Photocatalytic Reduction of CO2 with N-Doped TiO2-Based Photocatalysts Obtained in One-Pot Supercritical Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Catalysts
2.3. Characterization of Catalysts
2.4. Photocatalytic Reaction Tests
3. Results
3.1. N Content of the N-Doped Catalysts
3.2. Photocatalytic Activities
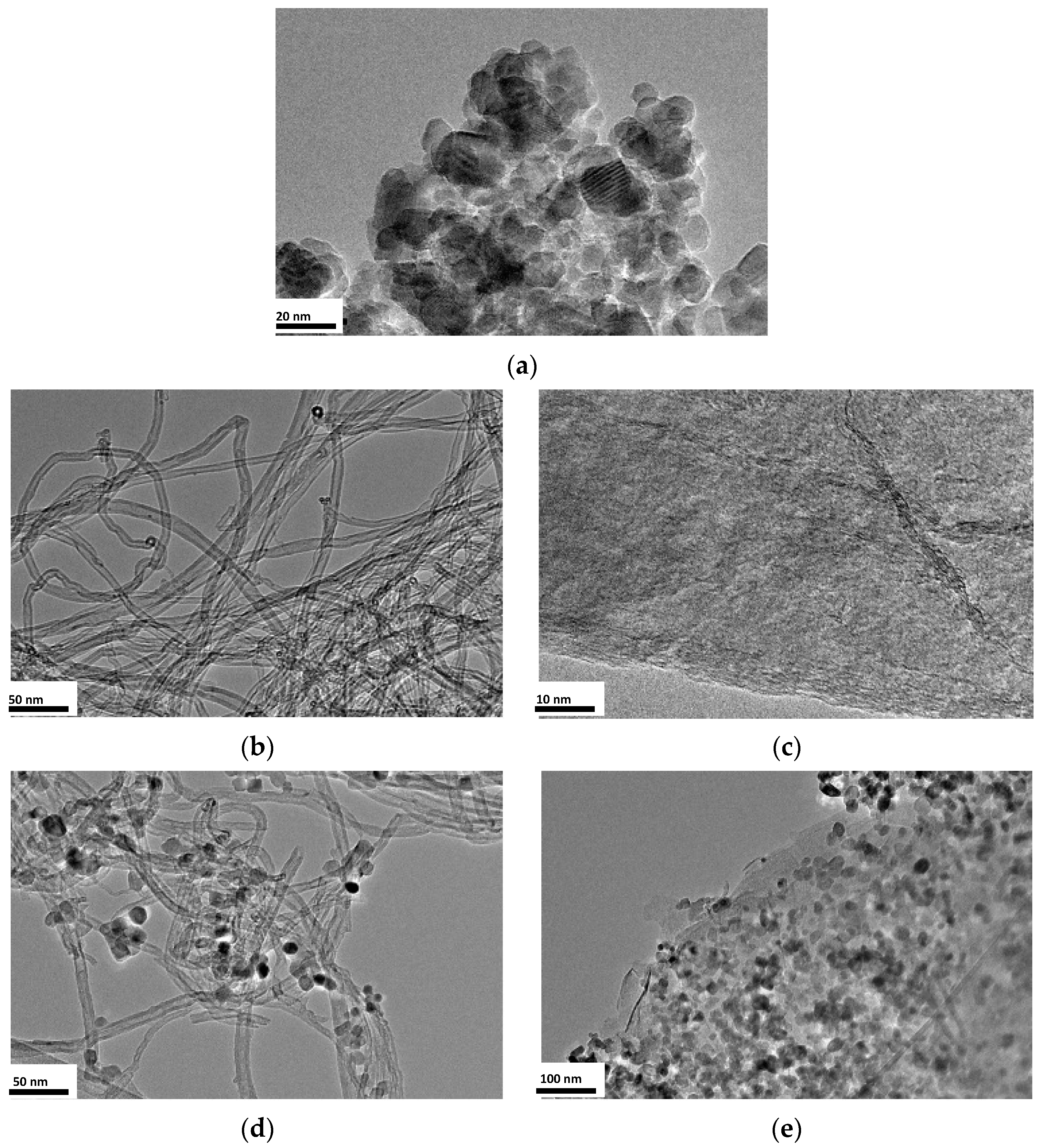
3.3. Surface Morphology Analysis (TEM)
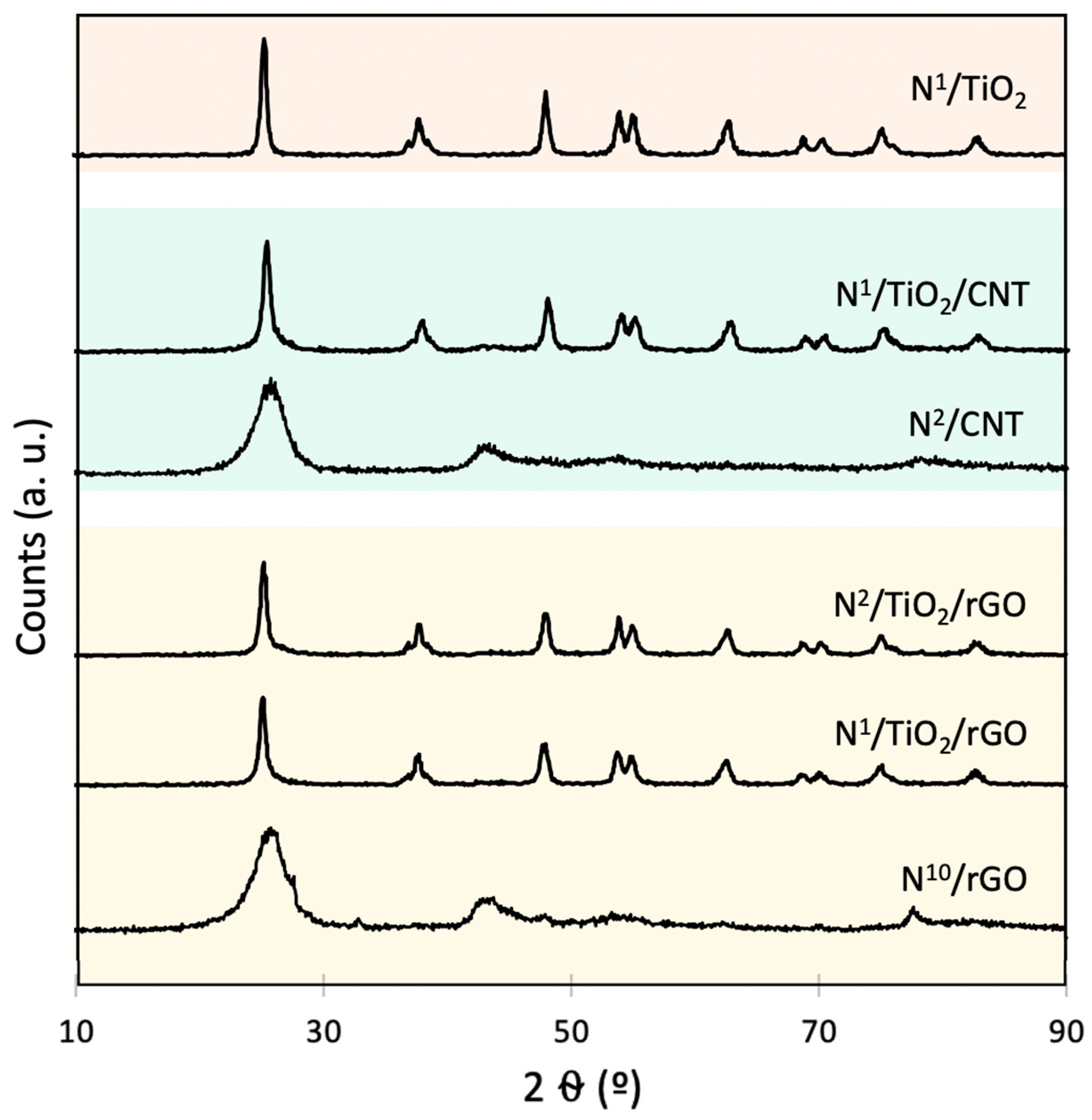
3.4. Crystalline Structure Analysis (XRD)
3.5. Surface Area Analysis (BET)
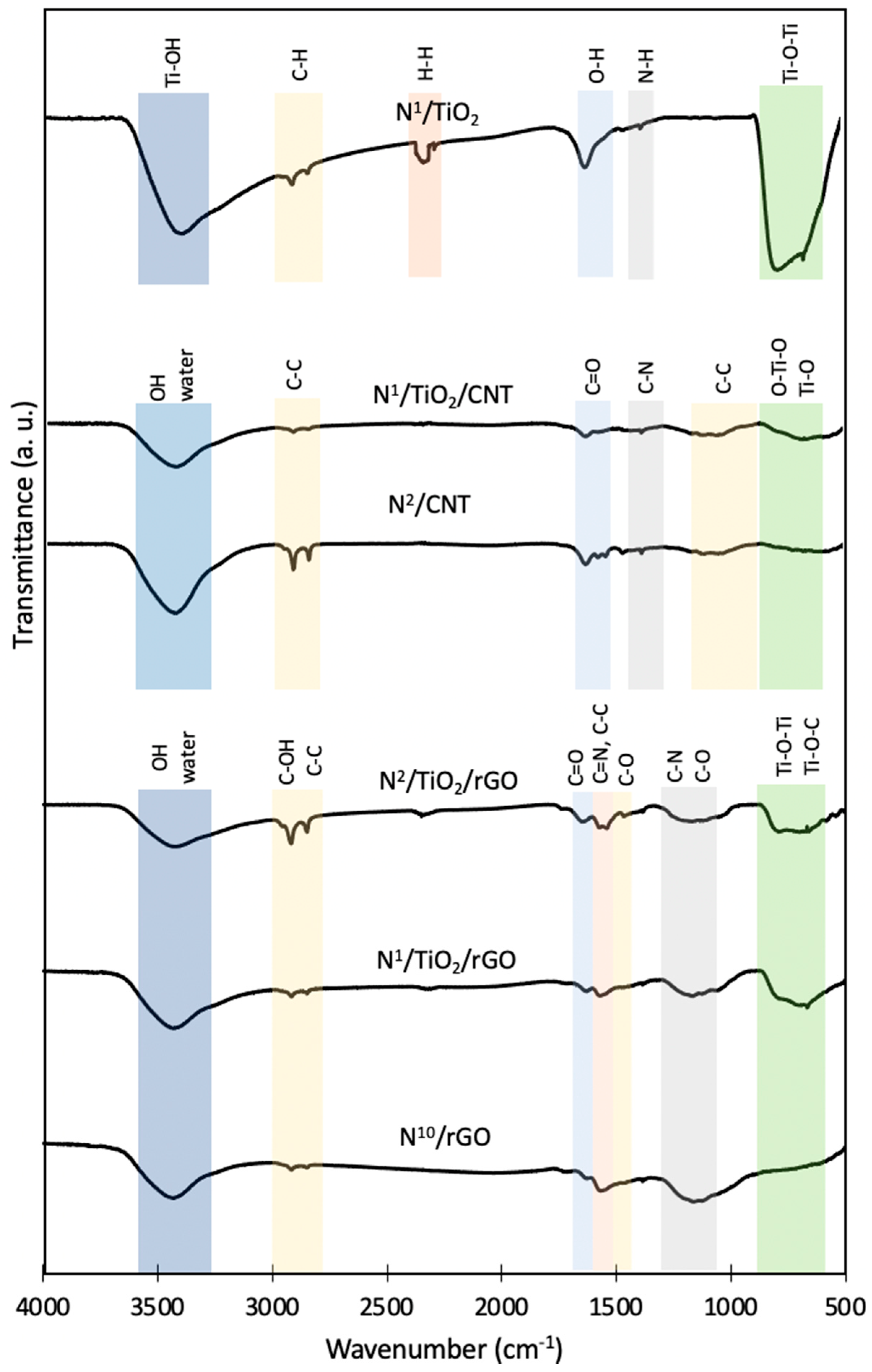
3.6. Surface Functional Groups Analysis (FTIR)
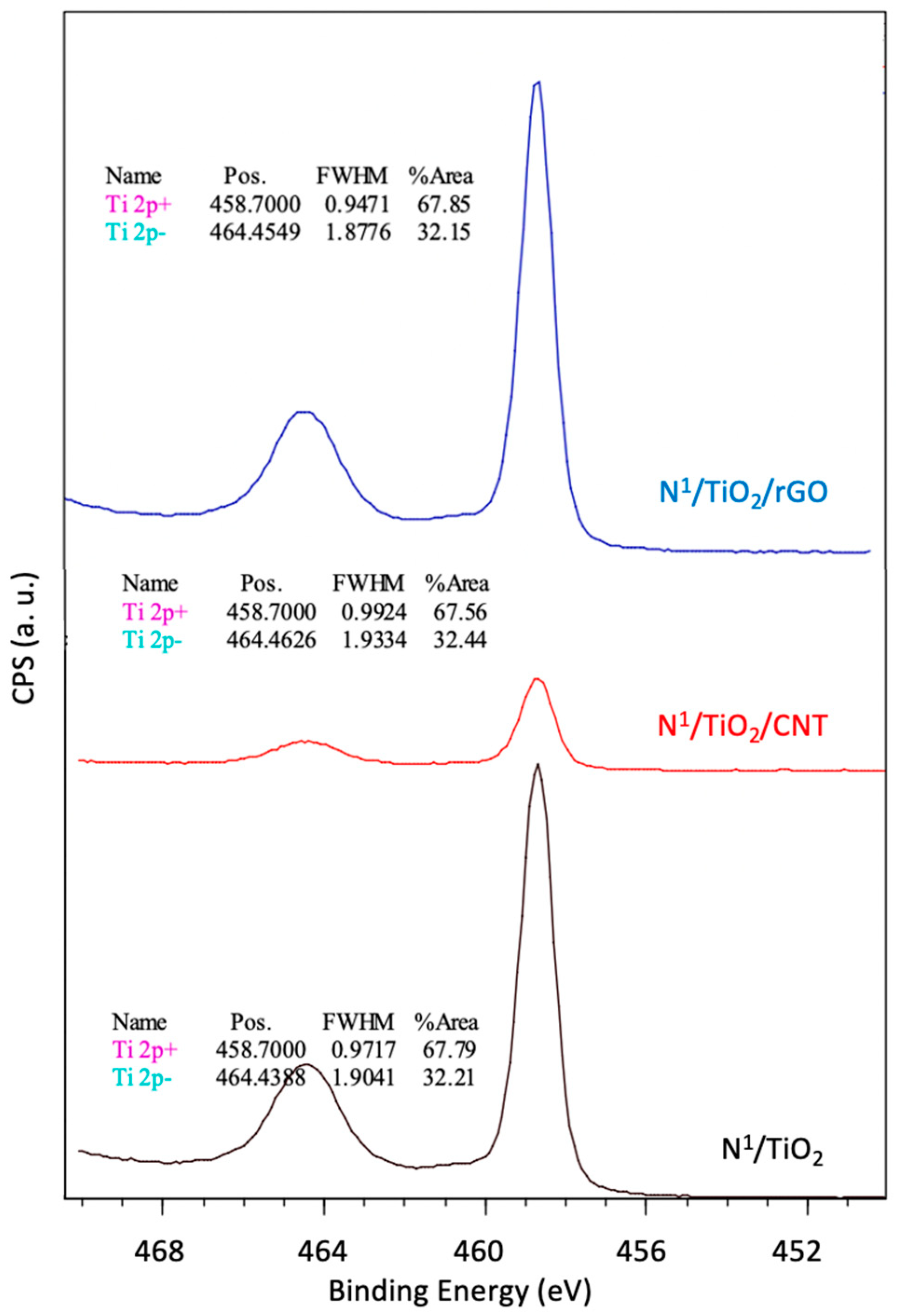
3.7. Surface Chemical Analysis (XPS)
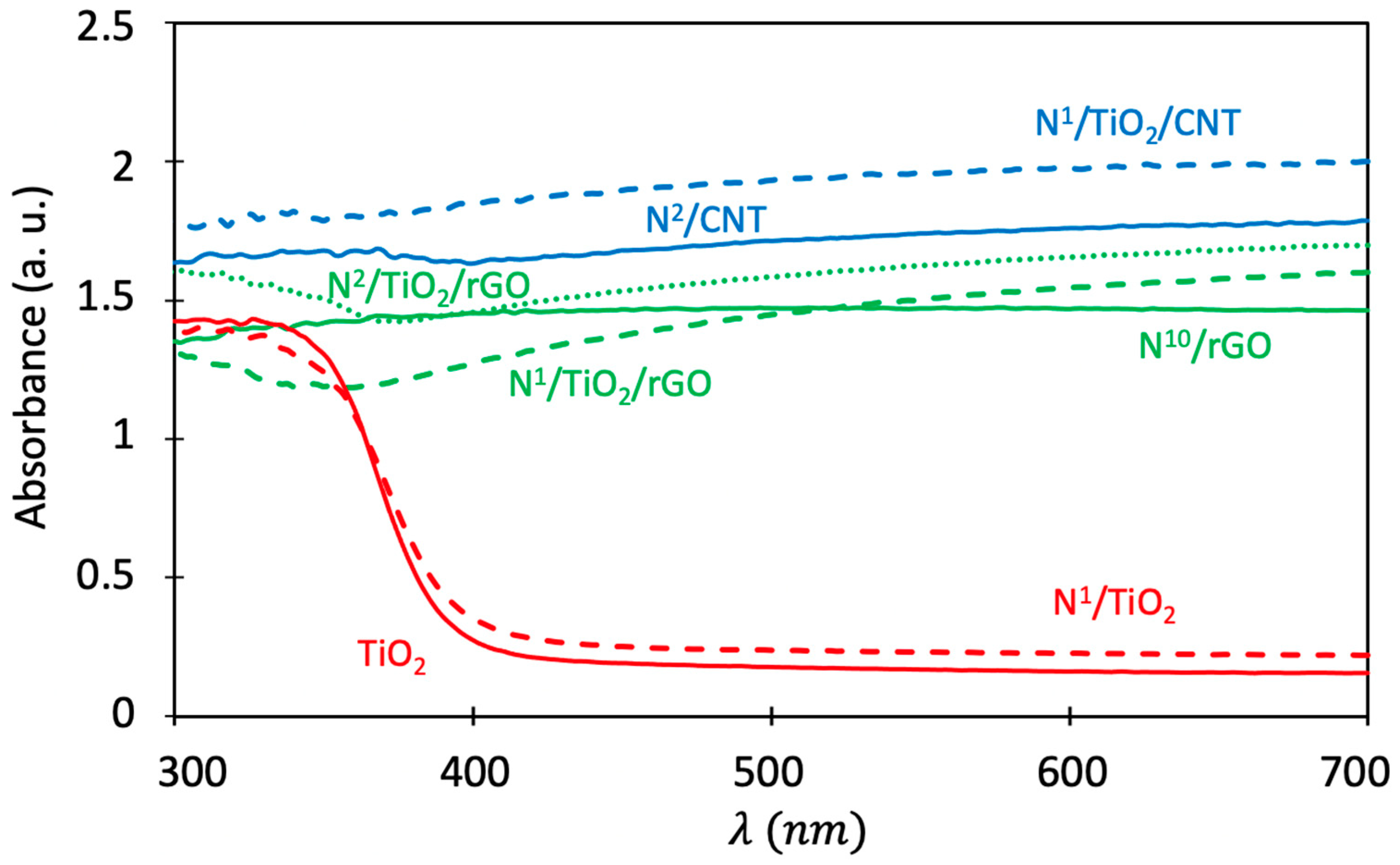
3.8. Optical Properties Analysis (DRS)
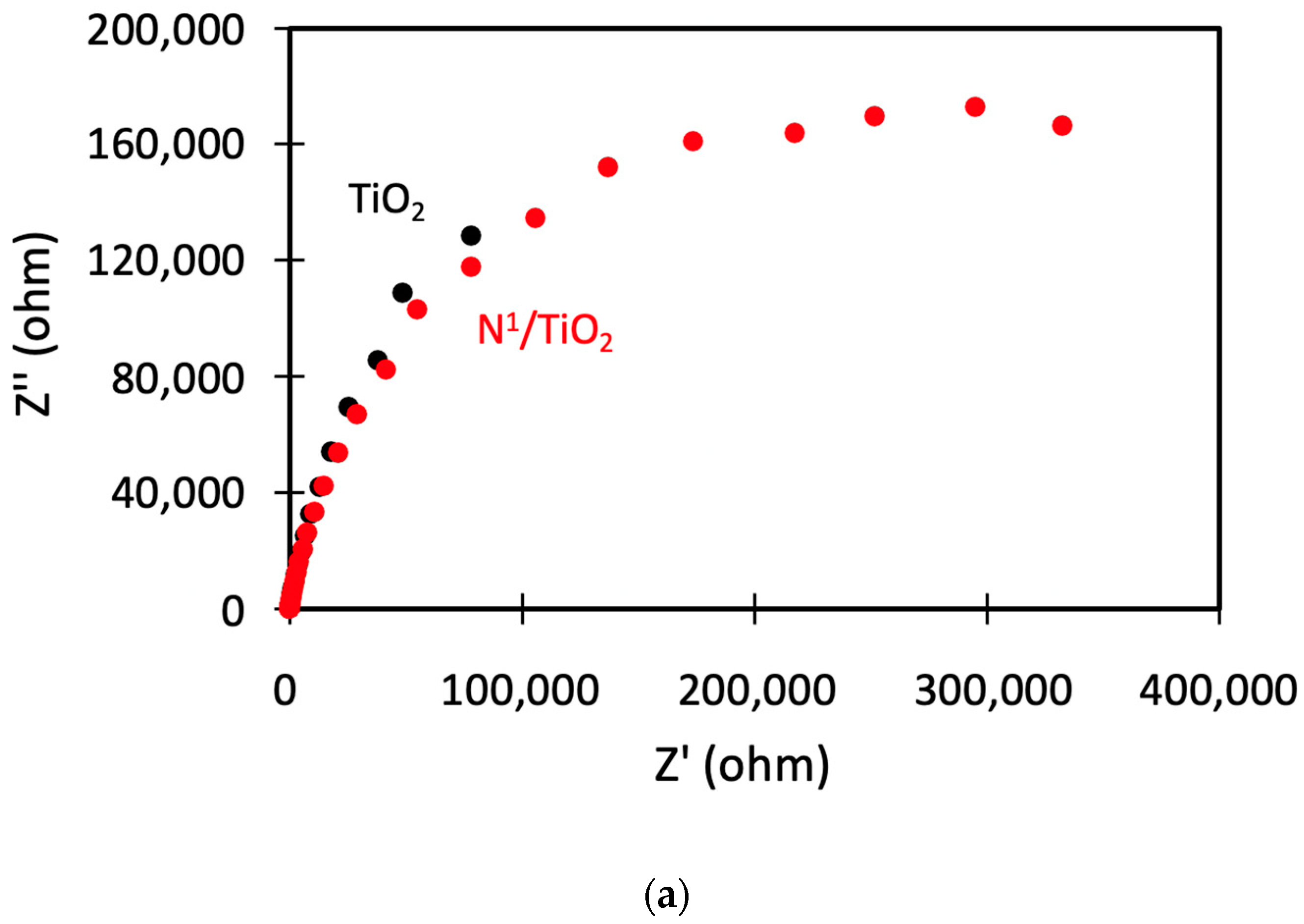
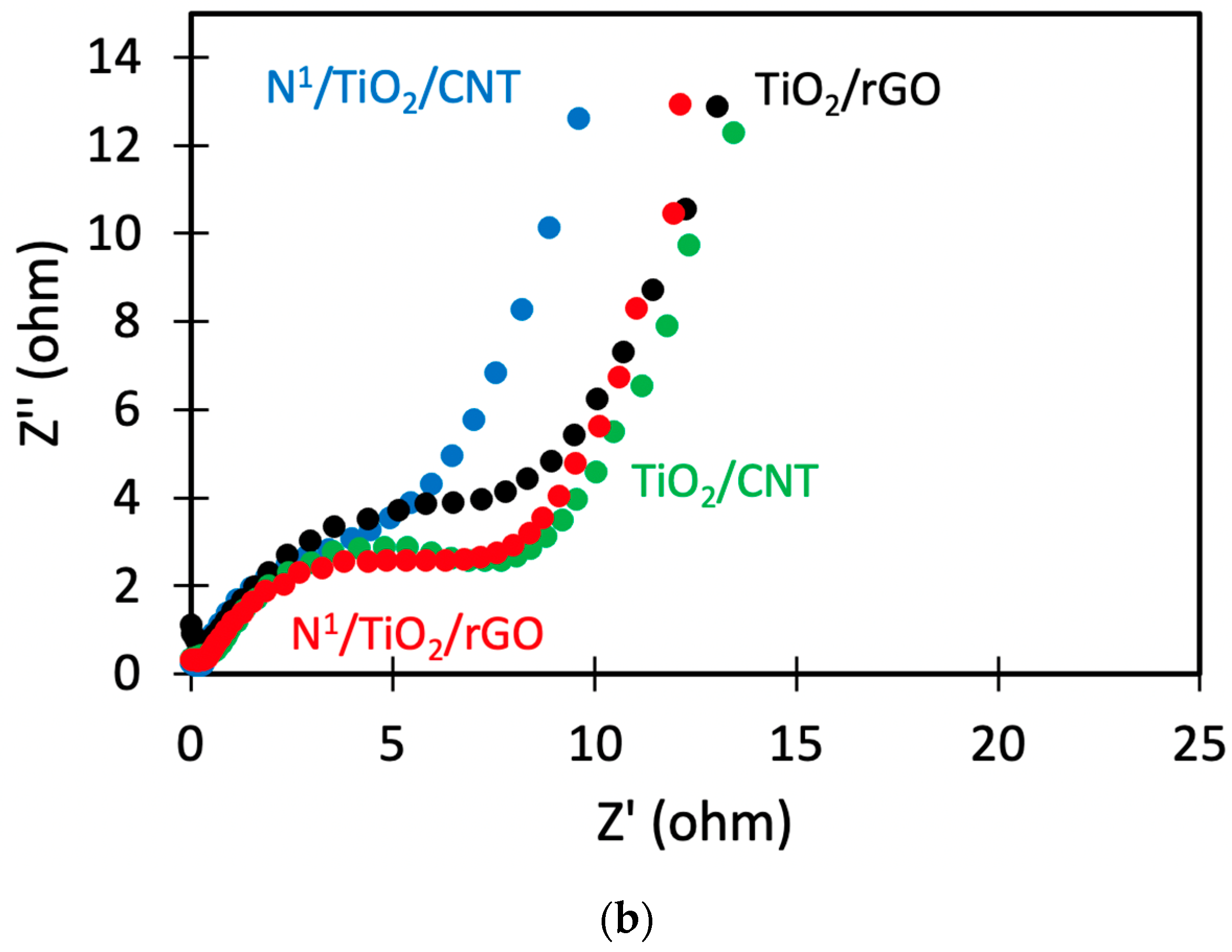
3.9. Electrical Properties Analysis
3.10. Summary of Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, A.A.; Mohd Noor, N.H.; Serrà, A.; Ibrahim, M.N.M. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Phongamwong, T.; Chareopanich, M.; Limtrakul, J. Role of chlorophyll in Spirulina on photocatalytic activity of CO2 reduction under visible light over modified N-doped TiO2 photocatalysts. Appl. Catal. B-Environ. 2015, 168–169, 114–124. [Google Scholar] [CrossRef]
- Camarillo, R.; Rincón, J. Photocatalytic discoloration of dyes: Relation between effect of operating parameters and dye structure. Chem. Eng. Technol. 2011, 34, 1675–1684. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, A.; Ibrahim, M.N.M.; Karri, R.R.; Rashid, M.; Ahamd, Z. Chapter 23—Synthesis of metal oxide–based nanocomposites for energy storage application. In Micro and Nano Technologies, Sustainable Nanotechnology for Environmental Remediation; Koduru, J.R., Karri, R.R., Mubarak, N.M., Bandala, E.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 611–635. [Google Scholar]
- Divyasri, Y.V.; Reddy, N.L.; Lee, K.; Sakar, M.; Rao, V.N.; Venkatramu, V.; Shankar, M.V.; Reddy, N.C.G. Optimization of N doping in TiO2 nanotubes for the enhanced solar light mediated photocatalytic H2 production and dye degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef]
- Tostón, S.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. Supercritical synthesis of planitum-modified titanium dioxide for solar fuel production from carbon dioxide. Chin. J. Catal. 2017, 38, 636–650. [Google Scholar] [CrossRef]
- Camarillo, R.; Rizaldos, D.; Jiménez, C.; Martínez, F.; Rincón, J. Enhancing the photocatalytic reduction of CO2 with undoped and Cu-doped TiO2 nanofibers synthesized in supercritical medium. J. Supercrit. Fluids 2019, 147, 70–80. [Google Scholar] [CrossRef]
- Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. CO2 photocatalytic reduction with CNT/TiO2 based nanocomposites prepared by high-pressure technology. J. Supercrit. Fluids 2020, 163, 104876. [Google Scholar] [CrossRef]
- Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. High-pressure synthesis of rGO/TiO2 and rGO/TiO2/Cu catalysts for efficient CO2 reduction under solar light. J. Supercrit. Fluids 2021, 174, 105265. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Wang, H.; Zhang, X.; Yang, Y.; Yang, C.; Gao, Z.; Wang, Z.; Jiang, K. Green synthesis of boron and nitrogen co-doped TiO2 with rich B-N motifs as Lewis acid-base couples for the effective artificial CO2 photoreduction under simulated sunlight. J. Colloid Interf. Sci. 2021, 585, 95–107. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Reddy, A.V.B. Toxicology and Environmental Application of Carbon Nanocomposite. In Environmental Remediation through Carbon Based Nano Composites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–18. [Google Scholar]
- De la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Removal of emerging pollutant dibutylhydroxytoluene from water with CNT/TiO2 catalysts in a visible LED photoreactor. Environ. Sci. Pollut. Res. 2021, 28, 23720–23730. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Multiwalled carbon nanotubes decorated with nitrogen, palladium co-doped TiO2 (MWCNT/N, Pd co-doped TiO2) for visible light photocatalytic degradation of Eosin Yellow in water. J. Nanopart. Res. 2012, 14, 776. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.M.; Park, S.-J. Synthesis and characterization of nitrogen-doped TiO2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity. Curr. Appl. Phys. 2018, 18, 163–169. [Google Scholar] [CrossRef]
- De la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and characterization of bimetallic TiO2/CNT/Pd-Cu for efficient remediation of endocrine disruptors under solar light. J. Environ. Chem. Eng. 2022, 10, 107245. [Google Scholar] [CrossRef]
- Bramhaiah, K.; Bhattacharyya, S. Challenges and future prospects of graphene-based hybrids for solar fuel generation: Moving towards next generation photocatalysts. Mater. Adv. 2022, 3, 142–172. [Google Scholar] [CrossRef]
- Li, J.; Hou, X.; Sun, T.; Han, J.; Liu, H.; Li, D. Hydrophilic, antibacterial and photocatalytic properties of TiO2 composite films modified by the methods of N+ ion implantation and doping of CNTs. Surf. Coat. Technol. 2019, 365, 123–128. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Osorio-Vargas, P.; Pulgarin, C.A. A critical review on N-modified TiO2 limits to treat chemical and biological contaminants in water. Evidence that enhanced visible light absorption does not lead to higher degradation rates under whole solar light. J. Hazard. Mater. 2022, 425, 127979. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, J.M.; Kochuveedu, S.T.; Han, T.H.; Jeong, H.Y.; Park, M.; Yun, J.M.; Kwon, J.; No, K.; Kim, D.H.; et al. Biomineralized N-doped CNT/TiO2 core/shell nanowires for visible light photocatalysis. ACS Nano 2012, 6, 935–943. [Google Scholar] [CrossRef]
- Lucky, R.A.; Charpentier, P.A. N-doped ZrO2/TiO2 bimetallic materials synthesized in supercritical CO2: Morphology and photocatalytic activity. Appl. Catal. B-Environ. 2010, 96, 516–523. [Google Scholar] [CrossRef]
- Rincón, J.; Martínez, F.; Rodríguez, L.; Ancillo, V. Recovery of triglycerides from used frying oil by extraction with liquid and supercritical ethane. J. Supercrit. Fluids 2011, 56, 72–79. [Google Scholar] [CrossRef]
- Mumin, M.A.; Moula, G.; Charpentier, P.A. Supercritical CO2 synthesized TiO2 nanowires covalently linked with core-shell CdS-ZnS quantum dots: Enhanced photocatalysis and stability. RSC Adv. 2015, 5, 67767–67779. [Google Scholar] [CrossRef]
- de la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and characterization of TiO2/CNT/Pd: An effective sunlight photocatalyst for neonicotinoids degradation. J. Environ. Chem. Eng. 2021, 9, 106278. [Google Scholar] [CrossRef]
- Camarillo, R.; Tostón, S.; Martínez, F.; Jiménez, C.; Rincón, J. Enhancing the photocatalytic reduction of CO2 through engineering of catalyst with high pressure technology: Pd/TiO2 photocatalysts. J. Supercrit. Fluids 2017, 123, 18–27. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvivhyanukul, P.; Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard. Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, J.; Philip, L. Photocatalytic degradation of lindane under UV and visible light using N-doped TiO2. Chem. Eng. J. 2010, 161, 83–92. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J. Phys. Chem. 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Production selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Bjelajac, A.; Kopac, D.; Fecant, A.; Tavernier, E.; Petrovic, R.; Lizokar, B.; Janackovic, D. Micro-kinetic modelling of photocatalytic CO2 reduction over undoped and N-doped TiO2. Catal. Sci. Technol. 2020, 10, 1688–1698. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Self-assembly of nitrogen-doped TiO2 with exposed {001} facets on a graphene scaffold as photo-active hybrid nanostructures for reduction of carbon dioxide to methane. Nano Res. 2014, 7, 1528–1547. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B-Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Nitrogen doped carbon nanotubes grafted TiO2 rutile nanofilms: Promising material for dye sensitized solar cell application. Optik 2021, 239, 166234. [Google Scholar] [CrossRef]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO2 nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef]
- Daraee, M.; Ghasemy, E.; Rashidi, A. Effective absorption of hydrogen sulfide by intercalation of TiO2 and N-doped TiO2 in graphene oxide. J. Environ. Chem. Eng. 2020, 8, 103836. [Google Scholar] [CrossRef]
- Hou, X.; Yao, K.; Wang, X.; Li, D.; Liao, B. Enhanced superhydrophilicity of N+ implanted multiwalled carbon nanotubes-TiO2 composite thin films. Vacuum 2014, 100, 74–77. [Google Scholar] [CrossRef]
- Camarillo, R.; Tostón, S.; Martínez, F.; Jiménez, C.; Rincón, J. Preparation of TiO2-based catalysts with supercritical fluid technology: Characterization and photocatalytic activity in CO2 reduction. J. Chem. Technol. Biotechnol. 2017, 92, 1710–1720. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, S.; Liu, X.; Gong, M.; Xu, T. Synthesis of carbon- and nitrogen-doped TiO2/carbon composite fibers by a surface-hydrolyzed PAN fiber and their photocatalytic property. J. Mater. Sci. 2020, 55, 2471–2481. [Google Scholar] [CrossRef]
- Gao, N.; Wan, T.; Xu, Z.; Ma, L.; Ramakrishna, S.; Liu, Y. Nitrogen doped TiO2/graphene nanofibers as DSSCs photoanode. Mater. Chem. Phys. 2020, 255, 123542. [Google Scholar] [CrossRef]
- Yadav, V.; Saini, V.K.; Sahrma, H. How different dopants leads to difference in photocatalytic activity in doped TiO2? Ceram. Internat. 2020, 46, 27308–27317. [Google Scholar] [CrossRef]
- Masa, J.; Bordoloi, A.; Muhler, M.; Schuhmann, W.; Xia, W. Enhanced electrocatalytic stability of platinum nanoparticles supported on a nitrogen-doped composite of carbon nanotubes and mesoporous titania under oxygen reduction conditions. ChemSusChem 2012, 5, 523–525. [Google Scholar] [CrossRef]
- Zikalala, S.A.; Selvaraj, R.; Al Marzouqi, F.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. Tailoring TiO2 through N doping and incorporation of amorphous carbon nanotubes via a microwave-assisted hydrothermal method. J. Environ. Chem. Eng. 2020, 8, 104082. [Google Scholar] [CrossRef]
- Carreño-Lizcano, M.I.; Guarldrón-Reyes, A.F.; Rodríguez-González, V.; Pedraza-Avella, J.A.; Niño-Gómez, M.E. Photoelectrocatalytic phenol oxidation employing nitrogen doped TiO2-rGO films as photoanodes. Catal. Today 2020, 341, 96–103. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Zhang, Y.; Ahmad, M. Nitrogen doped TiO2 nanoparticles decorated on graphene sheets for photocatalysis applications. Curr. Appl. Phys. 2012, 12, 1485–1492. [Google Scholar] [CrossRef]
- Ida, S.; Wilson, P.; Neppolian, B.; Sathish, M.; Mahammed Shanheer, A.R.; Ravi, P. Turning the type of nitrogen on N-RGO supported on N-TiO2 under ultrasonication/hydrothermal treatment for efficient hydrogen evolution—A mechanistic overview. Ultrason. Sonochem. 2020, 64, 104866. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murgesan, T.; Hussain, M. Improved interfacial bonding of graphene-TiO2 with enhanced photocatalytic reduction of CO2 into solar fuel. J. Environ. Chem. Eng. 2018, 6, 6947–6957. [Google Scholar] [CrossRef]
- Cao, G.; Aaron Deskins, N.; Yi, N. Carbon monoxide oxidation over copper and nitrogen modified titanium dioxide. Appl. Catal. B-Environ. 2021, 285, 119748. [Google Scholar] [CrossRef]
- Nada, A.A.; El Rouby, W.M.A.; Bekheet, M.F.; Antuch, M.; Weber, M.; Miele, P.; Viter, R.; Roualdes, S.; Millet, P.; Bechelany, M. Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl. Surf. Sci. 2020, 505, 144419. [Google Scholar] [CrossRef]
- Thirugnanam, L.; Palanisamy, M.; Kavery, S.; Ramaprabhu, S.; Pol, V.G.; Dutta, M. TiO2 nanoparticle embedded nitrogen doped electrospun helical carbon nanofiber-carbon nanotube hybrid anode for lithium-ion batteries. Int. J. Hydrogen Energy 2021, 46, 2464–2478. [Google Scholar] [CrossRef]
- Han, Z.; Peng, J.; Liu, L.; Wang, G.; Yu, F.; Guo, X. Few-layer TiO2-B nanosheets with N-doped graphene nanosheets as a highly robust anode for lithium-ion batteries. RSC Adv. 2017, 7, 7864–7869. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef]
- Kaur, N.; Shani, S.K.; Shani, J.S.; Sandhu, S.; Sharma, R.; Singh, V. Comprehensive review and future perspectives of efficient N-doped, Fe-doped and (N,Fe)-co-doped titania as visible light active photocatalysts. Vacuum 2020, 178, 109429. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Huang, J.; Ma, J.; Wang, X.; Wang, H.; Ma, R.; Xia, P.; Zhao, J. High performance of N-doped TiO2-magnetic activated carbon composites under visible light illumination: Synthesis and application in three-dimensional photoelectrochemical process. Electrochim. Acta 2016, 222, 1–11. [Google Scholar] [CrossRef]
- Wang, D.-H.; Jia, L.; Wu, X.-L.; Lu, L.-Q.; Xu, A.-W. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, J.; Shi, J.; Li, S.; Zhang, B.; Tan, X.; Zhang, F.; Liu, L.; Shao, D.; Han, B. Photocatalytic CO2 transformation to CH4 by Ag/Pd bimetals supported on N-doped TiO2 nanosheet. ACS Appl. Mater. Interfaces 2018, 10, 24516–24522. [Google Scholar] [CrossRef]
- Lei, Z.; Xiong, Z.; Wang, Y.; Chen, Y.; Cao, S.; Zhao, Y.; Zhang, J.; Zheng, C. Photocatalytic reduction of CO2 over facet engineered TiO2 nanocrystals supported by carbon nanofibers under simulated sunlight irradiation. Catal. Commun. 2018, 108, 27–32. [Google Scholar] [CrossRef]
- Min, Y.; He, G.; Li, R.; Zhao, W.; Chen, Y.; Zhang, Y. Doping nitrogen anion enhanced photocatalytic activity on TiO2 hybridized with graphene composite under solar light. Sep. Purif. Technol. 2013, 106, 97–104. [Google Scholar] [CrossRef]
- Camarillo, R.; Tostón, S.; Martínez, F.; Jiménez, C.; Rincón, J. Improving the photo-reduction of CO2 to fuels with catalysts synthesized under high pressure: Cu/TiO2. J. Chem. Technol. Biotechnol. 2018, 93, 1237–1248. [Google Scholar] [CrossRef]




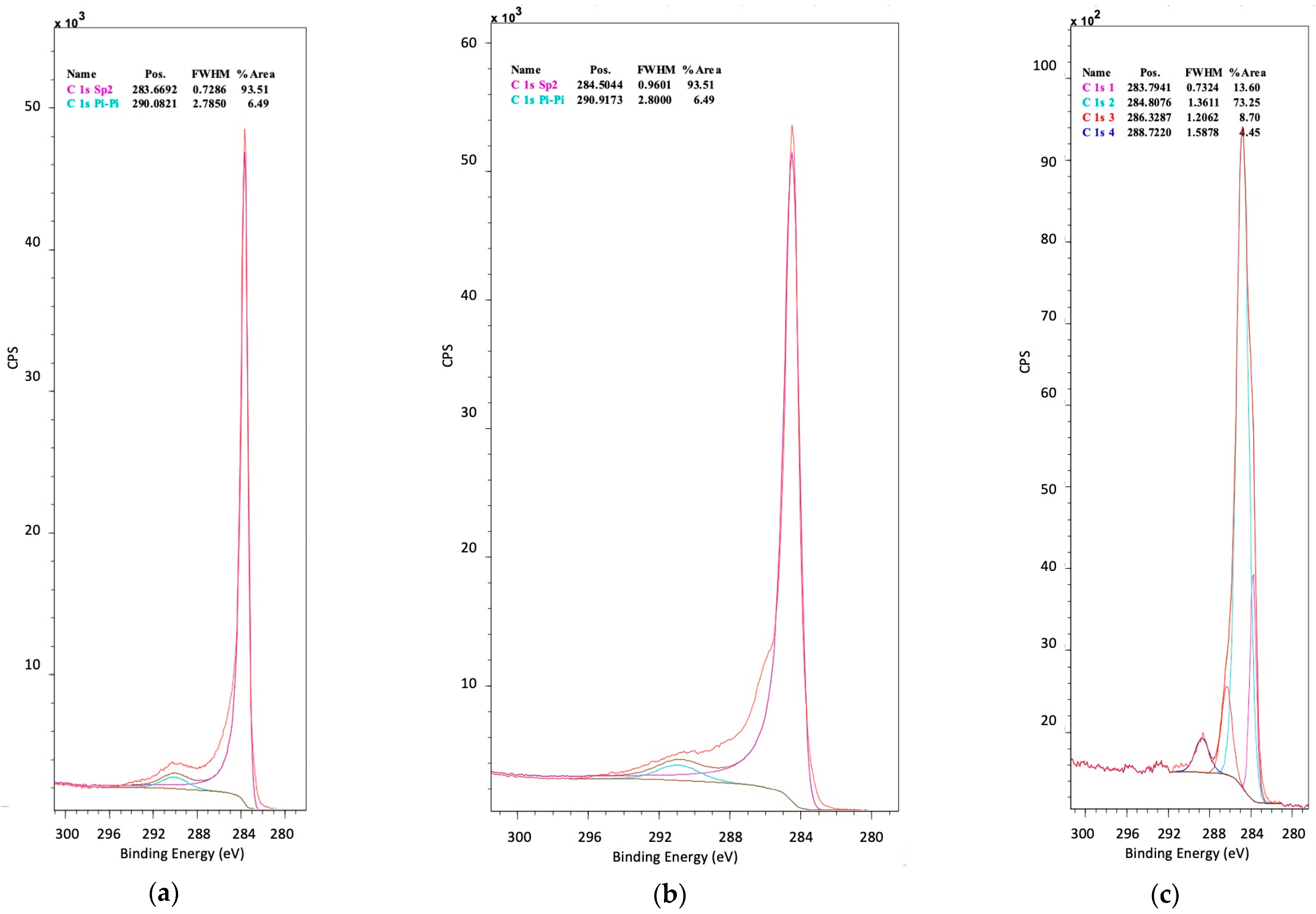

| Support | Nitrogen Precursor Used in the Synthesis | N Content (mg N/g Support) | Material Name (1) |
|---|---|---|---|
| CNT | 1 mL TEA 2 mL TEA 4 mL TEA 0.3 g urea | 1.0 2.1 2.0 1.9 | N1/CNT N2/CNT (a) N2/CNT (b) N2/CNT (c) |
| rGO | 1 mL TEA 2 mL TEA 4 mL TEA 0.3 g urea | 4.1 7.0 10.2 42.4 | N4/rGO N7/rGO N10/rGO N42/rGO |
| Photocatalyst | Nitrogen Precursor Used in the Synthesis | N Content (Measured by Elemental Analysis) (mg/g Photocatalyst) | N Content (Measured or Estimated) (mg N/g TiO2) | Photocatalyst Name (1) |
|---|---|---|---|---|
| N-doped TiO2 nanoparticles | 1 mL TEA 2 mL TEA 4 mL TEA 0.3 g urea | 1.1 1.0 1.0 2.1 | 1.1 1.0 1.0 2.1 | N1/TiO2 (a) N1/TiO2 (b) N1/TiO2 (c) N2/TiO2 |
| N-doped TiO2 nanoparticles supported on CNT | 1 mL TEA 2 mL TEA 4 mL TEA 0.3 g urea | 1.0 1.4 1.6 2.1 | 1 1 1 2 | N1/TiO2/CNT (a) N1/TiO2/CNT (b) N1/TiO2/CNT (c) N2/TiO2/CNT |
| N-doped TiO2 nanoparticles supported on rGO | 1 mL TEA 2 mL TEA 4 mL TEA 0.3 g urea | 2.6 4.0 5.6 22.2 | 1 1 1 2 | N1/TiO2/rGO (a) N1/TiO2/rGO (b) N1/TiO2/rGO (c) N2/TiO2/rGO |
| Catalyst | Products (µmol/h/g TiO2) | Conditions | Reference |
|---|---|---|---|
| N1/TiO2 | CO: 1.5 CH4: 3.7 C2H6 + C3H8: 0 Total: 5.2 | See Materials and methods | This work |
| 5 wt. % N-TiO2 (liquid phase) | CO: 0 CH4: 0.1 C2H6 + C3H8: Tr Total: 0.1 | 6 fluorescent bulbs (400–800 nm), 13 W, continuous flow stirred slurry reactor, 250 mL, 10 mL/min, catalyst concentration 1 g/L, 6 h | [2] |
| N-TiO2 (gas phase) | CO: 0.1 CH4: 0.2 C2H6 + C3H8: Tr Total: 0.3 | Continuous, catalyst 100–300 mg, CO2:H2O = 30:1, CO2 flow 0.3 mL/min, Xe lamp (315–600 nm), irradiation area 1.08 × 10−3 m2 | [29] |
| N1/TiO2/CNT | CO: 3.9 CH4: 0.1 C2H6 + C3H8: 0 Total: 4 | See Materials and methods | This work |
| N1/TiO2/rGO | CO: 7.5 CH4: 0.2 C2H6 + C3H8: 0 Total: 7.7 | See Materials and methods | This work |
| N-TiO2/graphene (gas phase) | CO: 0 CH4: 0.4 C2H6 + C3H8: 0 Total: 0.4 | Continuous gas flow reactor, visible light irradiation (15 W), 10 h, CO2 flow 5 mL/min | [30] |
| g-C3N4-N-TiO2 (gas phase) | CO: 4.8 CH4: 3.3 C2H6 + C3H8: 0 Total: 8.1 | Gas-closed circulation system, 780 mL, 0.1 g catalyst, 300 W Xe arc lamp | [31] |
| Samples | N Content (mg N/g TiO2) | TiO2 Crystallite Size (nm) | BET Area (m2/g) | Band Gap (eV) | Absorption Threshold (nm) |
|---|---|---|---|---|---|
| TiO2 | 0 | 11 | 152 | 3.10 | 400 |
| N1/TiO2 | 1 | 14 | 82 | 3.06 | 405 |
| N2/TiO2 | 2 | 14 | 81 | 3.06 | 405 |
| CNT | 0 | - | 216 | - | - |
| N1/CNT | 1 mg N/g CNT | - | 284 | - | - |
| N2/CNT | 2 mg N/g CNT | - | 280 | - | - |
| N1/TiO2/CNT | 1 | 10 | 145 | 2.12 | - |
| N2/TiO2/CNT | 2 | 10 | 140 | 2.10 | - |
| rGO | 0 | - | 163 | - | - |
| N4/rGO | 4 mg N/g rGO | - | 173 | - | - |
| N10/rGO | 10 mg N/g rGO | - | 171 | - | - |
| N1/TiO2/rGO | 1 | 14 | 85 | 2.45 | - |
| N2/TiO2/rGO | 2 | 13 | 84 | 2.40 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, Ó.R.; Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. Photocatalytic Reduction of CO2 with N-Doped TiO2-Based Photocatalysts Obtained in One-Pot Supercritical Synthesis. Nanomaterials 2022, 12, 1793. https://doi.org/10.3390/nano12111793
Andrade ÓR, Rodríguez V, Camarillo R, Martínez F, Jiménez C, Rincón J. Photocatalytic Reduction of CO2 with N-Doped TiO2-Based Photocatalysts Obtained in One-Pot Supercritical Synthesis. Nanomaterials. 2022; 12(11):1793. https://doi.org/10.3390/nano12111793
Chicago/Turabian StyleAndrade, Óscar R., Verónica Rodríguez, Rafael Camarillo, Fabiola Martínez, Carlos Jiménez, and Jesusa Rincón. 2022. "Photocatalytic Reduction of CO2 with N-Doped TiO2-Based Photocatalysts Obtained in One-Pot Supercritical Synthesis" Nanomaterials 12, no. 11: 1793. https://doi.org/10.3390/nano12111793








