Bactericidal and Antiviral Bionic Metalized Nanocoatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Eye Samples of Anopheles gambiae for Atomic Force Microscopy (AFM) and Proteomic Analyses
2.2. AFM
2.3. Mass-Spectrometry
2.4. Cloning of Genes for Retinin and Retinin-Like Proteins
2.5. Purification of Retinin and Retinin-Like Proteins
2.6. Fatty Acid Emulsion Preparation
2.7. Surface Treatment
2.7.1. Basic Protein-Based Nanocoatings
2.7.2. Post-Assembly Metallization
2.7.3. Pre-Assembly Metallization
2.8. SEM and EDS Analyzes
2.9. Anti-Infective Activity Measurement
2.9.1. Bactericidal Activity Measurement
2.9.2. Antiviral Activity Measurement
2.10. Cytotoxicity Test
2.11. Statistical Analysis
3. Results
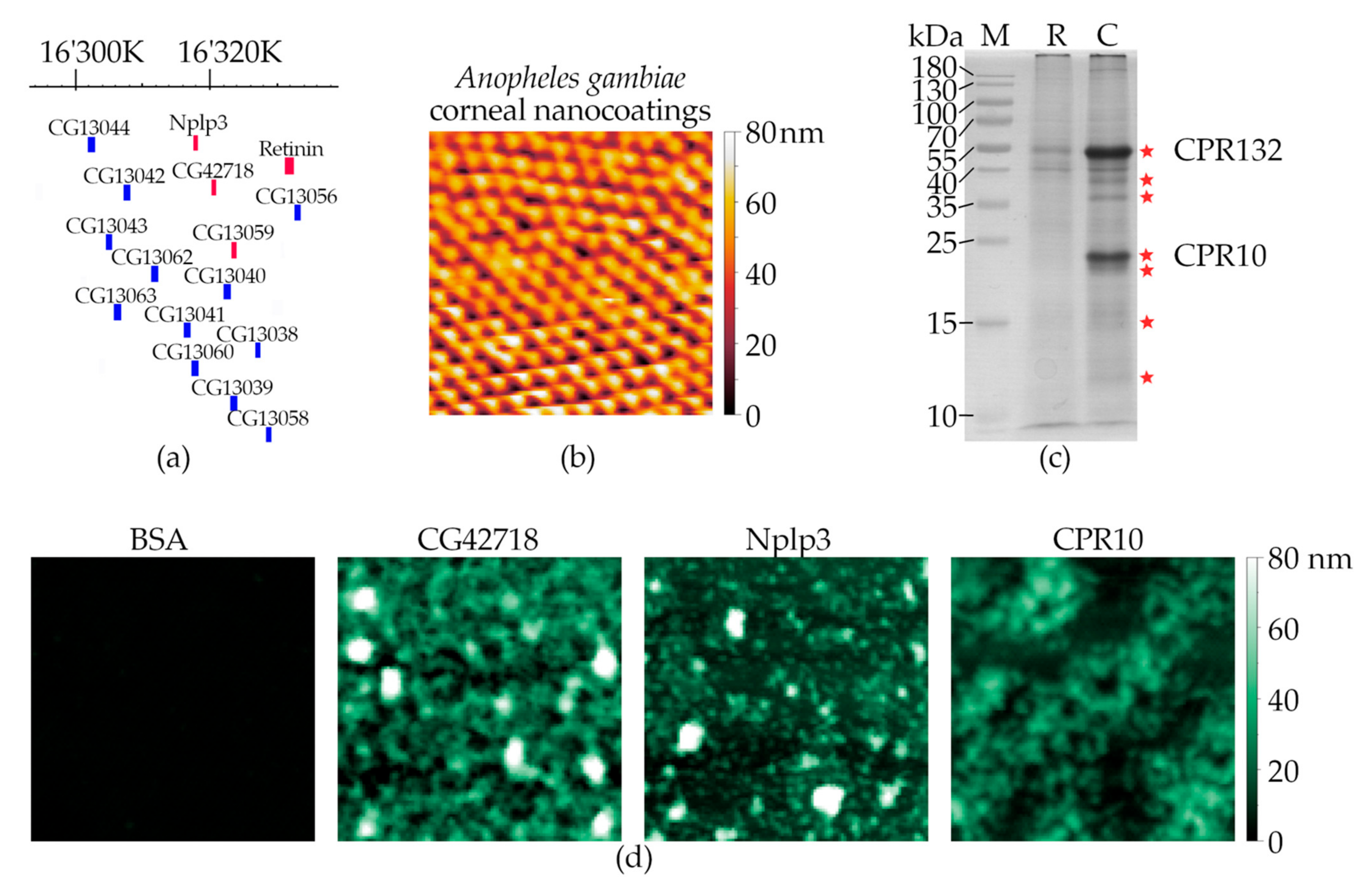
3.1. Diversification of the Basic Retinin-Based Nanocoatings
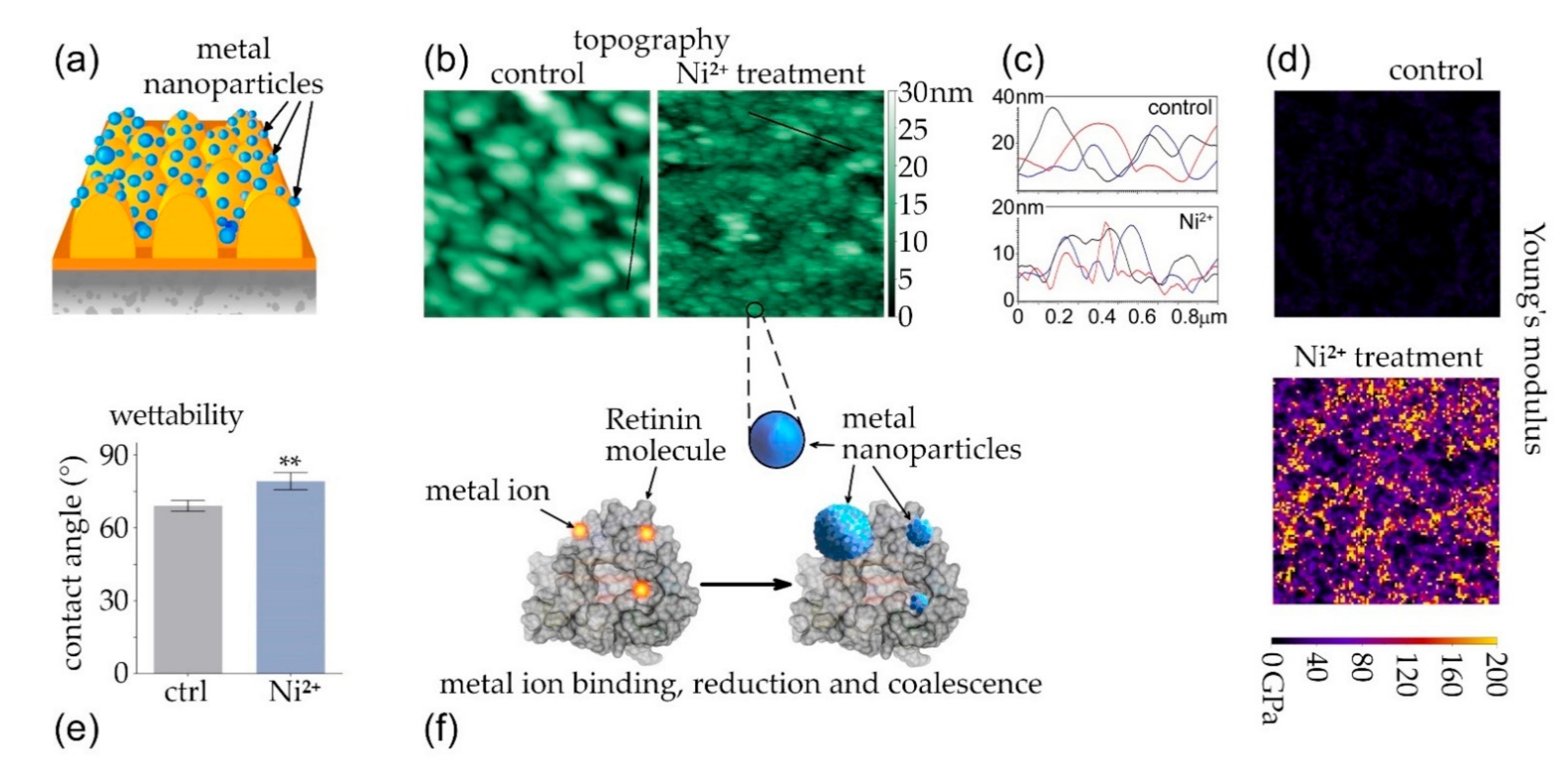
3.2. Coalescence of Metal Nanoparticles on Top of Protein-Based Nanocoatings
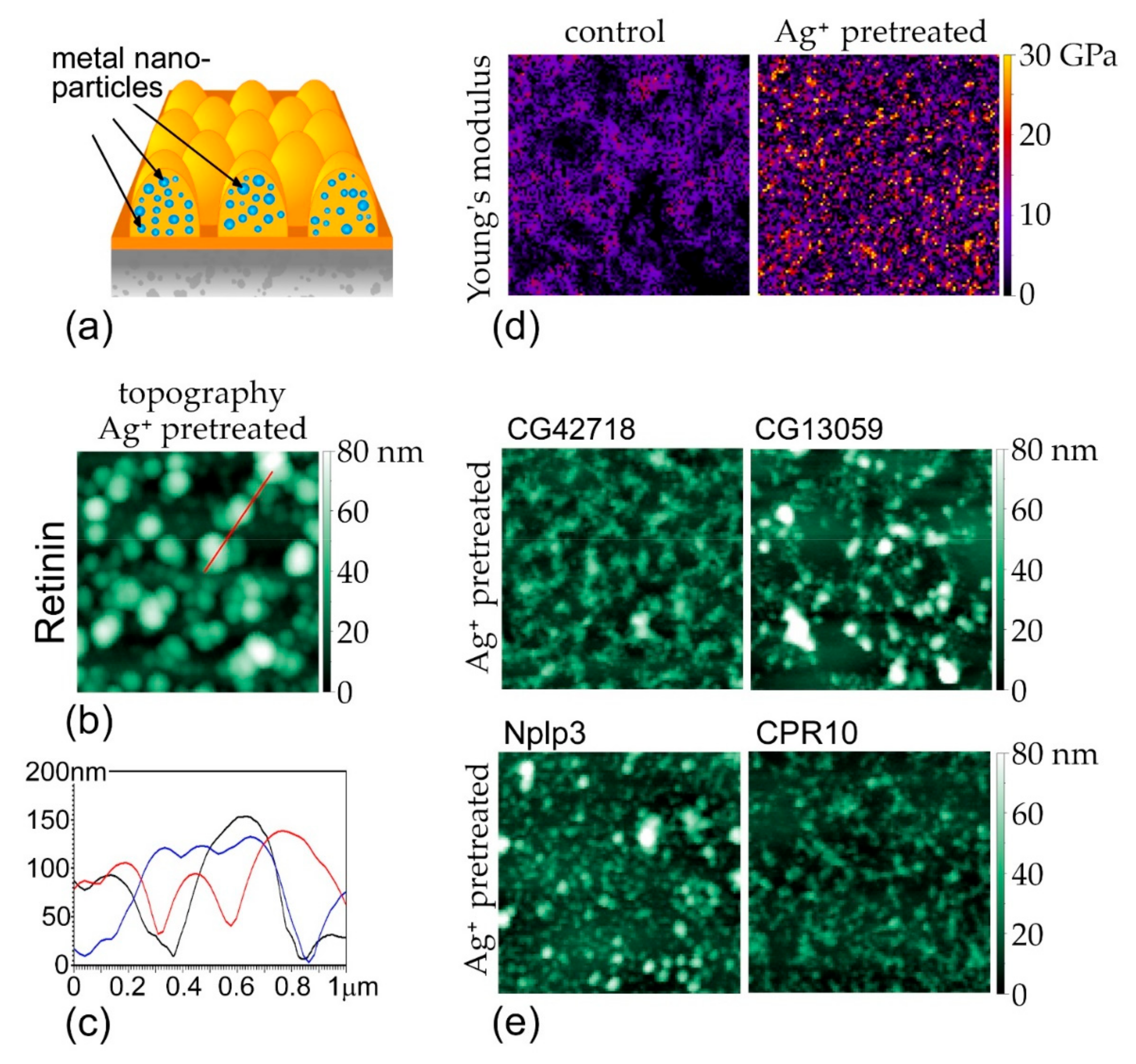
3.3. Metal-Conjugated Retinins Retain the Capacity of Self-Assembly
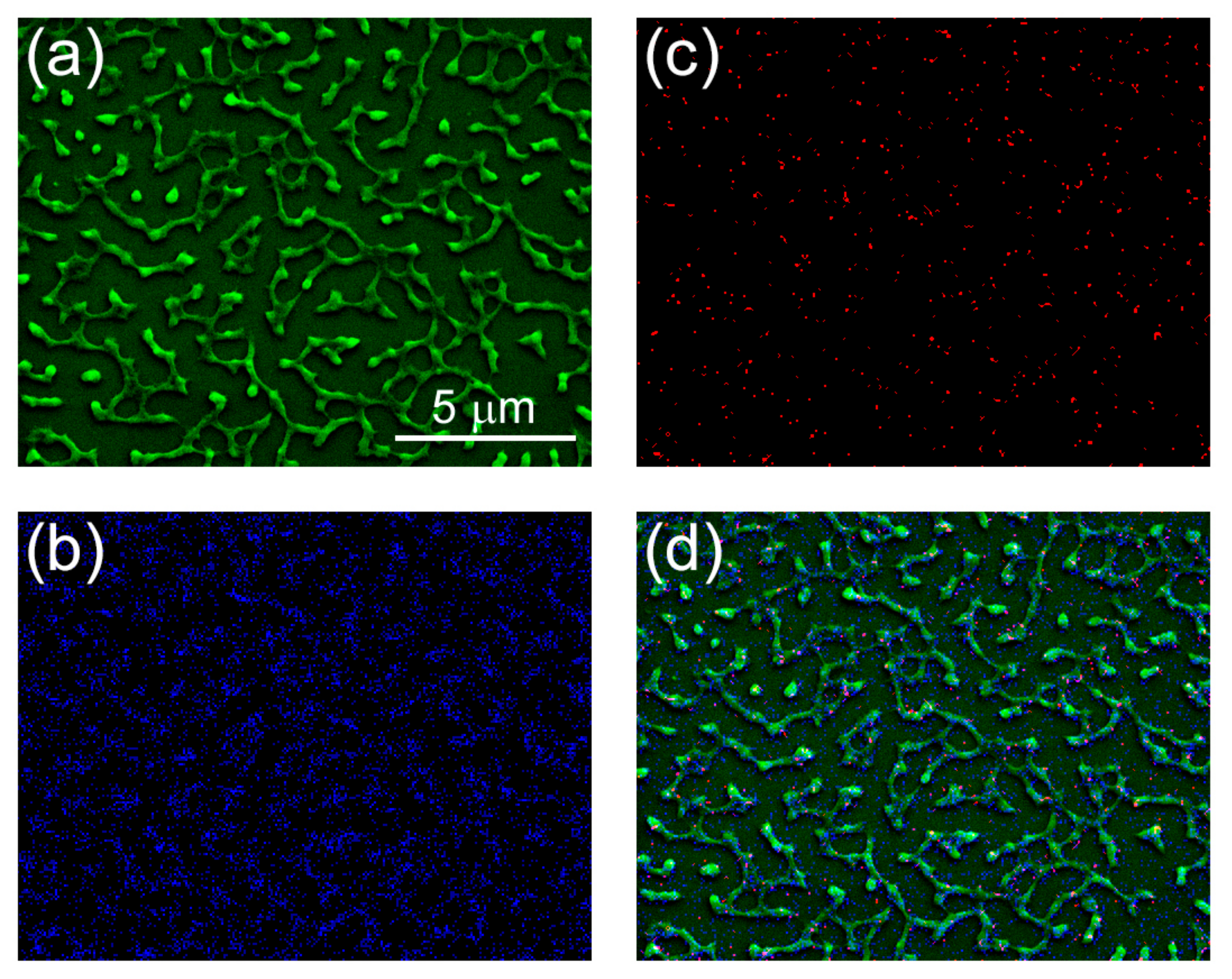
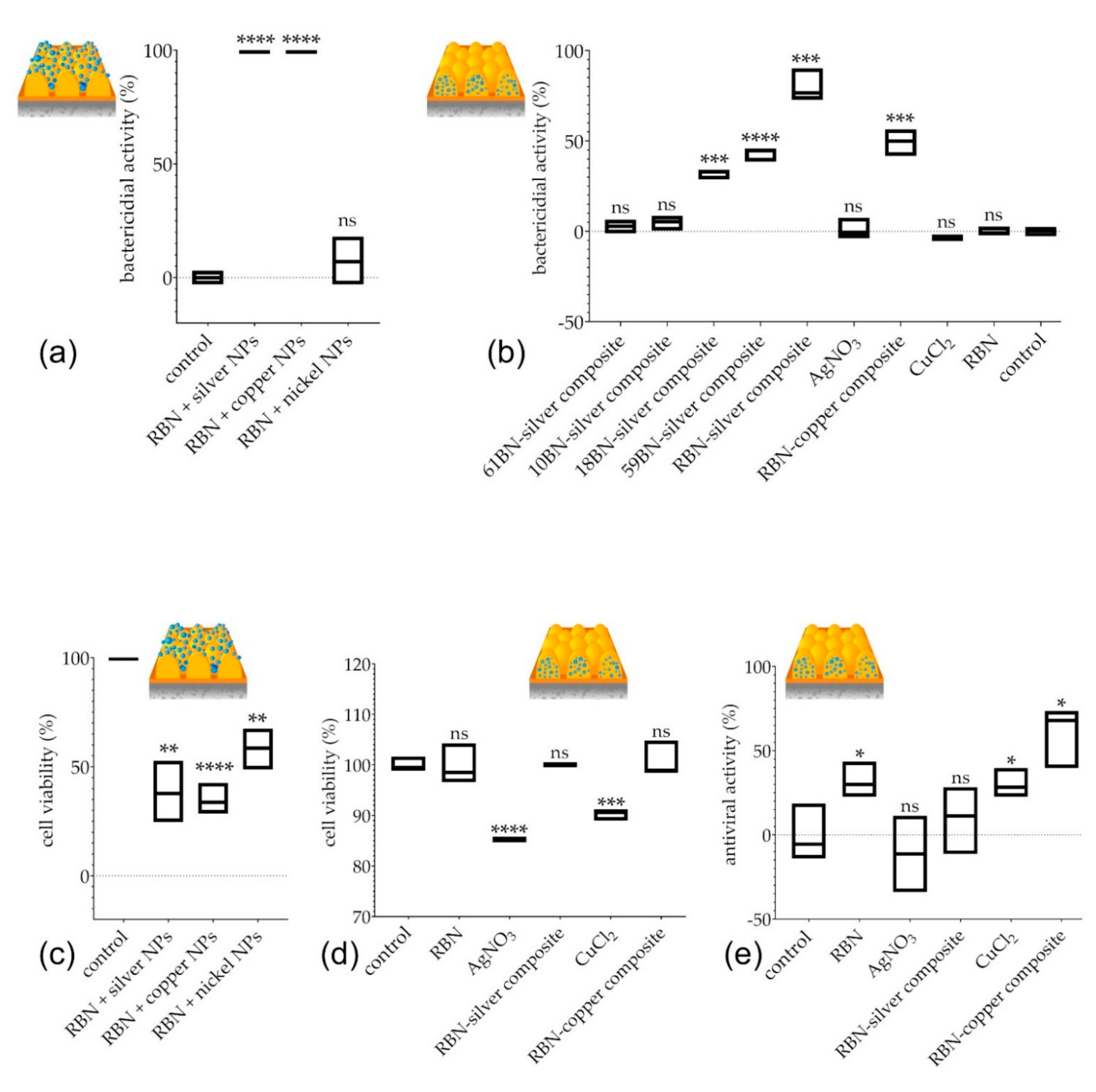
3.4. Bactericidal, Antiviral, and Cytotoxic Properties of Diverse Composite Nanocoatings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palasantzas, G.; De Hosson, J.T.M.; Michielsen, K.F.L.; Stavenga, D.G. Optical properties and wettability of nanostructured biomaterials: Moth eyes, lotus leaves, and insect wings. In Handbook of Nanostructured Biomaterials and their Applications in Nanobiotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2005; Volume 1, pp. 273–301. [Google Scholar]
- Arzt, E.; Gorb, S.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryuchkov, M.; Blagodatski, A.; Cherepanov, V.; Katanaev, V.L. Arthropod corneal nanocoatings: Diversity, mechanisms, and functions. In Functional Surfaces in Biology III: Diversity of the Physical Phenomena; Gorb, S.N., Gorb, E.V., Eds.; Springer International: Berlin/Heidelberg, Germany, 2017; pp. 29–52. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Johansen, V.E.; Onelli, O.D.; Steiner, L.M.; Vignolini, S. Photonics in nature: From order to disorder. In Functional Surfaces in Biology III: Diversity of the Physical Phenomena; Gorb, S.N., Gorb, E.V., Eds.; Springer International: Cham, Switzerland, 2017; pp. 53–89. [Google Scholar] [CrossRef] [Green Version]
- Turing, A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1952, 237, 37–72. [Google Scholar] [CrossRef]
- Kondo, S.; Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 2010, 329, 1616–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagodatski, A.; Sergeev, A.; Kryuchkov, M.; Lopatina, Y.; Katanaev, V.L. Diverse set of Turing nanopatterns coat corneae across insect lineages. Proc. Natl. Acad. Sci. USA 2015, 112, 10750–10755. [Google Scholar] [CrossRef] [Green Version]
- Buscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 527–532. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Katanaev, V.L.; Enin, G.A.; Sergeev, A.; Timchenko, A.A.; Serdyuk, I.N. Analysis of micro- and nano-structures of the corneal surface of Drosophila and its mutants by atomic force microscopy and optical diffraction. PLoS ONE 2011, 6, e22237. [Google Scholar] [CrossRef] [Green Version]
- Kryuchkov, M.; Bilousov, O.; Lehmann, J.; Fiebig, M.; Katanaev, V.L. Reverse and forward engineering of Drosophila corneal nanocoatings. Nature 2020, 585, 383–389. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Savitsky, V.; Wilts, B.D.; Gray, E.; Katanaev, V.L. Light Polarization by Biological Nanocoatings. ACS Appl. Mater. Interfaces 2021, 13, 23481–23488. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Lehmann, J.; Schaab, J.; Cherepanov, V.; Blagodatski, A.; Fiebig, M.; Katanaev, V.L. Alternative moth-eye nanostructures: Antireflective properties and composition of dimpled corneal nanocoatings in silk-moth ancestors. J. Nanobiotechnol. 2017, 15, 61. [Google Scholar] [CrossRef] [Green Version]
- Kryuchkov, M.; Lehmann, J.; Schaab, J.; Fiebig, M.; Katanaev, V.L. Antireflective nanocoatings for UV-sensation: The case of predatory owlfly insects. J. Nanobiotechnol. 2017, 15, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagodatski, A.; Kryuchkov, M.; Sergeev, A.; Klimov, A.A.; Shcherbakov, M.R.; Enin, G.A.; Katanaev, V.L. Under- and over-water halves of Gyrinidae beetle eyes harbor different corneal nanocoatings providing adaptation to the water and air environments. Sci. Rep. 2014, 4, 6004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antimicrobial_Resistance_Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. 2019. Available online: www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 25 May 2022).
- Meganck, R.M.; Baric, R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat. Med. 2021, 27, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Izmalkova, T.Y.; Sazonova, O.I.; Dymova, E.A.; Sokolov, S.L.; Gafarov, A.B. Playgrounds in city of pushchino with different types of coating as reservoir of antibiotic-resistant strains of Pseudomonas spp. Curr. Microbiol. 2022, 79, 80. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Arendsen Linda, P.; Thakar, R.; Sultan Abdul, H. The use of copper as an antimicrobial agent in health care, including obstetrics and gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging concern for silver nanoparticle resistance in acinetobacter baumannii and other bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef] [PubMed]
- Román-Kustas, J.; Hoffman, J.B.; Reed, J.H.; Gonsalves, A.E.; Oh, J.; Li, L.; Hong, S.; Jo, K.D.; Dana, C.E.; Miljkovic, N.; et al. Molecular and topographical organization: Influence on cicada wing wettability and bactericidal properties. Adv. Mater. Interfaces 2020, 7, 2000112. [Google Scholar] [CrossRef] [Green Version]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial nanomaterials and coatings: Current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2021, 13, 16. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef]
- Staneva, A.D.; Dimitrov, D.K.; Gospodinova, D.N.; Vladkova, T.G. Antibiofouling activity of graphene materials and graphene-based antimicrobial coatings. Microorganisms 2021, 9, 1839. [Google Scholar] [CrossRef]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface design for antibacterial materials: From fundamentals to advanced strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef]
- Molling, J.; Seezink, J.; Teunissen, B.; Muijrers, I.; Borm, P. Comparative performance of a panel of commercially available antimicrobial nanocoatings in Europe. Nanotechnol. Sci. Appl. 2014, 2014, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [Green Version]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.N.; Lee, J.Y.; Wang, D.I. Uncovering the design rules for peptide synthesis of metal nanoparticles. J. Am. Chem Soc. 2010, 132, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Necas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Appert-Collin, A.; Cotecchia, S.; Nenniger-Tosato, M.; Pedrazzini, T.; Diviani, D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 10140–10145. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.P.; Luce, M.J.; Reiser, J. Small-to large-scale production of lentivirus vectors. Methods Mol. Biol. 2003, 229, 43–55. [Google Scholar] [CrossRef]
- Perticaroli, S.; Nickels, J.D.; Ehlers, G.; O’Neill, H.; Zhang, Q.; Sokolov, A.P. Secondary structure and rigidity in model proteins. Soft Matter 2013, 9, 9548–9556. [Google Scholar] [CrossRef]
- Denver, H.; Heiman, T.; Martin, E.; Gupta, A.; Borca-Tasciuc, D.-A. Mechanical characterization of nickel nanoparticles elastomer composites. In Proceedings of the 2008 NSTI Nanotechnology Conference and Trade Show, Boston, MA, USA, 1–5 June 2008; Volume 1, pp. 301–303. [Google Scholar]
- Walch, E.B.T.; Roos, C. Measurement of the mechanical properties of silver and enamel thick films using nanoindentation. Int. J. Appl. Glass Sci. 2020, 11, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, M.D.; Nazreen, S.; Ali, N.M.; Amna, T. ZnO Nanocomposites of Juniperus procera and Dodonaea viscosa Extracts as Antiproliferative and Antimicrobial Agents. Nanomaterials 2022, 12, 664. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Mishra, A.; Yun, S.I.; Kim, H.C.; Kim, H.Y.; Khil, M.S. Fabrication, characterization and antibacterial effect of novel electrospun TiO2 nanorods on a panel of pathogenic bacteria. J. Biomed. Nanotechnol. 2012, 8, 394–404. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Yang, J.; Khil, M.S.; Song, K.D.; Oh, J.D.; Hwang, I. Virgin olive oil blended polyurethane micro/nanofibers ornamented with copper oxide nanocrystals for biomedical applications. Int. J. Nanomed. 2014, 9, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.J.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amna, T.; Yang, J.; Ryu, K.S.; Hwang, I.H. Electrospun antimicrobial hybrid mats: Innovative packaging material for meat and meat-products. J. Food Sci. Technol. 2015, 52, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Amna, T.; Yang, O.B.; El-Newehy, M.H.; Al-Deyab, S.S.; Khil, M.S. Smart copper oxide nanocrystals: Synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf. B Biointerfaces 2012, 97, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Hyeon, T.; Kim, D.-H.; Ruiz, R.; Tung, M.C.; Wong, H.S.P. Self-assembly for electronics. MRS Bull. 2020, 45, 807–814. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, H.-J.; Kim, J.Y.; Jung, W.-B.; Jeong, H.-S.; Kim, Y.H.; Shin, D.O.; Jeong, S.-J.; Shin, J.; Kim, S.O.; Jung, H.-T. Complex High-Aspect-Ratio Metal Nanostructures by Secondary Sputtering Combined with Block Copolymer Self-Assembly. Adv. Mater. 2016, 28, 8439–8445. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Joung, D.; Cho, J.-H. Plasma Triggered Grain Coalescence for Self-Assembly of 3D Nanostructures. Nano-Micro Lett. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryuchkov, M.; Adamcik, J.; Katanaev, V.L. Bactericidal and Antiviral Bionic Metalized Nanocoatings. Nanomaterials 2022, 12, 1868. https://doi.org/10.3390/nano12111868
Kryuchkov M, Adamcik J, Katanaev VL. Bactericidal and Antiviral Bionic Metalized Nanocoatings. Nanomaterials. 2022; 12(11):1868. https://doi.org/10.3390/nano12111868
Chicago/Turabian StyleKryuchkov, Mikhail, Jozef Adamcik, and Vladimir L. Katanaev. 2022. "Bactericidal and Antiviral Bionic Metalized Nanocoatings" Nanomaterials 12, no. 11: 1868. https://doi.org/10.3390/nano12111868
APA StyleKryuchkov, M., Adamcik, J., & Katanaev, V. L. (2022). Bactericidal and Antiviral Bionic Metalized Nanocoatings. Nanomaterials, 12(11), 1868. https://doi.org/10.3390/nano12111868







