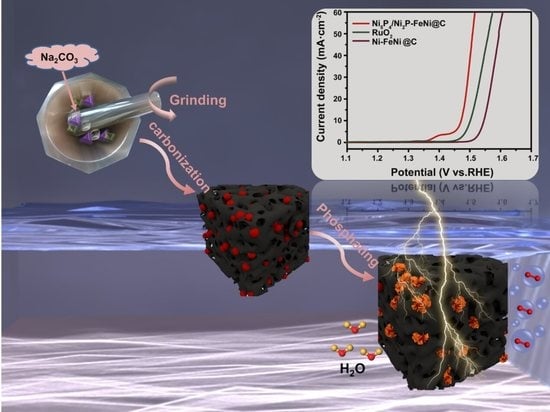
The Scalable Solid-State Synthesis of a Ni5P4/Ni2P–FeNi Alloy Encapsulated into a Hierarchical Porous Carbon Framework for Efficient Oxygen Evolution Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of the Ni–FeNi@C and FeNi3@AC Catalysts
2.3. Preparation of the Ni5P4/Ni2P–FeNi@C and Ni5P4/Ni2P–Fe–FeNi3@AC Catalysts
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Schematic Diagram of the Synthesis Process
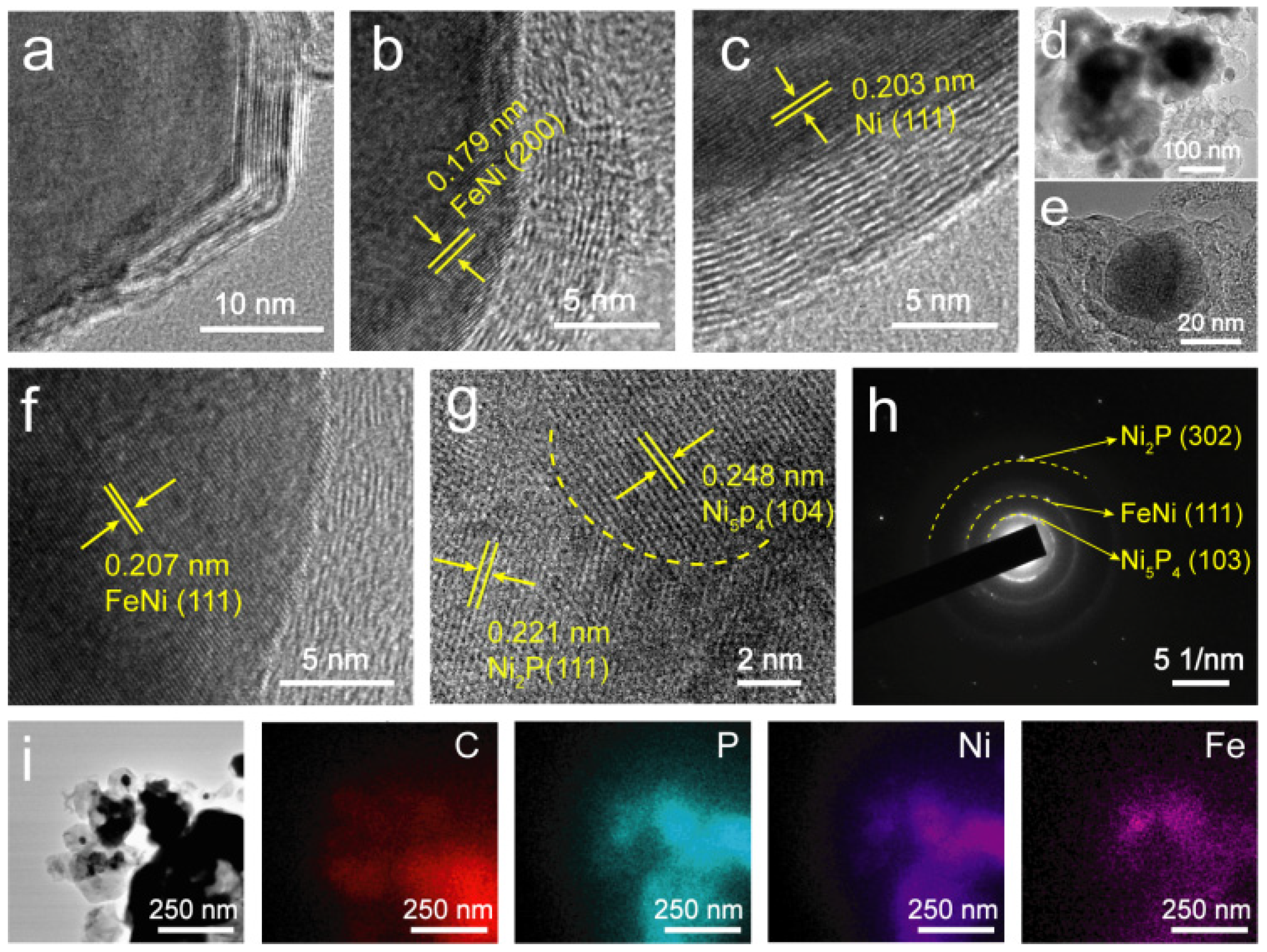
3.2. Structural Analysis
3.3. Electrochemical Measurements
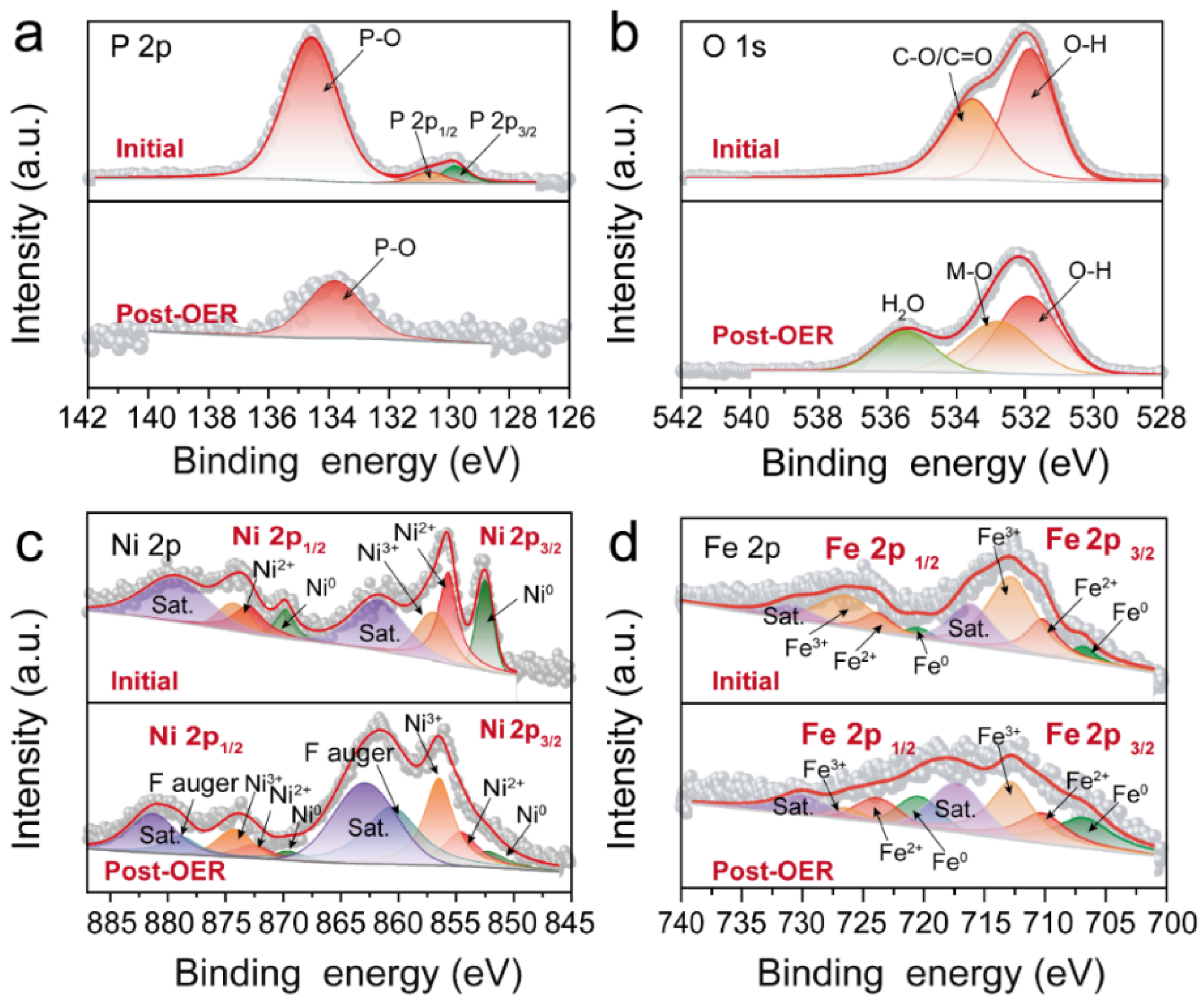
3.4. XPS Survey Spectrum of Ni5P4/Ni2P–FeNi@C before and after OER Reaction
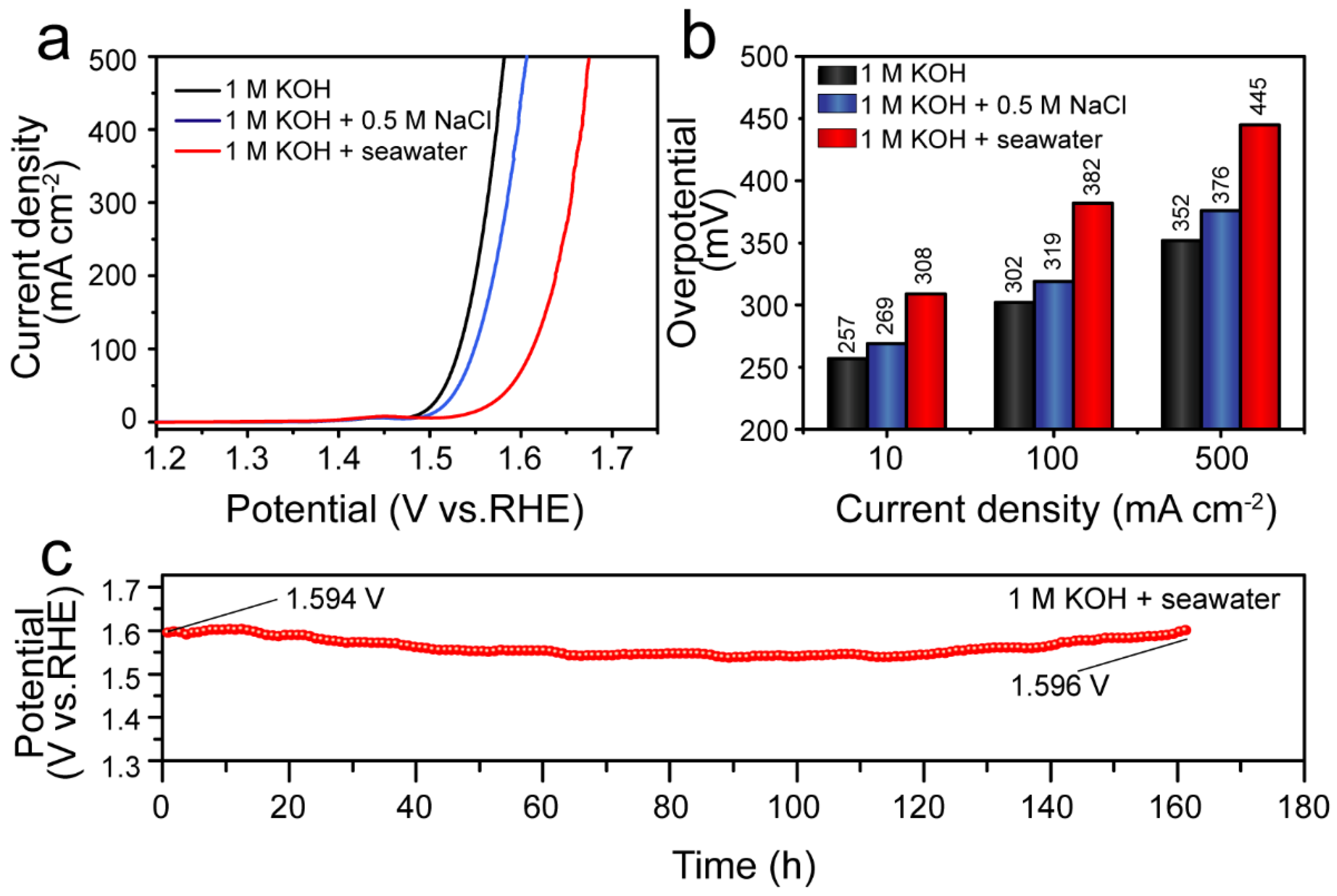
3.5. Electrolyzed Seawater Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Xie, Y.; Yu, Z.; Guo, S.; Yuan, M.; Yao, H.; Liang, Z.; Lu, Y.R.; Chan, T.-S.; Li, C.; et al. Self-supported NiFe-LDH@CoSX nanosheet arrays grown on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 419, 129512. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.J.; Li, Z.Y.; Bu, X.H. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, 2003916. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, Y.; Chen, H.M.; Hu, Z.; Hung, S.F.; Ma, N.; Dai, J.; Lin, H.J.; Chen, C.T.; Zhou, W.; et al. An Amorphous Nickel-Iron-Based Electrocatalyst with Unusual Local Structures for Ultrafast Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1900883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Cai, C.; Wang, M.; Zhao, Z.L.; Li, M.; Huang, X.; Han, S.; Zhou, H.; Feng, Z.; et al. Single Iridium Atom Doped Ni2P Catalyst for Optimal Oxygen Evolution. J. Am. Chem. Soc. 2021, 143, 13605–13615. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Wu, D.; Hao, J.; Wang, W.; Yu, Y.; Fu, X.Z.; Luo, J.L. Energy-saving H2 Generation Coupled with Oxidative Alcohol Refining over Bimetallic Phosphide Ni2P-CoP Junction Bifunctional Electrocatalysts. ChemSusChem 2021, 14, 5450–5459. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Jiao, L. Multifunctional Transition Metal-Based Phosphides in Energy-Related Electrocatalysis. Adv. Energy Mater. 2020, 10, 1902104. [Google Scholar] [CrossRef]
- Li, S.H.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Nanostructured metal phosphides: From controllable synthesis to sustainable catalysis. Chem. Soc. Rev. 2021, 50, 7539–7586. [Google Scholar] [CrossRef]
- Liu, S.; Sankar, K.V.; Kundu, A.; Ma, M.; Kwon, J.Y.; Jun, S.C. Honeycomb-Like Interconnected Network of Nickel Phosphide Heteronanoparticles with Superior Electrochemical Performance for Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 21829–21838. [Google Scholar] [CrossRef]
- Xu, S.; Du, Y.; Liu, X.; Yu, X.; Teng, C.; Cheng, X.; Chen, Y.; Wu, Q. Three-dimensional (3D) hierarchical coral-like Mn-doped Ni2P–Ni5P4/NF catalyst for efficient oxygen evolution. J. Alloy. Compd. 2020, 826, 154210. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Bao, K.; Xu, T.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Free-standing porous Ni2P-Ni5P4 heterostructured arrays for efficient electrocatalytic water splitting. J. Colloid Interface Sci. 2019, 552, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2020, 31, 2006484. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, C.; Xu, L.; Lu, C.; Yang, J.; Pang, H.; Hou, W. Encapsulation of Janus-structured Ni/Ni2P nanoparticles within hierarchical wrinkled N-doped carbon nanofibers: Interface engineering induces high-efficiency water oxidation. Appl. Catal. B Environ. 2021, 298, 120578. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Q.; Zhang, H.; Qiu, L.; Wu, H.B.; Yu, X.-Y. Deeply reconstructed hierarchical and defective NiOOH/FeOOH nanoboxes with accelerated kinetics for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 15586–15594. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Ji, P.; Hu, W.; Liu, J.; Mu, S. Transition-Metal Phosphides: Activity Origin, Energy-Related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Y.; Liang, Q.; Pang, Y.; Miao, L.; Liu, X.; Kou, Z.; He, J.; Pennycook, S.J.; Mu, S.; et al. Surface nitridation of nickel-cobalt alloy nanocactoids raises the performance of water oxidation and splitting. Appl. Catal. B Environ. 2020, 270, 118889. [Google Scholar] [CrossRef]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.; Hu, J.-S. Scalable Solid-State Synthesis of Highly Dispersed Uncapped Metal (Rh, Ru, Ir) Nanoparticles for Efficient Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801698. [Google Scholar] [CrossRef]
- Yamada, N.; Kitano, S.; Yato, Y.; Kowalski, D.; Aoki, Y.; Habazaki, H. In Situ Activation of Anodized Ni–Fe Alloys for the Oxygen Evolution Reaction in Alkaline Media. ACS Appl. Energy Mater. 2020, 3, 12316–12326. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Wang, T.; Ola, O.; Dapaah, M.F.; Lu, Y.; Niu, Q.; Cheng, L.; Wang, N.; Zhu, Y. Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research. Nanomaterials 2022, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Sun, X.; Liang, Q.; Li, X.; He, Z.; Hu, X.; Zhang, J.; Wang, M.; Li, Q.; Yang, H.; et al. Enhanced Oxygen Evolution Reaction Activity by Encapsulating NiFe Alloy Nanoparticles in Nitrogen-Doped Carbon Nanofibers. ACS Appl. Mater. Interfaces 2020, 12, 31503–31513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.; Peng, T.; Liu, M.; Zhang, L.; Zhang, J. Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc-Air Batteries. Nanomaterials 2022, 12, 381. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, M.; Singh, R. NaCl-Assisted CVD Growth of Large-Area High-Quality Trilayer MoS2 and the Role of the Concentration Boundary Layer. Cryst. Growth Des. 2021, 21, 4940–4946. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hassandoost, R.; Khataee, A.; Luque, R.; Assadi, M.H.N. Hard-templated metal-organic frameworks for advanced applications. Chem. Soc. Rev. 2021, 50, 2927–2953. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Zhang, L.; Zhang, L.; Li, L.; Tian, J.; Wang, M.; Xu, J.; Dai, B.; Li, Y. Fe-leaching induced surface reconstruction of Ni-Fe alloy on N-doped carbon to boost oxygen evolution reaction. Chem. Eng. J. 2020, 394, 124977. [Google Scholar] [CrossRef]
- Huan, Y.; Shi, J.; Zou, X.; Gong, Y.; Xie, C.; Yang, Z.; Zhang, Z.; Gao, Y.; Shi, Y.; Li, M.; et al. Scalable Production of Two-Dimensional Metallic Transition Metal Dichalcogenide Nanosheet Powders Using NaCl Templates toward Electrocatalytic Applications. J. Am. Chem. Soc. 2019, 141, 18694–18703. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Fan, G.; Yang, L.; Li, F. A robust core-shell nanostructured nickel-iron alloy@nitrogen-containing carbon catalyst for the highly efficient hydrogenation of nitroarenes. Dalton Trans. 2018, 47, 13668–13679. [Google Scholar] [CrossRef]
- Fan, A.; Qin, C.; Zhang, X.; Dai, X.; Dong, Z.; Luan, C.; Yu, L.; Ge, J.; Gao, F. Phosphorus-Doped FeNi Alloys/NiFe2O4 Imbedded in Carbon Network Hollow Bipyramid as Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 7, 2285–2295. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Yuan, W.; Li, C.M. Tannic Acid-Mediated In Situ Controlled Assembly of NiFe Alloy Nanoparticles on Pristine Graphene as a Superior Oxygen Evolution Catalyst. ACS Appl. Energy Mater. 2020, 3, 3966–3977. [Google Scholar] [CrossRef]
- Chang, M.C.; Ho, P.H.; Tseng, M.F.; Lin, F.Y.; Hou, C.H.; Lin, I.K.; Wang, H.; Huang, P.P.; Chiang, C.H.; Yang, Y.C.; et al. Fast growth of large-grain and continuous MoS2 films through a self-capping vapor-liquid-solid method. Nat. Commun. 2020, 11, 3682. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Q.; Zhong, L.; Wang, X.; Chai, L.; Li, Q.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Thermal conversion of hollow nickel-organic framework into bimetallic FeNi3 alloy embedded in carbon materials as efficient oer electrocatalyst. Electrochim. Acta 2020, 354, 136716. [Google Scholar] [CrossRef]
- Lim, D.; Oh, E.; Lim, C.; Shim, S.E.; Baeck, S.-H. Bimetallic NiFe alloys as highly efficient electrocatalysts for the oxygen evolution reaction. Catal. Today 2020, 352, 27–33. [Google Scholar] [CrossRef]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Liu, M.; Yong, J.; Qiao, H.; Jin, A.; Li, Z.; Huang, X.; Wang, H.; Zhang, X. Strongly Coupled FeNi Alloys/NiFe2O4@Carbonitride Layers-Assembled Microboxes for Enhanced Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 34396–34404. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zeng, L.; Xu, J.; Zhang, Z.; Zhiliang, z.; Wang, Y.; Yuan, X.-Z.; Li, H.; Wang, H. NaCl template-directed approach to ultrathin lamellar molybdenum phosphide-carbon hybrids for efficient hydrogen production. J. Power Sources 2019, 438, 227048. [Google Scholar] [CrossRef]
- Arunkumar, P.; Gayathri, S.; Han, J.H. A Complementary Co-Ni Phosphide/Bimetallic Alloy-Interspersed N-Doped Graphene Electrocatalyst for Overall Alkaline Water Splitting. ChemSusChem 2021, 14, 1921–1935. [Google Scholar] [CrossRef]
- Gao, X.; Chen, D.; Qi, J.; Li, F.; Song, Y.; Zhang, W.; Cao, R. NiFe Oxalate Nanomesh Array with Homogenous Doping of Fe for Electrocatalytic Water Oxidation. Small 2019, 15, 1904579. [Google Scholar] [CrossRef]
- Li, M.; Huang, Y.; Lin, J.; Li, M.; Jiang, M.; Ding, L.; Sun, D.; Huang, K.; Tang, Y. Carbon Nanotubes Interconnected NiCo Layered Double Hydroxide Rhombic Dodecahedral Nanocages for Efficient Oxygen Evolution Reaction. Nanomaterials 2022, 12, 1015. [Google Scholar] [CrossRef]
- Mukhiya, T.; Muthurasu, A.; Tiwari, A.P.; Chhetri, K.; Chae, S.H.; Kim, H.; Dahal, B.; Lee, B.M.; Kim, H.Y. Integrating the Essence of a Metal-Organic Framework with Electrospinning: A New Approach for Making a Metal Nanoparticle Confined N-Doped Carbon Nanotubes/Porous Carbon Nanofibrous Membrane for Energy Storage and Conversion. ACS Appl Mater. Interfaces 2021, 13, 23732–23742. [Google Scholar] [CrossRef]
- Ji, S.M.; Muthurasu, A.; Chhetri, K.; Yong Kim, H. Metal-organic framework assisted vanadium oxide nanorods as efficient electrode materials for water oxidation. J. Colloid Interface Sci. 2022, 618, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Thangasamy, P.; Nam, S.; Oh, S.; Randriamahazaka, H.; Oh, I.K. Boosting Oxygen Evolution Reaction on Metallocene-based Transition Metal Sulfides Integrated with N-doped Carbon Nanostructures. ChemSusChem 2021, 14, 5004–5020. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Ko, T.H.; Seo, M.K.; Khil, M.S.; Kim, H.Y.; Kim, B.S. Oxalic acid assisted rapid synthesis of mesoporous NiCo2O4 nanorods as electrode materials with higher energy density and cycle stability for high-performance asymmetric hybrid supercapacitor applications. J. Colloid Interface Sci. 2020, 564, 65–76. [Google Scholar] [CrossRef]





| Catalyst | Substrate | Electrolyte | η (mV) 10 mA/cm2 | Tafel Slope (mV/Decade) | Reference |
|---|---|---|---|---|---|
| G@Ni9Fe | GCE 1 | 1.0 M KOH | 246 | 46 | [30] |
| P–Ni0.5Fe@C | GCE 1 | 1.0 M KOH | 256 | 65 | [29] |
| F–NiFe alloys | Ni plate | 1.0 M KOH | 260 | 53 | [19] |
| NiFeC-800-5 | GCE 1 | 1.0 M KOH | 269 | 72 | [32] |
| Ni/Ni2P@N–CNF | GCE 1 | 1.0 M KOH | 285 | 45.2 | [14] |
| Ni2P | NF 2 | 1.0 M KOH | 290 | 47 | [34] |
| NiFe@NCNFs | GCE 1 | 1.0 M KOH | 294 | 52 | [22] |
| NiFe alloys | GCE 1 | 1.0 M KOH | 298 | 51.9 | [33] |
| NiFe/NiFe2O4@NC | GCE 1 | 1.0 M KOH | 316 | 60 | [35] |
| Ni5P4/Ni2P–FeNi@C | GCE 1 | 1.0 M KOH | 242 | 46 | This work |
| Commercial RuO2 | GCE 1 | 1.0 M KOH | 272 | 65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Yi, P.; Sun, J.; Li, C.; Liu, R.; Sun, J.-K. The Scalable Solid-State Synthesis of a Ni5P4/Ni2P–FeNi Alloy Encapsulated into a Hierarchical Porous Carbon Framework for Efficient Oxygen Evolution Reactions. Nanomaterials 2022, 12, 1848. https://doi.org/10.3390/nano12111848
Tian X, Yi P, Sun J, Li C, Liu R, Sun J-K. The Scalable Solid-State Synthesis of a Ni5P4/Ni2P–FeNi Alloy Encapsulated into a Hierarchical Porous Carbon Framework for Efficient Oxygen Evolution Reactions. Nanomaterials. 2022; 12(11):1848. https://doi.org/10.3390/nano12111848
Chicago/Turabian StyleTian, Xiangyun, Peng Yi, Junwei Sun, Caiyun Li, Rongzhan Liu, and Jian-Kun Sun. 2022. "The Scalable Solid-State Synthesis of a Ni5P4/Ni2P–FeNi Alloy Encapsulated into a Hierarchical Porous Carbon Framework for Efficient Oxygen Evolution Reactions" Nanomaterials 12, no. 11: 1848. https://doi.org/10.3390/nano12111848