Influence of Temperature Parameters on Morphological Characteristics of Plasma Deposited Zinc Oxide Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Plasma–Chemical Synthesis
2.3. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.4. X-ray Structural Analysis
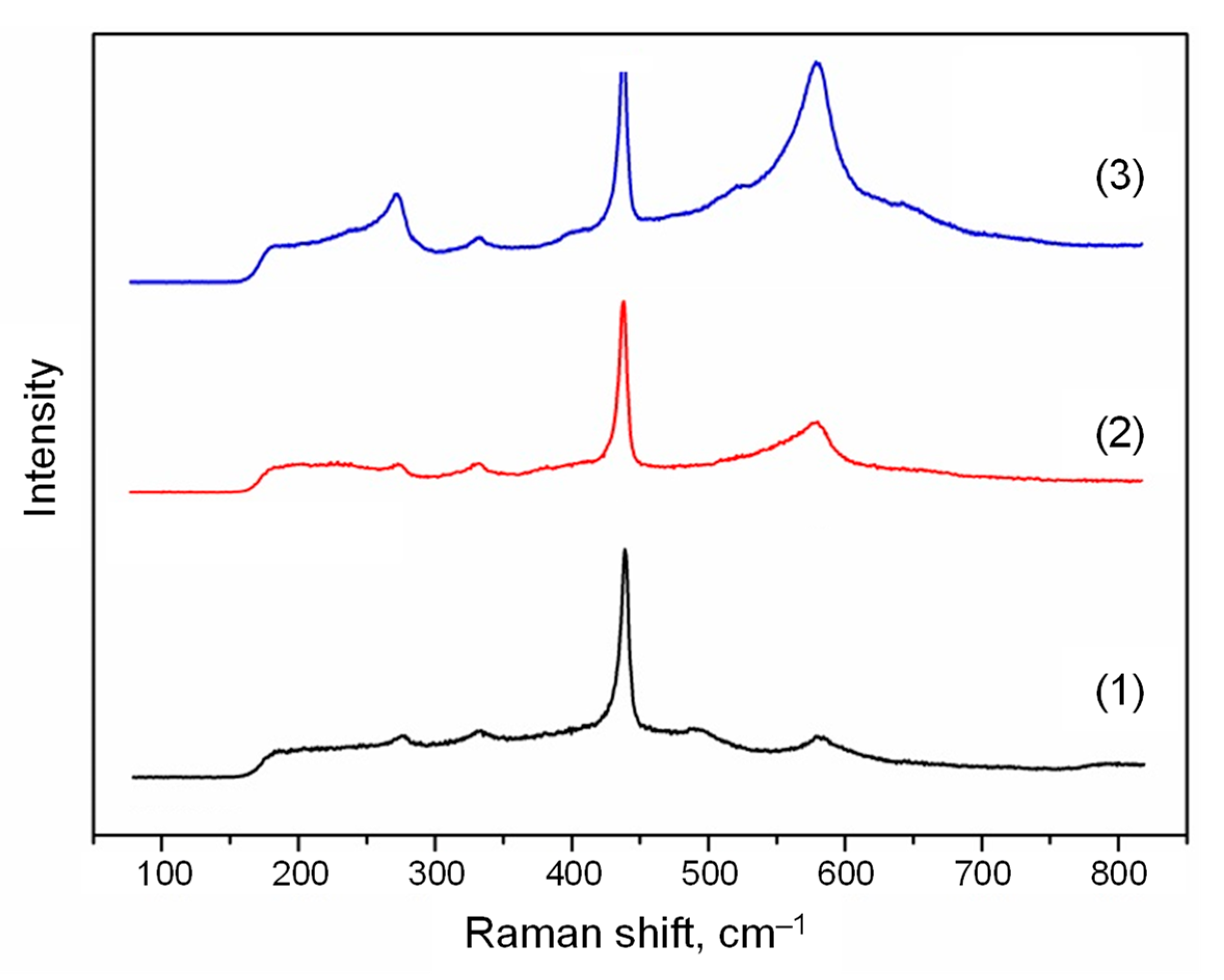
2.5. Raman Spectroscopy
2.6. Varying Temperature Parameters in Plasma–Chemical Deposition
3. Results and Discussion
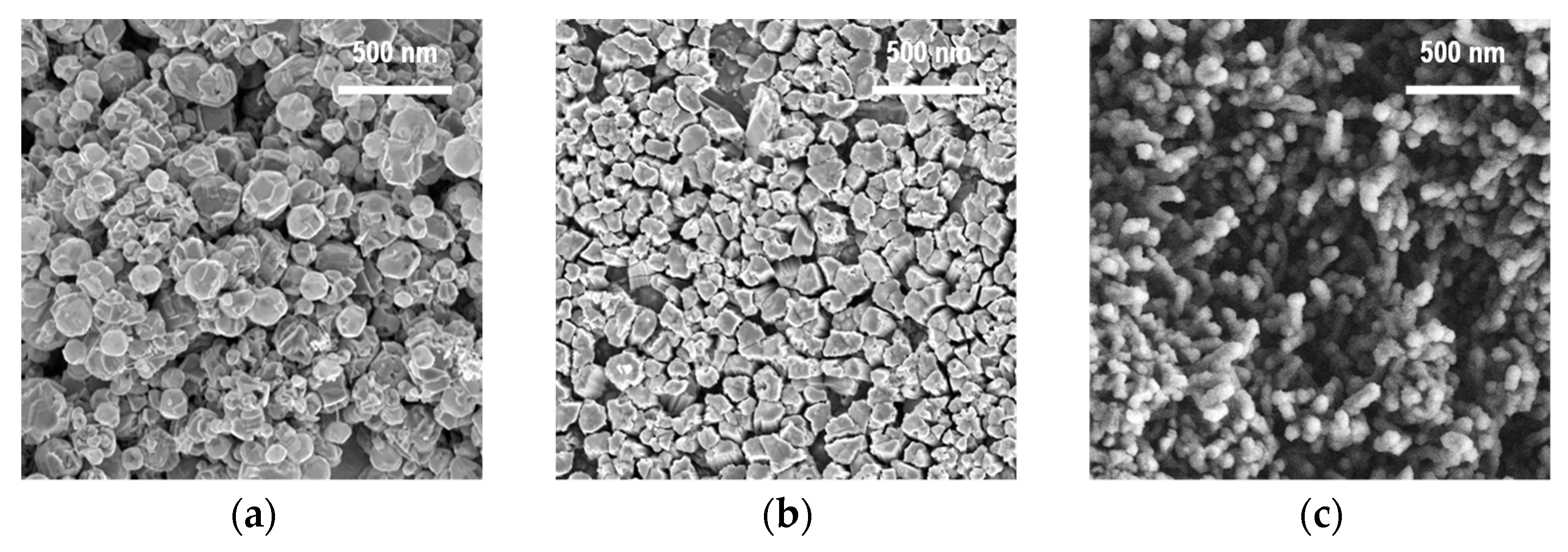
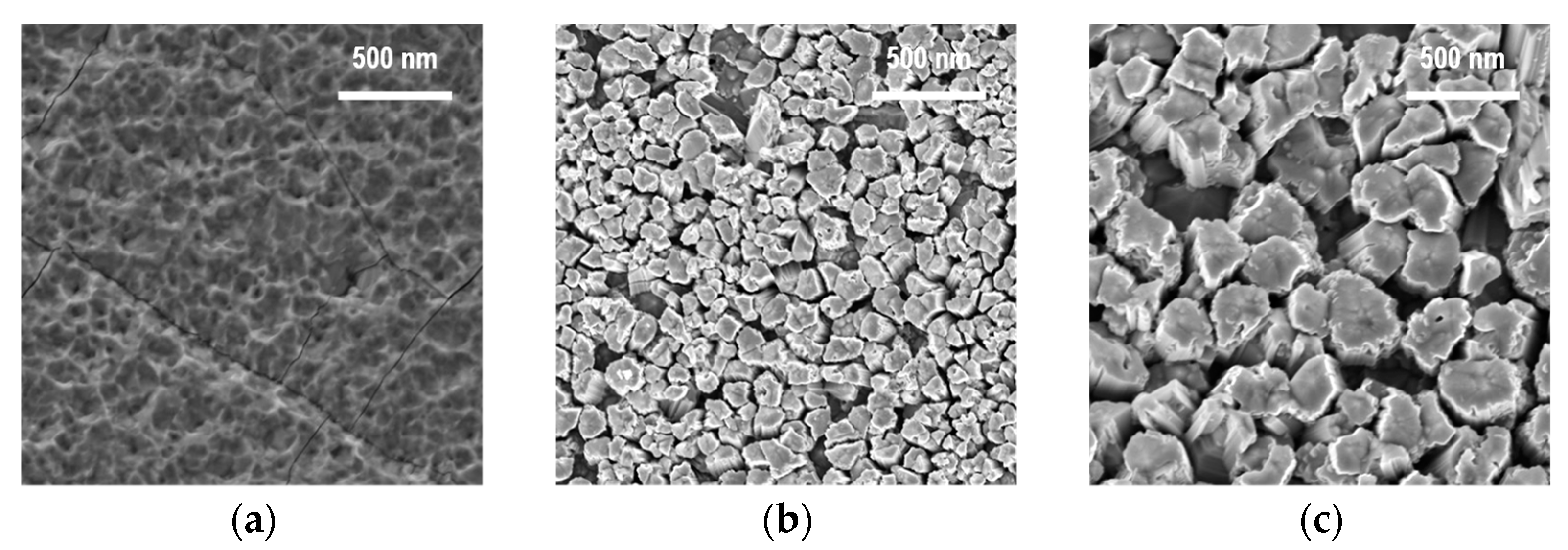
3.1. Zinc Source Temperature and Morphology of ZnO Nanoparticles
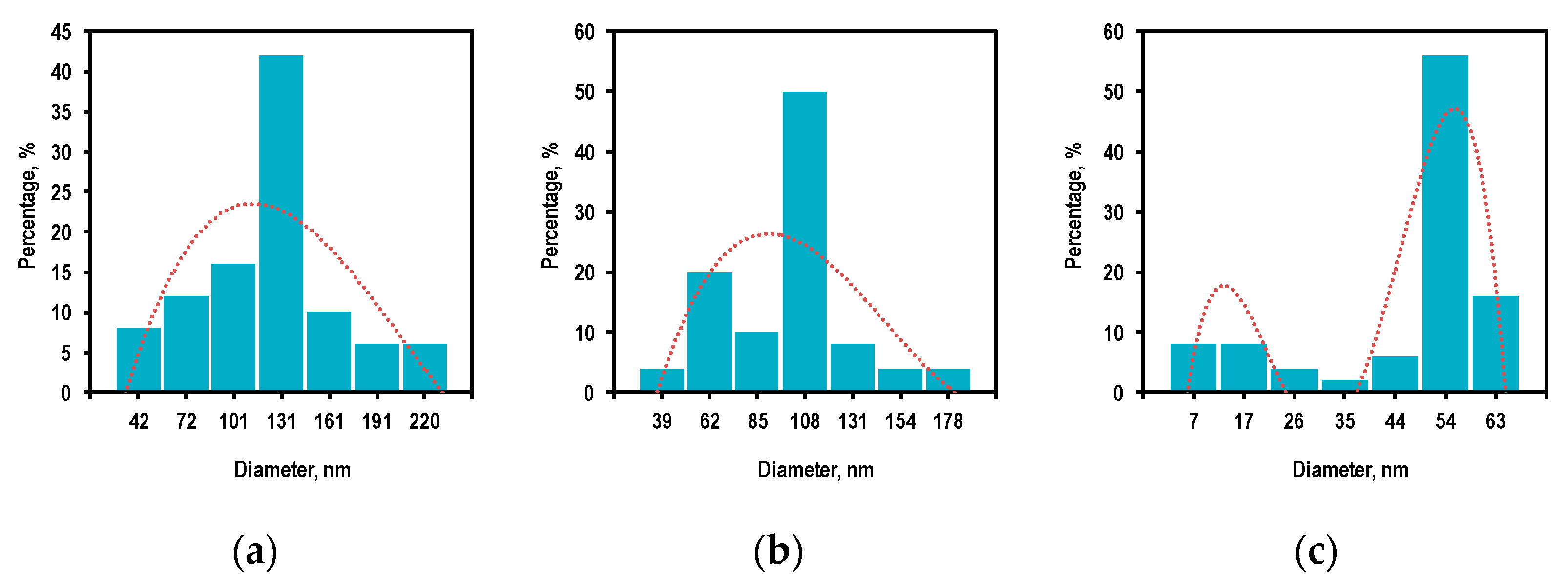
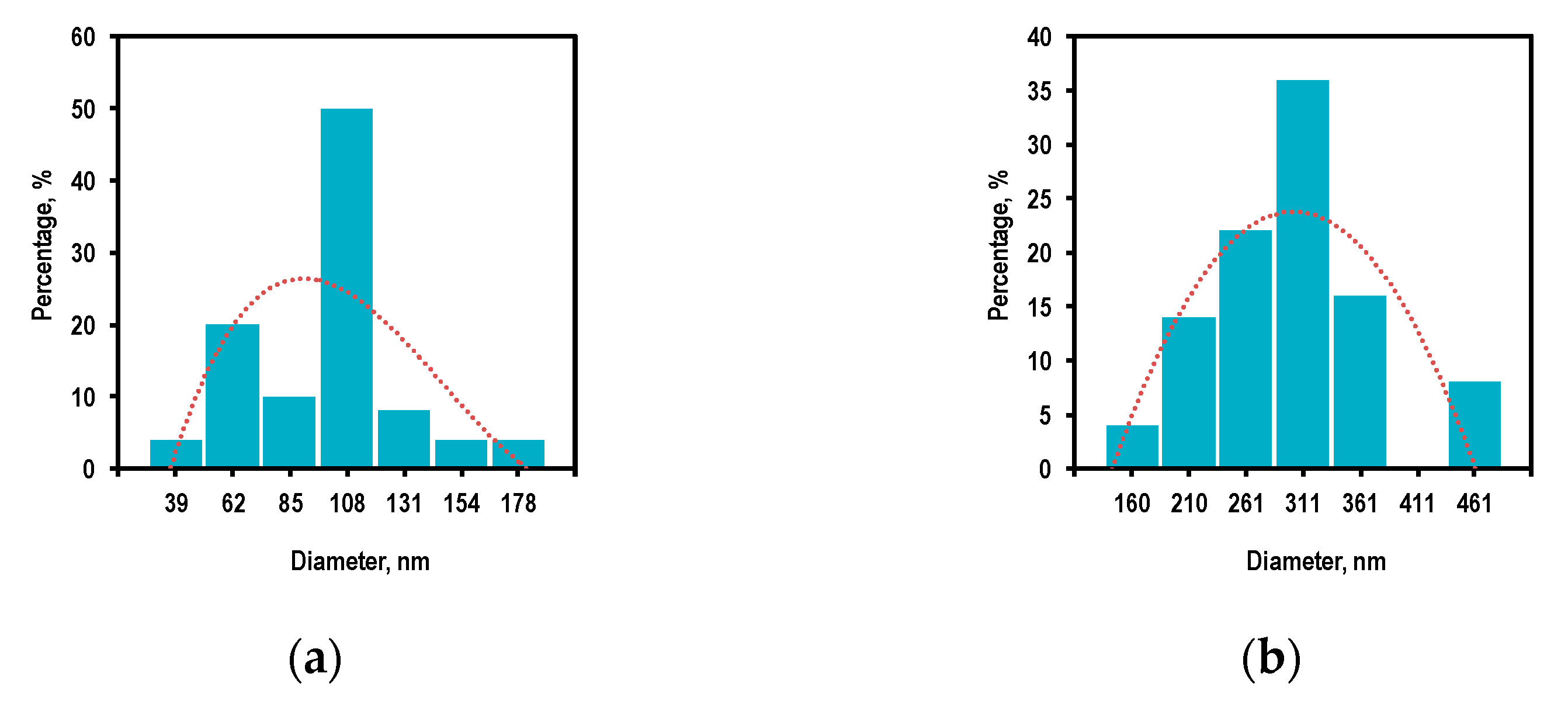
3.2. Reactor Temperature and Size Distribution of ZnO Nanoparticles
3.3. Controlling the Size and Morphological Characteristics of ZnO Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Borysiewicz, M.A. ZnO as a functional material, a review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef] [PubMed]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Silva, J.M.P.; Andrade Neto, N.F.; Oliveira, M.C.; Ribeiro, R.A.P.; de Lazaro, S.R.; Gomes, Y.F.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Recent progress and approaches on the synthesis of Mn-doped zinc oxide nanoparticles: A theoretical and experimental investigation on the photocatalytic performance. New J. Chem. 2020, 44, 8805–8812. [Google Scholar] [CrossRef]
- Tinoco, J.C.; Hernández, S.A.; Rodríguez-Bernal, O.; Vega-Poot, A.G.; Rodríguez-Gattorno, G.; Olvera, M.d.l.L.; Martinez-Lopez, A.G. Fabrication of Schottky barrier diodes based on ZnO for flexible electronics. J. Mater. Sci. Mater. Electron. 2020, 31, 7373–7377. [Google Scholar] [CrossRef]
- Alexandrov, A.; Zvaigzne, M.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Al-, Ga-, Mg-, or Li-doped zinc oxide nanoparticles as electron transport layers for quantum dot light-emitting diodes. Sci. Rep. 2020, 10, 7496. [Google Scholar] [CrossRef]
- Kumar, A. Sol gel synthesis of zinc oxide nanoparticles and their application as nano-composite electrode material for supercapacitor. J. Mol. Struct. 2020, 1220, 128654. [Google Scholar] [CrossRef]
- Jaballah, S.; Benamara, M.; Dahman, H.; Lahem, D.; Debliquy, M.; El Mir, L. Formaldehyde sensing characteristics of calcium-doped zinc oxide nanoparticles-based gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 8230–8239. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Hammad, T.M.; Salem, J.K.; Harrison, R.G. The influence of annealing temperature on the structure, morphologies and optical properties of ZnO nanoparticles. Superlattices Microstruct. 2010, 47, 335–340. [Google Scholar] [CrossRef]
- Mornani, E.G.; Mosayebian, P.; Dorranian, D.; Behzad, K. Effect of calcination temperature on the size and optical properties of synthesized ZnO nanoparticles. J. Ovonic Res. 2016, 12, 75–80. [Google Scholar]
- An, L.; Zhang, D.; Zhang, L.; Feng, G. Effect of nanoparticle size on the mechanical properties of nanoparticle assemblies. Nanoscale 2019, 11, 9563–9573. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Chen, Y.; Lv, S.; Liao, X.; Yao, Y. Size effects of Ag nanoparticle for N2 photofixation over Ag/g-C3N4: Built-in electric fields determine photocatalytic performance. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127053. [Google Scholar] [CrossRef]
- Sorichetti, V.; Hugouvieux, V.; Kob, W. Structure and dynamics of a polymer–nanoparticle composite: Effect of nanoparticle size and volume fraction. Macromolecules 2018, 51, 5375–5391. [Google Scholar] [CrossRef]
- Ismail, A.M.; Menazea, A.A.; Kabary, H.A.; El-Sherbiny, A.E.; Samy, A. The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol–Gel method. J. Mol. Struct. 2019, 1196, 332–337. [Google Scholar] [CrossRef]
- Cai, B.; Pei, J.; Dong, J.; Zhuang, H.-L.; Gu, J.; Cao, Q.; Hu, H.; Lin, Z.; Li, J.-F. (Bi,Sb)2Te3/SiC nanocomposites with enhanced thermoelectric performance: Effect of SiC nanoparticle size and compositional modulation. Sci. China Mater. 2021, 64, 2551–2562. [Google Scholar] [CrossRef]
- Ramya, M.; Nideep, T.K.; Nampoori, V.P.N.; Kailasnath, M. The impact of ZnO nanoparticle size on the performance of photoanodes in DSSC and QDSSC: A comparative study. J. Mater. Sci. Mater. Electron. 2021, 32, 3167–3179. [Google Scholar] [CrossRef]
- Mahpudz, A.; Lim, S.L.; Inokawa, H.; Kusakabe, K.; Tomoshige, R. Cobalt nanoparticle supported on layered double hydroxide: Effect of nanoparticle size on catalytic hydrogen production by NaBH4 hydrolysis. Environ. Pollut. 2021, 290, 117990. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Vorotyntsev, V. Structural and optical properties of As–Se–Te chalcogenide films prepared by plasma-enhanced chemical vapor deposition. Mater. Res. Express 2019, 6, 56407. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Markin, A.; Kitnis, A.; Vorotyntsev, V. Characteristics of the Te-based chalcogenide films dependently on the parameters of the PECVD process. Opt. Quantum Electron. 2020, 52, 197. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Prokhorov, I.; Sazanova, T.; Kudrin, A.; Yunin, P.; Zelentsov, S.; Letnianchik, A.; Starostin, N.; Boreman, G.; et al. Plasma-Chemical Synthesis of Lead Sulphide Thin Films for Near-IR Photodetectors. Plasma Chem. Plasma Process. 2021, 41, 493–506. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, A.; Kaushal, A.; Kaur, D.; Pandey, A.; Goyal, R.N. In situ high temperature XRD studies of ZnO nanopowder prepared via cost effective ultrasonic mist chemical vapour deposition. Bull. Mater. Sci. 2008, 31, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Purica, M. Optical and structural investigation of ZnO thin films prepared by chemical vapor deposition (CVD). Thin Solid Film. 2002, 403–404, 485–488. [Google Scholar] [CrossRef]
- Kocyigit, A.; Orak, I.; Çaldıran, Z.; Turut, A. Current–voltage characteristics of Au/ZnO/n-Si device in a wide range temperature. J. Mater. Sci. Mater. Electron. 2017, 28, 17177–17184. [Google Scholar] [CrossRef]
- Wu, J.-J.; Wen, H.-I.; Tseng, C.-H.; Liu, S.-C. Well-Aligned ZnO Nanorods via Hydrogen Treatment of ZnO Films. Adv. Funct. Mater. 2004, 14, 806–810. [Google Scholar] [CrossRef]
- Savaloni, H.; Savari, R. Nano-structural variations of ZnO:N thin films as a function of deposition angle and annealing conditions: XRD, AFM, FESEM and EDS analyses. Mater. Chem. Phys. 2018, 214, 402–420. [Google Scholar] [CrossRef]
- Franklin, J.B.; Zou, B.; Petrov, P.; McComb, D.W.; Ryan, M.P.; McLachlan, M.A. Optimised pulsed laser deposition of ZnO thin films on transparent conducting substrates. J. Mater. Chem. 2011, 21, 8178. [Google Scholar] [CrossRef]
- Fan, H.; Jia, X. Selective detection of acetone and gasoline by temperature modulation in zinc oxide nanosheets sensors. Solid State Ion. 2011, 192, 688–692. [Google Scholar] [CrossRef]
- Mondal, B.; Mandal, S.P.; Kundu, M.; Adhikari, U.; Roy, U.K. Synthesis and characterization of nano−zinc wire using a self designed unit galvanic cell in aqueous medium and its reactivity in propargylation of aldehydes. Tetrahedron 2019, 75, 4669–4675. [Google Scholar] [CrossRef]
- Friedrich, F.; Nickel, N.H. Resonant Raman scattering in hydrogen and nitrogen doped ZnO. Appl. Phys. Lett. 2007, 91, 111903. [Google Scholar] [CrossRef]
- Sann, J.; Stehr, J.; Hofstaetter, A.; Hofmann, D.M.; Neumann, A.; Lerch, M.; Haboeck, U.; Hoffmann, A.; Thomsen, C. Zn interstitial related donors in ammonia-treated ZnO powders. Phys. Rev. B 2007, 76, 195203. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, D.N.; Hortelano, V.; Martínez, O.; Martínez-Tomas, M.C.; Sallet, V.; Muñoz-Sanjosé, V.; Jiménez, J. Non-radiative recombination centres in catalyst-free ZnO nanorods grown by atmospheric-metal organic chemical vapour deposition. J. Phys. D Appl. Phys. 2013, 46, 235302. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Mochalov, L.A.; Logunov, A.A.; Fukina, D.G.; Vorotyntsev, I.V. Influence of plasma power on the size distribution of deposited zinc oxide nanorods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1155, 12093. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazanova, T.S.; Mochalov, L.A.; Logunov, A.A.; Kudryashov, M.A.; Fukina, D.G.; Vshivtsev, M.A.; Prokhorov, I.O.; Yunin, P.A.; Smorodin, K.A.; Atlaskin, A.A.; et al. Influence of Temperature Parameters on Morphological Characteristics of Plasma Deposited Zinc Oxide Nanoparticles. Nanomaterials 2022, 12, 1838. https://doi.org/10.3390/nano12111838
Sazanova TS, Mochalov LA, Logunov AA, Kudryashov MA, Fukina DG, Vshivtsev MA, Prokhorov IO, Yunin PA, Smorodin KA, Atlaskin AA, et al. Influence of Temperature Parameters on Morphological Characteristics of Plasma Deposited Zinc Oxide Nanoparticles. Nanomaterials. 2022; 12(11):1838. https://doi.org/10.3390/nano12111838
Chicago/Turabian StyleSazanova, Tatyana Sergeevna, Leonid Alexandrovich Mochalov, Alexander Alexandrovich Logunov, Mikhail Alexandrovich Kudryashov, Diana Georgievna Fukina, Maksim Anatolevich Vshivtsev, Igor Olegovich Prokhorov, Pavel Andreevich Yunin, Kirill Alexandrovich Smorodin, Artem Anatolevich Atlaskin, and et al. 2022. "Influence of Temperature Parameters on Morphological Characteristics of Plasma Deposited Zinc Oxide Nanoparticles" Nanomaterials 12, no. 11: 1838. https://doi.org/10.3390/nano12111838
APA StyleSazanova, T. S., Mochalov, L. A., Logunov, A. A., Kudryashov, M. A., Fukina, D. G., Vshivtsev, M. A., Prokhorov, I. O., Yunin, P. A., Smorodin, K. A., Atlaskin, A. A., & Vorotyntsev, A. V. (2022). Influence of Temperature Parameters on Morphological Characteristics of Plasma Deposited Zinc Oxide Nanoparticles. Nanomaterials, 12(11), 1838. https://doi.org/10.3390/nano12111838






