Large Enhancement in Thermal Conductivity of Solvent−Cast Expanded Graphite/Polyetherimide Composites
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Fabrication of the EG/Polymer Composites
3. Characterization
4. Result and Discussion
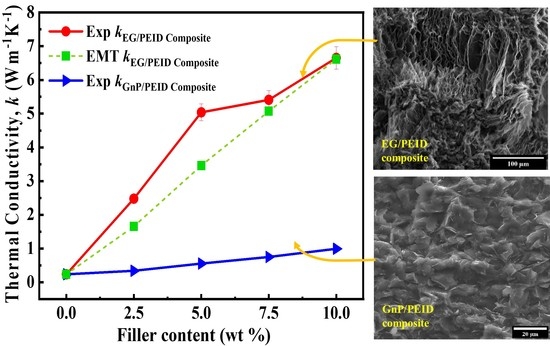
4.1. Thermal Conductivity Results
| Filler | Matrix | Fraction | Enhancement (%) | Preparation Method | Ref. | ||
|---|---|---|---|---|---|---|---|
| GnPs/MWCNT | PS | (3.5/1.5) vol% | 1.02 | 0.19 | 437 | Melt mixing + Synergistic effect | [70] |
| Graphite | PP | 40 wt% | 5.4 | 0.25 | 2060 | Compression molding | [71] |
| Multilayer GnP | Epoxy | 10 vol% | 5.1 | 0.21 | 2300 | Solvent casting, higher sheer mixing | [8] |
| fGO | Epoxy | 5 wt% | 0.21 | 0.16 | 34 | Solution casting | [72] |
| EG | LLDP | 5.78 wt% | 1.85 | 0.33 | 461 | Melt mixing, 3D network formation | [48] |
| EG | PMMA | 7 wt% | 0.47 | 0.125 | 276 | Water-assistedmelt extrusion | [73] |
| EG | LDPE | 10 wt% | 0.5 | 0.32 | 56 | Melt mixing | [74] |
| EG | PDMS | 10 wt% | 4.7 | 0.18 | 2511 | Solvent casting, hot press | [45] |
| EG | PEG | 10 wt% | 1.324 | 0.298 | 344 | Melt mixing | [75] |
| EG | Paraffin | 25 wt% | 3.16 | 0.18 | 1695 | Melt mixing | [46] |
| EG | PEID | 30 wt% | 1.6 | 0.2 | 700 | Solvent mixing, melt mixing followed by injection molding | [76] |
| EG | PEID | 10 wt% | 6.6 | 0.23 | 2770 | Solution casting | This work |
4.2. Effective Medium Model for Thermal Conductivity Prediction
4.3. Morphologies of EG Filler and EG/PEID Composite
4.4. Analysis of Crystal Structure by XRD and Raman Spectroscopy
4.5. XPS Analysis of GIC and EG Filler
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Tian, B.; Liang, J.; Wu, W. Recent advances in printed flexible heaters for portable and wearable thermal management. Mater. Horiz. 2021, 8, 1634–1656. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, M.; Flohn, J.; Hu, Y. Thermal management materials for energy-efficient and sustainable future buildings. Chem. Commun. 2021, 57, 12236–12253. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, M.; Kumar, C.R.; Joshi, G.M. Polymer composites for thermal management: A review. Compos. Interfaces 2016, 23, 847–872. [Google Scholar] [CrossRef]
- Wang, K.; Amin, K.; An, Z.; Cai, Z.; Chen, H.; Chen, H.; Dong, Y.; Feng, X.; Fu, W.; Gu, J. Advanced functional polymer materials. Mater. Chem. Front. 2020, 4, 1803–1915. [Google Scholar] [CrossRef]
- Tarannum, F.; Muthaiah, R.; Annam, R.S.; Gu, T.; Garg, J. Effect of alignment on enhancement of thermal conductivity of polyethylene–graphene nanocomposites and comparison with effective medium theory. Nanomater. 2020, 10, 1291. [Google Scholar] [CrossRef]
- Muthaiah, R.; Garg, J. Temperature effects in the thermal conductivity of aligned amorphous polyethylene—A molecular dynamics study. J. Appl. Phys. 2018, 124, 105102. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.S.; Perrier, T.; Barani, Z.; Kargar, F.; Balandin, A.A. Thermal interface materials with graphene fillers: Review of the state of the art and outlook for future applications. Nanotechnology 2021, 32, 142003. [Google Scholar] [CrossRef]
- Shahil, K.M.; Balandin, A.A. Graphene–multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Sudhindra, S.; Rashvand, F.; Wright, D.; Barani, Z.; Drozdov, A.D.; Baraghani, S.; Backes, C.; Kargar, F.; Balandin, A.A. Specifics of Thermal Transport in Graphene Composites: Effect of Lateral Dimensions of Graphene Fillers. ACS Appl. Mater. Interfaces 2021, 13, 53073–53082. [Google Scholar] [CrossRef]
- Sudhindra, S.; Kargar, F.; Balandin, A.A. Noncured Graphene Thermal Interface Materials for High-Power Electronics: Minimizing the Thermal Contact Resistance. Nanotechnology 2021, 11, 1699. [Google Scholar] [CrossRef]
- Bernal, M.M.; Di Pierro, A.; Novara, C.; Giorgis, F.; Mortazavi, B.; Saracco, G.; Fina, A. Edge-Grafted Molecular Junctions between Graphene Nanoplatelets: Applied Chemistry to Enhance Heat Transfer in Nanomaterials. Adv. Funct. Mater. 2018, 28, 1706954. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A. Phononics of Graphene and Related Materials. ACS Nano 2020, 14, 5170–5178. [Google Scholar] [CrossRef] [PubMed]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.-Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal Properties of the Binary-Filler Hybrid Composites with Graphene and Copper Nanoparticles. Adv. Funct. Mater. 2020, 30, 1904008. [Google Scholar] [CrossRef]
- An, F.; Li, X.; Min, P.; Liu, P.; Jiang, Z.-G.; Yu, Z.-Z. Vertically Aligned High-Quality Graphene Foams for Anisotropically Conductive Polymer Composites with Ultrahigh Through-Plane Thermal Conductivities. ACS Appl. Mater. Interfaces 2018, 10, 17383–17392. [Google Scholar] [CrossRef] [PubMed]
- Saeidijavash, M.; Garg, J.; Grady, B.; Smith, B.; Li, Z.; Young, R.J.; Tarannum, F.; Bel Bekri, N. High thermal conductivity through simultaneously aligned polyethylene lamellae and graphene nanoplatelets. Nanoscale 2017, 9, 12867–12873. [Google Scholar] [CrossRef]
- Yan, H.; Tang, Y.; Long, W.; Li, Y. Enhanced thermal conductivity in polymer composites with aligned graphene nanosheets. J. Mater. Sci. 2014, 49, 5256–5264. [Google Scholar] [CrossRef]
- Wu, S.; Ladani, R.B.; Zhang, J.; Bafekrpour, E.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. Aligning multilayer graphene flakes with an external electric field to improve multifunctional properties of epoxy nanocomposites. Carbon 2015, 94, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Liao, Q.; Liu, Z.; Liu, W.; Deng, C.; Yang, N. Extremely High Thermal Conductivity of Aligned Carbon Nanotube-Polyethylene Composites. Sci. Rep. 2015, 5, 16543. [Google Scholar] [CrossRef] [Green Version]
- Lian, G.; Tuan, C.-C.; Li, L.; Jiao, S.; Wang, Q.; Moon, K.-S.; Cui, D.; Wong, C.-P. Vertically Aligned and Interconnected Graphene Networks for High Thermal Conductivity of Epoxy Composites with Ultralow Loading. Chem. Mater. 2016, 28, 6096–6104. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Li, W.; Qiu, S.; Zhu, C.; Wei, X.; Chen, M.; Liu, C.; Liao, S.; Gong, Y.; et al. Ultrahigh Thermal Conductivity of Assembled Aligned Multilayer Graphene/Epoxy Composite. Chem. Mater. 2014, 26, 4459–4465. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal Percolation Threshold and Thermal Properties of Composites with High Loading of Graphene and Boron Nitride Fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Hwang, H.S.; Lee, J.; Park, I. Azelaic Acid/Expanded Graphite Composites with High Latent Heat Storage Capacity and Thermal Conductivity at Medium Temperature. ACS Omega 2021, 6, 8469–8476. [Google Scholar] [CrossRef]
- Deng, S.; Zhu, Y.; Qi, X.; Yu, W.; Chen, F.; Fu, Q. Preparation of polyvinylidene fluoride/expanded graphite composites with enhanced thermal conductivity via ball milling treatment. RSC Adv. 2016, 6, 45578–45584. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Kim, W.Y.; Lee, H.S.; Kim, S.Y.; Khil, M.-S. Volume control of expanded graphite based on inductively coupled plasma and enhanced thermal conductivity of epoxy composite by formation of the filler network. Carbon 2017, 119, 40–46. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Liu, L.; Liang, J.; Liu, W. Polymer/expanded graphite-based flexible phase change material with high thermal conductivity for battery thermal management. J. Clean. Prod. 2022, 331, 130014. [Google Scholar] [CrossRef]
- Nayak, S.K.; Mohanty, S.; Nayak, S.K. A new way synthesis of expanded graphite as a thermal filler to enhance the thermal conductivity of DGEBA resin as thermal interface material. High Perform. Polym. 2019, 32, 506–523. [Google Scholar] [CrossRef]
- Raza, G.; Shi, Y.; Deng, Y. Expanded graphite as thermal conductivity enhancer for paraffin wax being used in thermal energy storage systems. In Proceedings of the 2016 13th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 12–16 January 2016; pp. 1–12. [Google Scholar]
- Moon, S.; Choi, J.; Farris, R.J. Preparation of aligned polyetherimide fiber by electrospinning. J. Appl. Polym. Sci. 2008, 109, 691–694. [Google Scholar] [CrossRef]
- Sato, D.M.; Guerrini, L.M.; De Oliveira, M.P.; de Oliveira Hein, L.R.; Botelho, E.C. Production and characterization of polyetherimide mats by an electrospinning process. Mater. Res. Express 2018, 5, 115302. [Google Scholar] [CrossRef] [Green Version]
- Khanbareh, H.; Hegde, M.; Bijleveld, J.; Van Der Zwaag, S.; Groen, P. Functionally graded ferroelectric polyetherimide composites for high temperature sensing. J. Mater. Chem. C 2017, 5, 9389–9397. [Google Scholar] [CrossRef] [Green Version]
- Preethika, M.; Shetty, B.H.; Govindasamy, M.; Sundramoorthy, A.K. Recent Trends in the Applications of Thermally Expanded Graphite for Energy Storage and Sensors–A Review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar]
- Huang, J.; Zhao, Z.; Chen, T.; Zhu, Y.; Lv, Z.; Gong, X.; Niu, Y.; Ma, B. Preparation of highly dispersed expandable graphite/polystyrene composite foam via suspension polymerization with enhanced fire retardation. Carbon 2019, 146, 503–512. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Wang, S.; Ding, Y.; Gao, X.; Zhang, Z.; Fang, Y. SiO2 hydrophilic modification of expanded graphite to fabricate form-stable ternary nitrate composite room temperature phase change material for thermal energy storage. Chem. Eng. J. 2021, 413, 127549. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, Q. N-eicosane/expanded graphite as composite phase change materials for electro-driven thermal energy storage. J. Energy Storage 2020, 29, 101339. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, X.; Su, S.; Ma, J.; Hu, Y.; Liu, Y.; Kang, F.; He, Y.-B. Expanded-graphite embedded in lithium metal as dendrite-free anode of lithium metal batteries. J. Mater. Chem. A 2019, 7, 15871–15879. [Google Scholar] [CrossRef]
- Xu, T.; Wang, D.; Qiu, P.; Zhang, J.; Wang, Q.; Xia, B.; Xie, X. In situ synthesis of porous Si dispersed in carbon nanotube intertwined expanded graphite for high-energy lithium-ion batteries. Nanoscale 2018, 10, 16638–16644. [Google Scholar] [CrossRef]
- Manea, F.; Radovan, C.; Corb, I.; Pop, A.; Burtica, G.; Malchev, P.; Picken, S.; Schoonman, J. Simultaneous Determination of 4-Chlorophenol and Oxalic Acid Using an Expanded Graphite-Epoxy Composite Electrode. Electroanal. An Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1719–1722. [Google Scholar] [CrossRef]
- Dhakate, S.; Sharma, S.; Borah, M.; Mathur, R.; Dhami, T. Expanded graphite-based electrically conductive composites as bipolar plate for PEM fuel cell. Int. J. Hydrog. Energy 2008, 33, 7146–7152. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zeng, T.; Zhang, Y.; Wan, Q.; Li, Y.; Yang, N. Three-dimensional catalyst systems from expanded graphite and metal nanoparticles for electrocatalytic oxidation of liquid fuels. Nanoscale 2019, 11, 7952–7958. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, C.; Li, Y.; Chen, H.; Shao, Z. Self-adhesive Co3O4/expanded graphite paper as high-performance flexible anode for Li-ion batteries. Carbon 2015, 95, 494–496. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Lin, S.; Chen, Z.; Wang, Y.; Zhao, X.; Wu, T.; Zhang, J.; Liu, W.; Lu, H. Building vertically-structured, high-performance electrodes by interlayer-confined reactions in accordion-like, chemically expanded graphite. Nano Energy 2020, 70, 104482. [Google Scholar] [CrossRef]
- Xiong, C.; Lin, X.; Liu, H.; Li, M.; Li, B.; Jiao, S.; Zhao, W.; Duan, C.; Dai, L.; Ni, Y. Fabrication of 3D expanded graphite-based (MnO2 nanowalls and PANI nanofibers) hybrid as bifunctional material for high-performance supercapacitor and sensor. J. Electrochem. Soc. 2019, 166, A3965. [Google Scholar] [CrossRef]
- He, J.; Chen, S.; Yang, S.; Song, W.; Yu, C.; Song, L. Fabrication of MoS2 loaded on expanded graphite matrix for high-efficiency pH-universal hydrogen evolution reaction. J. Alloy. Compd. 2020, 828, 154370. [Google Scholar] [CrossRef]
- Wu, S.; Li, Q.-Y.; Ikuta, T.; Morishita, K.; Takahashi, K.; Wang, R.; Li, T. Thermal conductivity measurement of an individual millimeter-long expanded graphite ribbon using a variable-length T-type method. Int. J. Heat Mass Transf. 2021, 171, 121115. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, H.; Li, X.; Liu, Z.; Guo, Q. Expanded graphite/polydimethylsiloxane composites with high thermal conductivity. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.; Zou, B.; Qiao, G.; Zhang, T.; Cong, L.; Jiang, F.; Li, C.; Huang, Y.; Ding, Y. Expanded graphite–Paraffin composite phase change materials: Effect of particle size on the composite structure and properties. Appl. Therm. Eng. 2020, 171, 115015. [Google Scholar] [CrossRef]
- Song, Z.; Deng, Y.; Li, J.; Nian, H. Expanded graphite for thermal conductivity and reliability enhancement and supercooling decrease of MgCl2⋅6H2O phase change material. Mater. Res. Bull. 2018, 102, 203–208. [Google Scholar] [CrossRef]
- Wei, B.; Yang, S. Polymer composites with expanded graphite network with superior thermal conductivity and electromagnetic interference shielding performance. Chem. Eng. J. 2021, 404, 126437. [Google Scholar] [CrossRef]
- Che, J.; Wu, K.; Lin, Y.; Wang, K.; Fu, Q. Largely improved thermal conductivity of HDPE/expanded graphite/carbon nanotubes ternary composites via filler network-network synergy. Composites Part A Appl. Sci. Manuf. 2017, 99, 32–40. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, S.; Wang, Q. High-performance thermal and electrical conductive composites from multilayer plastic packaging waste and expanded graphite. J. Mater. Chem. C 2018, 6, 11209–11218. [Google Scholar] [CrossRef]
- Termentzidis, K.; Giordano, V.M.; Katsikini, M.; Paloura, E.; Pernot, G.; Verdier, M.; Lacroix, D.; Karakostas, I.; Kioseoglou, J. Enhanced thermal conductivity in percolating nanocomposites: A molecular dynamics investigation. Nanoscale 2018, 10, 21732–21741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghossoub, M.G.; Lee, J.-H.; Baris, O.T.; Cahill, D.G.; Sinha, S. Percolation of thermal conductivity in amorphous fluorocarbons. Phys. Rev. B 2010, 82, 195441. [Google Scholar] [CrossRef]
- Jang, J.-u.; Nam, H.E.; So, S.O.; Lee, H.; Kim, G.S.; Kim, S.Y.; Kim, S.H. Thermal Percolation Behavior in Thermal Conductivity of Polymer Nanocomposite with Lateral Size of Graphene Nanoplatelet. Polymers 2022, 14, 323. [Google Scholar] [CrossRef]
- Konatham, D.; Papavassiliou, D.V.; Striolo, A. Thermal boundary resistance at the graphene–graphene interface estimated by molecular dynamics simulations. Chem. Phys. Lett. 2012, 527, 47–50. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of yellowing: Carbonyl formation during hygrothermal aging in a common amine epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.; Heo, Y.; Yoo, Y.; Kim, J. The addition of functionalized graphene oxide to polyetherimide to improve its thermal conductivity and mechanical properties. Polym. Adv. Technol. 2014, 25, 1155–1162. [Google Scholar] [CrossRef]
- Tarannum, F.; Muthaiah, R.; Danayat, S.; Foley, K.; Annam, R.S.; Walters, K.B.; Garg, J. Chemically Edge-Carboxylated Graphene Enhances the Thermal Conductivity of Polyetherimide–Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2022, 14, 14753–14763. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Effect of oxidation degree on the thermal properties of graphene oxide. J. Mater. Res. Technol. 2020, 9, 13740–13748. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.-Q.; Huang, P.; Cao, H.-J.; Zeng, W.; Li, J.; Li, F.; Sun, B.-G.; Shi, H.-Q.; Zhou, Z.-l. Temperature-dependent mechanical properties of polyetherimide composites reinforced by graphene oxide-coated short carbon fibers. Compos. Struct. 2021, 270, 114075. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Q.; Liao, W.; Wang, G.; Wei, W.; Li, C. Green preparation of expandable graphite and its application in flame-resistance polymer elastomer. Ind. Eng. Chem. Res. 2017, 56, 5253–5261. [Google Scholar] [CrossRef]
- Tarannum, F.; Danayat, S.; Nayal, A.; Muthaiah, R.; Annam, R.S.; Garg, J. Thermally expanded graphite polyetherimide composite with superior electrical and thermal conductivity. Arxiv Prepr. 2022, arXiv:2204.12075. [Google Scholar] [CrossRef]
- Kuan, C.F.; Tsai, K.C.; Chen, C.H.; Kuan, H.C.; Liu, T.Y.; Chiang, C.L. Preparation of expandable graphite via H2O2-hydrothermal process and its effect on properties of high-density polyethylene composites. Polym. Compos. 2012, 33, 872–880. [Google Scholar] [CrossRef]
- Graphite Store. Available online: https://www.graphitestore.com/gs-3570-expandable-graphite (accessed on 8 March 2021).
- GRAPHENE SUPERMARKET. Available online: https://www.graphene-supermarket.com/products/graphene-nanopowder-ao-4-60nm-flakes (accessed on 12 August 2020).
- HEXION. Available online: https://www.hexion.com/en-us/chemistry/epoxy-resins-curing-agents-modifiers/system-recommendations/wind-composites (accessed on 21 July 2019).
- Alfa Aesar. Available online: https://www.alfa.com/en/catalog/A10924/ (accessed on 14 September 2019).
- MilliporeSigma. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/700207 (accessed on 14 September 2019).
- Mokhtari, M.; Archer, E.; Bloomfield, N.; Harkin-Jones, E.; McIlhagger, A. High-performance and cost-effective melt blended poly (ether ether ketone)/expanded graphite composites for mass production of antistatic materials. Polym. Int. 2021, 70, 1137–1145. [Google Scholar] [CrossRef]
- Su, Y.; Li, J.J.; Weng, G.J. Theory of thermal conductivity of graphene-polymer nanocomposites with interfacial Kapitza resistance and graphene-graphene contact resistance. Carbon 2018, 137, 222–233. [Google Scholar] [CrossRef]
- Wu, K.; Lei, C.; Huang, R.; Yang, W.; Chai, S.; Geng, C.; Chen, F.; Fu, Q. Design and preparation of a unique segregated double network with excellent thermal conductive property. ACS Appl. Mater. Interfaces 2017, 9, 7637–7647. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ni, H.; Chen, J.; Yang, W. Facile method to fabricate highly thermally conductive graphite/PP composite with network structures. ACS Appl. Mater. Interfaces 2016, 8, 19732–19738. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Yang, Z.; Zhu, T.; Wu, T.; Dong, C.; Liu, G. Tuning the oxidation degree of graphite toward highly thermally conductive graphite/epoxy composites. Chem. Mater. 2018, 30, 7473–7483. [Google Scholar] [CrossRef]
- Wu, M.; Huang, H.-X.; Tong, J.; Ke, D.-Y. Enhancing thermal conductivity and mechanical properties of poly (methyl methacrylate) via adding expanded graphite and injecting water. Compos. Part A Appl. Sci. Manuf. 2017, 102, 228–235. [Google Scholar] [CrossRef]
- Kratochvíla, J.; Boudenne, A.; Krupa, I. Effect of filler size on thermophysical and electrical behavior of nanocomposites based on expanded graphite nanoparticles filled in low-density polyethylene matrix. Polym. Compos. 2013, 34, 149–155. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Preparation and thermal properties of polyethylene glycol/expanded graphite blends for energy storage. Appl. Energy 2009, 86, 1479–1483. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Z.-K.; Zhang, Y.-Y.; Li, Y.-Q.; Fu, Y.-Q.; Sun, B.-G.; Shi, H.-Q.; Huang, P.; Hu, N.; Fu, S.-Y. Mechanical, tribological and thermal properties of injection molded short carbon fiber/expanded graphite/polyetherimide composites. Compos. Sci. Technol. 2021, 201, 108498. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Prog. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Effect of graphene content on the properties of poly (lactic acid) nanocomposites. RSC Adv. 2015, 5, 28410–28423. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C.; Lu, J.; Wang, P.; Chen, X. Preparation and characterization of graphite nanosheets from ultrasonic powdering technique. Carbon 2004, 42, 753–759. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413–1422. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Z.-H.; Yu, X.; Shen, W.; Zheng, Y.; Kang, F. Mildly expanded graphite for anode materials of lithium ion battery synthesized with perchloric acid. Electrochim. Acta 2014, 116, 170–174. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, R. Preparation of boric acid modified expandable graphite and its influence on polyethylene combustion characteristics. J. Chil. Chem. Soc. 2016, 61, 2767–2771. [Google Scholar] [CrossRef]
- Tao, S.; Wei, S.; Yulan, Y. Characterization of expanded graphite microstructure and fabrication of composite phase-change material for energy storage. J. Mater. Civ. Eng. 2015, 27, 04014156. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Dong, L.; Sun, C.; Zhao, X.; Ruan, Y.; Lu, H. Interlayer polymerization in chemically expanded graphite for preparation of highly conductive, mechanically strong polymer composites. Chem. Mater. 2017, 29, 3412–3422. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Wang, Q.; Xiong, D.; Song, J.; Tang, Z.; Liu, X. The graphite foam/erythritol composites with ultrahigh thermal conductivity for medium temperature applications. Sol. Energy Mater. Sol. Cells 2021, 230, 111135. [Google Scholar] [CrossRef]
- Peng, T.; Liu, B.; Gao, X.; Luo, L.; Sun, H. Preparation, quantitative surface analysis, intercalation characteristics and industrial implications of low temperature expandable graphite. Appl. Surf. Sci. 2018, 444, 800–810. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Xia, Z.; Bellani, V.; Sun, J.; Palermo, V. Electrochemical exfoliation of graphite in H2SO4, Li2SO4 and NaClO4 solutions monitored in situ by Raman microscopy and spectroscopy. Faraday Discuss. 2021, 227, 291–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.; Gong, P.; Mi, Y.; Wang, J.; Yang, S. Scalable fabrication of high quality graphene by exfoliation of edge sulfonated graphite for supercapacitor application. RSC Adv. 2014, 4, 35914–35918. [Google Scholar] [CrossRef]







| Material Parameters | Values |
|---|---|
| Average graphene lateral length, l, | 10 μm |
| Average graphene thickness | 10 nm |
| Aspect ratio of the graphene filler | 0.001 |
| Thermal conductivity of polymer phase | 0.23 |
| Thermal conductivity of graphene filler, and () | 2000 and 10 |
| Thermal conductivity of interlayer with Kapitza resistance | |
| Thermal conductivity of the interlayer with a firmly developed graphene–graphene contact state, |
| Atomic Composition by XPS (at%) | |||
|---|---|---|---|
| C (285.08 eV) | O (532.08 eV) | S (169.11 eV) | |
| GIC | 85.14 | 13.16 | 1.7 |
| EG | 95.76 | 4.24 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarannum, F.; Danayat, S.S.; Nayal, A.; Muthaiah, R.; Annam, R.S.; Garg, J. Large Enhancement in Thermal Conductivity of Solvent−Cast Expanded Graphite/Polyetherimide Composites. Nanomaterials 2022, 12, 1877. https://doi.org/10.3390/nano12111877
Tarannum F, Danayat SS, Nayal A, Muthaiah R, Annam RS, Garg J. Large Enhancement in Thermal Conductivity of Solvent−Cast Expanded Graphite/Polyetherimide Composites. Nanomaterials. 2022; 12(11):1877. https://doi.org/10.3390/nano12111877
Chicago/Turabian StyleTarannum, Fatema, Swapneel S. Danayat, Avinash Nayal, Rajmohan Muthaiah, Roshan Sameer Annam, and Jivtesh Garg. 2022. "Large Enhancement in Thermal Conductivity of Solvent−Cast Expanded Graphite/Polyetherimide Composites" Nanomaterials 12, no. 11: 1877. https://doi.org/10.3390/nano12111877