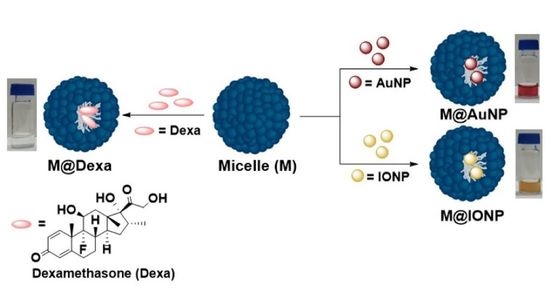
Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization Methods
- −
- Nuclear magnetic resonance (NMR) spectra were recorded on a BRUKER AMX-500 apparatus (Billerica, MA, USA). Deuterated chloroform, water, dimethylsulfoxide and methanol were used and indicated in parentheses for each compound. The chemical shift values (δ) were referred to tetramethylsilane used as an internal reference.
- −
- High resolution mass spectrometry (HRMS) was recorded on a Q Exactive Hybrid Quadrupole-Orbitrap apparatus from ThermoFisher Scientific (Waltham, MA, USA).
- −
- Transmission electron microscopy (TEM) images were obtained on an HR Fei Talos 200X microscope (Waltham, MA, USA) operated at an accelerating voltage of 100 kV. TEM samples were prepared by dropping a solution of the corresponding nanosystem onto a carbon-coated copper grid without staining. The mean sizes were calculated on an average of 100 nanoparticles measured.
- −
- The intensity distribution of the nanosystems was measured on a Zetasizer Nano ZS90 from Malvern Panalytical (Malvern, UK). The dynamic light scattering (DLS) measurements were performed on a cell type: ZEN0040 disposable cuvette, setting a refractive index of 2.3 for iron oxide NPs, 0.2 for the AuNPs and 1.40 for the micelles without metallic NPs. The measurement duration was set as automatic and 3 as the number of measurements. As analysis model, the general purpose (normal resolution) was chosen. The nanosystems were suspended in the corresponding solvent at a concentration of 1 mg/L of micelles and/or metallic NP.
- −
- HPLC was performed on a reversed phase column ZORBAX Eclipse XDB-C18, Narrow-Bore 2.1 × 150 mm, 3.5 Micron from Agilent (Santa Clara, CA, USA). The elution was carried out using water, 0.1% FA (A)-methanol, 0.1% FA (B) with the following elution gradient: 20% B (0.5 min), linear gradient from 20 to 100% of B for 11.5 min, isocratic gradient to 100% of B (1.5 min) and return to initial conditions (20% of B) for up to 15 min with constant flow of 0.3 mL/min and injection volume of 10 µL.
2.2. Encapsulation and Release of Dexamethasone
2.3. Synthesis of Iron Oxide NPs
2.4. Synthesis of Au NPs
2.5. Functionalization of Metallic NPs
3. Results and Discussion
3.1. Loading and Drug Release
3.2. Functionalization of Metallic NPs
3.3. Characterization of the Functionalized Metallic NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Liu, T.; Chen, H.; Yin, B.; Zhang, Z.; Russell, T.P.; Shi, S. Molecular Brush Surfactants: Versatile Emulsifiers for Stabilizing and Structuring Liquids. Angew. Chem. 2021, 133, 19778–19782. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanodrug Delivery Systems: A Promising Technology for Detection, Diagnosis, and Treatment of Cancer. AAPS PharmSciTech 2014, 15, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Yergeri, M.; Kapse-Mistry, S.; Srivastava, R.; Govender, T. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 159. [Google Scholar] [CrossRef] [Green Version]
- Renugalakshmi, A.; Vinothkumar, T.S.; Kandaswamy, D. Nanodrug delivery systems in dentistry: A review on current status and future perspectives. Curr. Drug Deliv. 2011, 8, 586–594. [Google Scholar] [CrossRef]
- Cihan, E.; Polat, M.; Polat, H. Designing of spherical chitosan nano-shells with micellar cores for solvation and safeguarded delivery of strongly lipophilic drugs. Colloids Surf. A 2017, 529, 815. [Google Scholar] [CrossRef]
- Li, R.; Xie, Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release 2017, 251, 49. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of curcumin loaded nanostructured lipid carrier based thermosensitive in situ gel for dermal delivery. Colloids Surf. A 2016, 506, 356. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [Green Version]
- Boullanger, P. ChemInform Abstract: Amphiphilic Carbohydrates as a Tool for Molecular Recognition in Organized Systems. ChemInform 2010, 28, 275–312. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J. Molecular Biology of the Cell, 4th ed.; Taylor & Francis: San Francisco, CA, USA, 2002. [Google Scholar]
- Mavossaghian, S.; Merkel, M.O.; Torchilin, P.V. Applications of polymer micelles for imaging and drug delivery. WIREs Nanomed. Nanobiotechnol. 2015, 7, 691. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.I. Development of copolymers of poly(D,L-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Biointerfaces 1999, 16, 161. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Zhao, J.; Pang, X.; Xi, Y.; Zhai, G. Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloids Surf. B Biointerfaces 2014, 121, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Nag, M.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Transferrin functionalized chitosan-PEG nanoparticles for targeted delivery of paclitaxel to cancer cells. Coll. Surf. B Biointerfaces 2016, 148, 363–370. [Google Scholar] [CrossRef]
- David, A. Peptide ligand-modified nanomedicines for targeting cells at the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 119, 120–142. [Google Scholar] [CrossRef]
- Alibolandi, M.; Ramezani, M.; Abnous, K.; Sadeghi, F.; Atyabi, F.; Asouri, M.; Ahmadi, A.A.; Hadizadeh, F. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J. Control. Release 2015, 209, 88–100. [Google Scholar] [CrossRef]
- Han, Y.; An, Y.; Jia, G.; Wang, X.; He, C.; Ding, Y.; Tang, Q. Theranostic micelles based on up conversion nanoparticles for dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Nanoscale 2018, 10, 6511. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yang, C.; Xue, Z.; Huang, Y.; Luo, H.; Zu, X.; Zhang, L.; Yi, G. Controlled construction of gold nanoparticles in situ from β-cyclodextrin based unimolecular micelles for in vitro computed tomography imaging. J. Coll. Interface Sci. 2018, 528, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.P.; Muñoz-Hernández, C.; Berry, C.C.; García-Martín, M.L. In vivo pharmacokinetics of T2 contrast agents based on iron oxide nanoparticles: Optimization of blood circulation times. RSC Adv. 2015, 5, 76883–76891. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Dalmases, M.; Figuerola, A.; García-Martín, M.L.; Leal, M.P. Highly water-stable rare ternary Ag–Au–Se nanocomposites as long blood circulation time X-ray computed tomography contrast agents. Nanoscale 2017, 9, 7242–7251. [Google Scholar] [CrossRef] [Green Version]
- Zalipsky, S.; Brandeis, E.; Newman, M.S.; Woodle, M.C. Long circulating, cationic liposomes containing amino-PEG-phosphatidylethanolamine. FEBS Lett. 1994, 353, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Lou, B.; Zhao, J.; Jin, R.; Zhao, P.; Lic, J.; Ren, J. Self-assembled micelles of PEG–poly(disulphide carbamate amine) copolymers for intracellular dual-responsive drug delivery. J. Mater. Chem. B 2016, 4, 902–909. [Google Scholar] [CrossRef]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Leal, M.P.; García-Martín, M.L. Comprehensive Toxicity Assessment of PEGylated Magnetic Nanoparticles for in vivo applications. Coll. Surf. B Biointerfaces 2019, 177, 253–259. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.; Prasad, P.N.; Mark, T. Synthesis of Monodisperse Au, Ag, and Au–Ag Alloy Nanoparticles with Tunable Size and Surface Plasmon Resonance Frequency. Chem. Mater. 2011, 23, 4098. [Google Scholar] [CrossRef]
- Valdivia, V.; Paggiaro, C.; Fernández, I. Synthesis and Characterization of New Biocompatible Amino Amphiphilic Compounds Derived from Oleic Acid as Nanovectors for Drug Delivery. Proceedings 2019, 41, 1. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Torres, E.; Caro, C.; Avasthi, A.; Páez-Muñoz, J.M.; García-Martín, M.L.; Fernández, I.; Leal, M.P. Clickable iron oxide NPs based on catechol derived ligands: Synthesis and characterization. Soft Matter 2020, 16, 3257–3266. [Google Scholar] [CrossRef]
- Kang, W.; Zhu, T.; Wang, P.; Hou, X.; Zhao, Y.; Zhang, X.; Yang, H. A pH-responsive anionic wormlike micelle based on ion release effect of phosphate. J. Mol. Liq. 2019, 286, 110946. [Google Scholar] [CrossRef]
- Gessi, S.; Merighi, S.; Borea, P.A. Glucocorticoids Pharmacology: Past, Present and Future. Curr. Pharm. Des. 2010, 16, 3540–3553. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ghafoor, D.; Khan, A.; Ualiyeva, D.; Khan, S.; Bilal, H.; Khan, B.; Sajjad, W. Diagnostic approaches and potential therapeutic options for coronavirus disease 2019. New Microbes New Infect. 2020, 38, 100770. [Google Scholar] [CrossRef] [PubMed]






| M3@Dexa | Size (nm) | Std. Dev. | PDI |
|---|---|---|---|
| pH 10 | 884 | 182.3 | 0.6 |
| pH 7 | 98.5 | 1.6 | 0.37 |
| pH 3 | 43.1 | 0.8 | 0.38 |
| Size (nm) | PDI | Std. Dev. | |
|---|---|---|---|
| M3@AuNPs | 240.7 | 0.25 | 7.4 |
| M3@IONPs | 101.6 | 0.18 | 1.4 |
| AuNPs | 14.6 | 0.01 | 0.04 |
| IONPs | 29.9 | 0.22 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivia, V.; Gimeno-Ferrero, R.; Pernia Leal, M.; Paggiaro, C.; Fernández-Romero, A.M.; González-Rodríguez, M.L.; Fernández, I. Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles. Nanomaterials 2022, 12, 1753. https://doi.org/10.3390/nano12101753
Valdivia V, Gimeno-Ferrero R, Pernia Leal M, Paggiaro C, Fernández-Romero AM, González-Rodríguez ML, Fernández I. Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles. Nanomaterials. 2022; 12(10):1753. https://doi.org/10.3390/nano12101753
Chicago/Turabian StyleValdivia, Victoria, Raúl Gimeno-Ferrero, Manuel Pernia Leal, Chiara Paggiaro, Ana María Fernández-Romero, María Luisa González-Rodríguez, and Inmaculada Fernández. 2022. "Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles" Nanomaterials 12, no. 10: 1753. https://doi.org/10.3390/nano12101753
APA StyleValdivia, V., Gimeno-Ferrero, R., Pernia Leal, M., Paggiaro, C., Fernández-Romero, A. M., González-Rodríguez, M. L., & Fernández, I. (2022). Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles. Nanomaterials, 12(10), 1753. https://doi.org/10.3390/nano12101753