Obtained by Self-Assemblies of Hybrid Oligosaccharide-block-poly(N-isopropylacrylamide) Copolymer Systems. Langmuir 2009, 26, 2325–2332. [Google Scholar] [CrossRef]
- Ree, B.J.; Satoh, Y.; Isono, T.; Satoh, T. Bicyclic Topology Transforms Self-Assembled Nanostructures in Block Copolymer Thin Films. Nano Lett. 2020, 20, 6520–6525. [Google Scholar] [CrossRef]
- Saito, K.; Isono, T.; Sun, H.-S.; Kakuchi, T.; Chen, W.-C.; Satoh, T. Rod–coil type miktoarm star copolymers consisting of polyfluorene and polylactide: Precise synthesis and structure–morphology relationship. Polym. Chem. 2015, 6, 6959–6972. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Kaizawa, N.; Ree, B.J.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. One-Shot Intrablock Cross-Linking of Linear Diblock Copolymer to Realize Janus-Shaped Single-Chain Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 18122–18128. [Google Scholar] [CrossRef]
- Watanabe, K.; Katsuhara, S.; Mamiya, H.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. Downsizing feature of microphase-separated structures via intramolecular crosslinking of block copolymers. Chem. Sci. 2019, 10, 3330–3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Katsuhara, S.; Mamiya, H.; Kawamura, Y.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. Highly asymmetric lamellar nanostructures from nanoparticle–linear hybrid block copolymers. Nanoscale 2020, 12, 16526–16534. [Google Scholar] [CrossRef] [PubMed]
- Poelma, J.E.; Ono, K.; Miyajima, D.; Aida, T.; Satoh, K.; Hawker, C.J. Cyclic Block Copolymers for Controlling Feature Sizes in Block Copolymer Lithography. ACS Nano 2012, 6, 10845–10854. [Google Scholar] [CrossRef]
- Ree, B.J.; Satoh, Y.; Isono, T.; Satoh, T. Highly Ordered Nanoscale Film Morphologies of Block Copolymers Governed by Nonlinear Topologies. ACS Macro Lett. 2021, 10, 811–818. [Google Scholar] [CrossRef]
- Ree, B.J.; Satoh, Y.; Isono, T.; Satoh, T. Correlations of nanoscale film morphologies and topological confinement of three-armed cage block copolymers. Polym. Chem. 2021, 12, 3451–3460. [Google Scholar] [CrossRef]
- Ree, B.J.; Satoh, Y.; Isono, T.; Satoh, T. Influence of Topological Confinement on Nanoscale Film Morphologies of Tricyclic Block Copolymers. Macromolecules 2021, 54, 4120–4127. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.; Lazzari, M. Polydimethylsiloxane thermal degradation Part Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, J.K.; Zhao, B.; Duan, C.; Li, W. Morphology Transitions of Linear A1B1A2B2 Tetrablock Copolymers at Symmetric Overall Volume Fraction. Macromolecules 2018, 51, 4415–4421. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Thomas, E.L.; Anderson, D.M.; Henkee, C.S.; Hoffman, D.J. Periodic area-minimizing surfaces in block copolymers. Nature 1988, 334, 598–601. [Google Scholar] [CrossRef]
- Lee, B.; Park, I.; Yoon, J.; Park, S.; Kim, J.; Kim, K.-W.; Chang, A.T.; Ree, M. Structural Analysis of Block Copolymer Thin Films with Grazing Incidence Small-Angle X-ray Scattering. Macromolecules 2005, 38, 4311–4323. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Zhang, H.; Kang, D.-J.; Kahng, S.-H.; Tall, B.D.; Lee, N.Y. Fabrication of Polymerase Chain Reaction Plastic Lab-on-a-Chip Device for Rapid Molecular Diagnoses. Int. Neurourol. J. 2016, 20, S38–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yu, B.; Jiang, S.; Jiang, L.; Qian, L. UV/ozone-assisted tribochemistry-induced nanofabrication on Si(100) surfaces. RSC Adv. 2017, 7, 39651–39656. [Google Scholar] [CrossRef] [Green Version]





| Sample | Mn,NMRa | Ð b | fDMSnc | Bulk State Morphology | ||
|---|---|---|---|---|---|---|
| Annealing Condition | Morphology | D d/nm | ||||
| Glc1-b-DMSn-b-Glc1 | 1610 | 1.08 | 0.82 | non | GYR | 5.0 e |
| 80 °C, 6 h | GYR | 5.0 e | ||||
| 130 °C, 6 h | GYR | 5.0 e | ||||
| Glc2-b-DMSn-b-Glc2 | 1950 | 1.08 | 0.73 | non | LAM | 5.6 |
| 80 °C, 6 h | LAM | 5.7 | ||||
| 130 °C, 6 h | LAM | 5.7 | ||||
| Glc3-b-DMSn-b-Glc3 | 2350 | 1.12 | 0.62 | non | LAM | 5.9 |
| 80 °C, 6 h | LAM | 5.9 | ||||
| 130 °C, 6 h | LAM | 5.9 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, T.; Katsuhara, S.; Lee, C.; Ree, B.J.; Borsali, R.; Yamamoto, T.; Tajima, K.; Satoh, T.; Isono, T. Fabrication of Ultrafine, Highly Ordered Nanostructures Using Carbohydrate-Inorganic Hybrid Block Copolymers. Nanomaterials 2022, 12, 1653. https://doi.org/10.3390/nano12101653
Nishimura T, Katsuhara S, Lee C, Ree BJ, Borsali R, Yamamoto T, Tajima K, Satoh T, Isono T. Fabrication of Ultrafine, Highly Ordered Nanostructures Using Carbohydrate-Inorganic Hybrid Block Copolymers. Nanomaterials. 2022; 12(10):1653. https://doi.org/10.3390/nano12101653
Chicago/Turabian StyleNishimura, Taiki, Satoshi Katsuhara, Chaehun Lee, Brian J. Ree, Redouane Borsali, Takuya Yamamoto, Kenji Tajima, Toshifumi Satoh, and Takuya Isono. 2022. "Fabrication of Ultrafine, Highly Ordered Nanostructures Using Carbohydrate-Inorganic Hybrid Block Copolymers" Nanomaterials 12, no. 10: 1653. https://doi.org/10.3390/nano12101653
APA StyleNishimura, T., Katsuhara, S., Lee, C., Ree, B. J., Borsali, R., Yamamoto, T., Tajima, K., Satoh, T., & Isono, T. (2022). Fabrication of Ultrafine, Highly Ordered Nanostructures Using Carbohydrate-Inorganic Hybrid Block Copolymers. Nanomaterials, 12(10), 1653. https://doi.org/10.3390/nano12101653