Phase Composition, Structure and Properties of the Spark Plasma Sintered Ceramics Obtained from the Al12Mg17-B-Si Powder Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Process to Obtain Dense Composite Materials
2.3. Characterization
3. Results and Discussions
3.1. Microstructure, Phase Composition and Dispersity of the Al12Mg17-B Powder
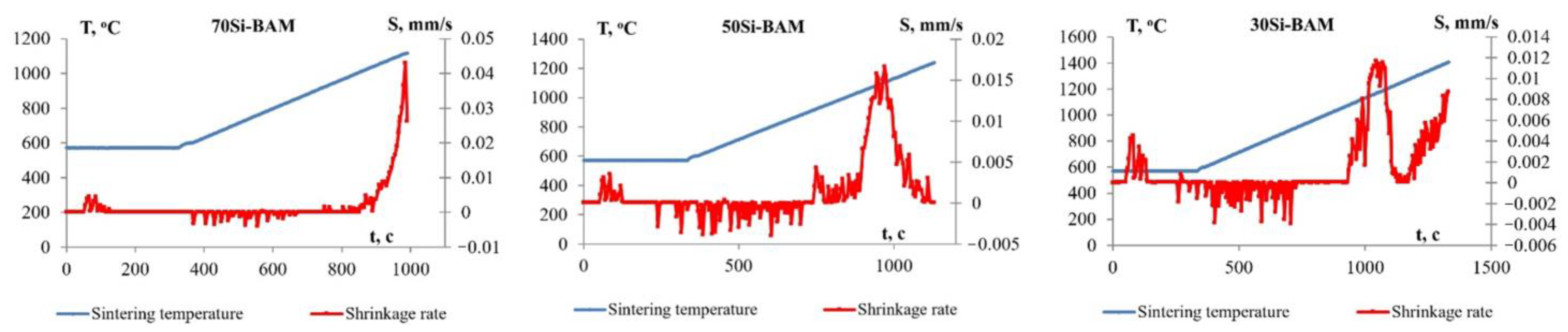
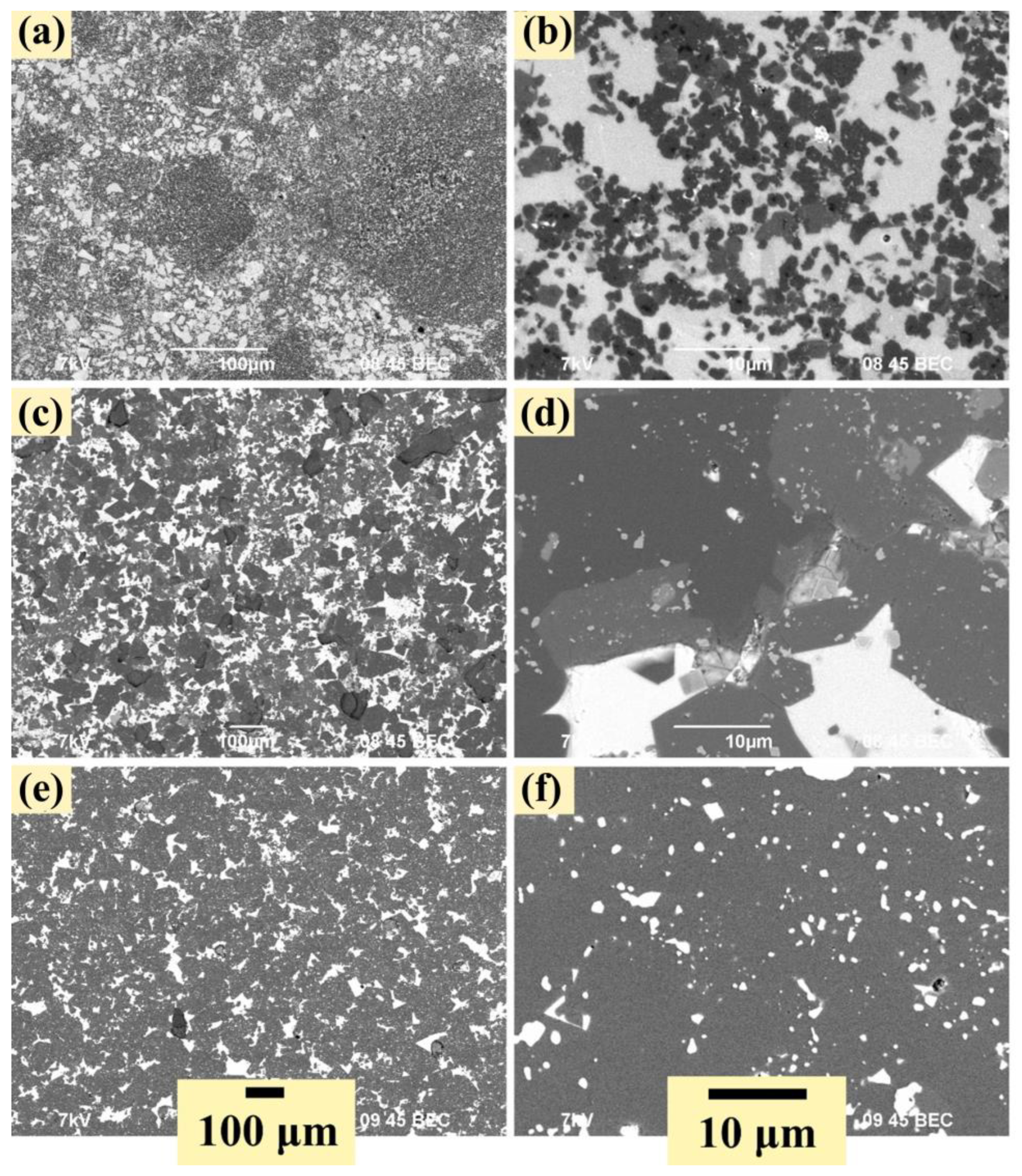
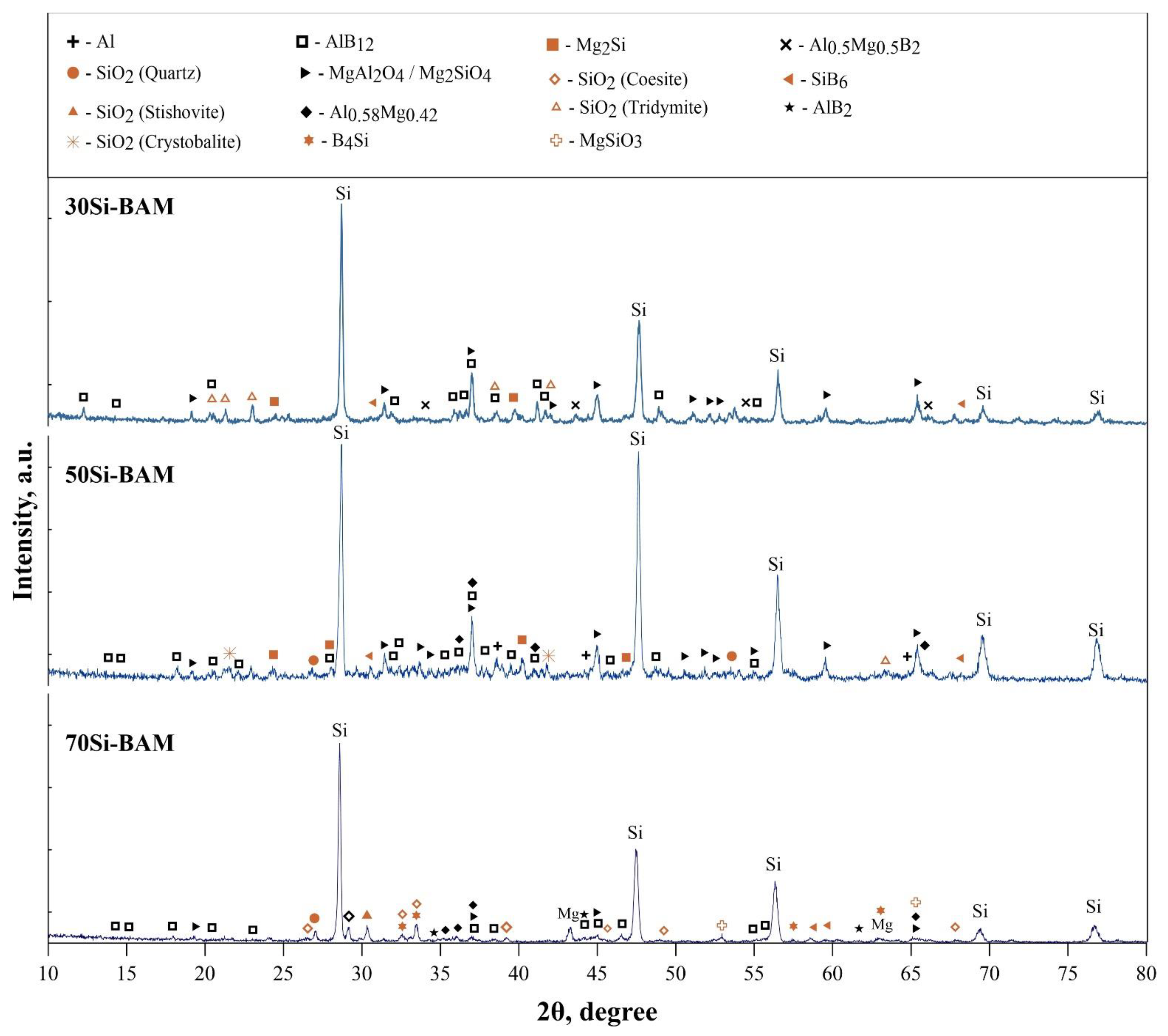
3.2. Sintering Features, Microstructure and Phase Composition of the Obtained Specimens
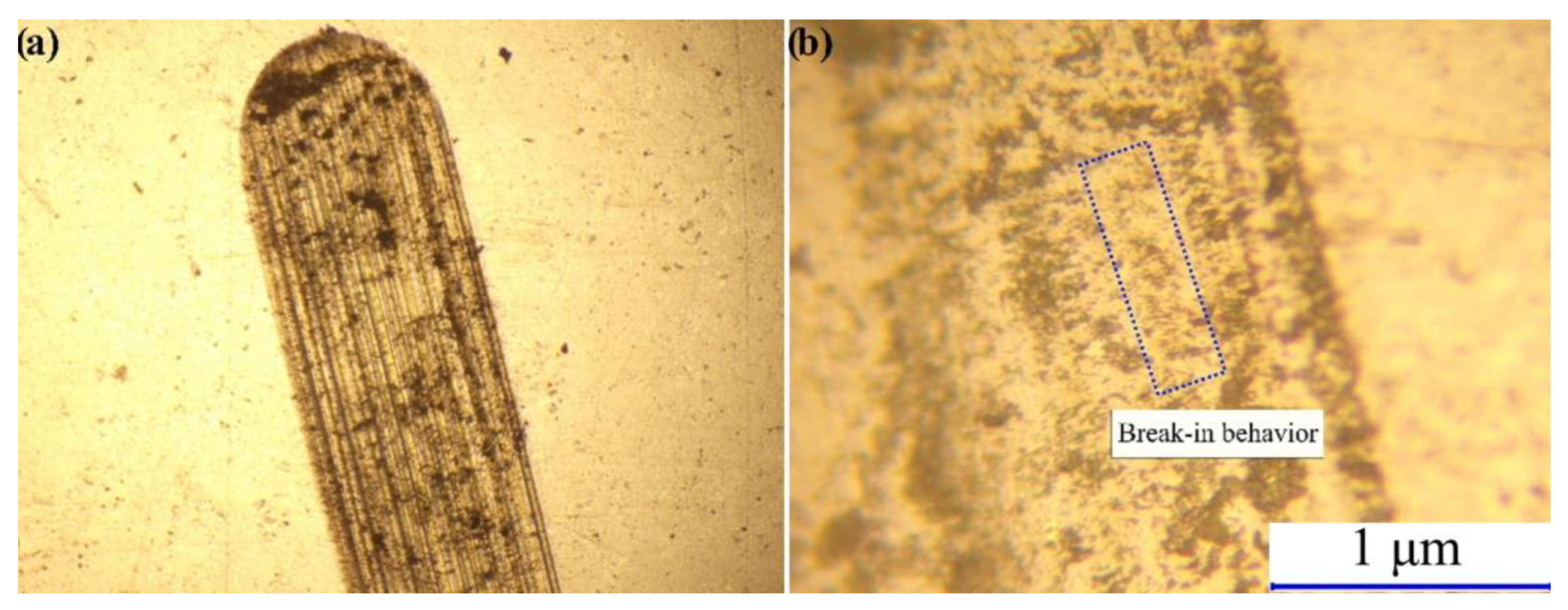
3.3. Mechanical Properties of the Sintered Specimens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, B.; Raju, G.B.; Suri, A.K. Processing and properties of monolithic TiB2 based materials. Int. Mater. Rev. 2006, 51, 352–374. [Google Scholar] [CrossRef]
- Matveev, A.E.; Zhukov, I.A.; Ziatdinov, M.H.; Zhukov, A.S. Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials 2020, 13, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talash, V.M.; Vasiliev, O.O.; Muratov, V.B.; Rudenko, Y.B.; Malyshevska, G.I. Electrochemical Behavior of AlB12–AlN Composites in Natural Environments. Powder Metall. Met. Ceram. 2019, 58, 329–333. [Google Scholar] [CrossRef]
- Cherednichenko, K.A.; Solozhenko, V.L. Thermal expansion of α-boron and some boron-rich pnictides. Solid State Commun. 2019, 303, 113735. [Google Scholar] [CrossRef] [Green Version]
- Prikhna, T.A.; Barvitskyi, P.P.; Maznaya, A.V.; Muratov, V.B.; Devin, L.N.; Neshpor, A.V.; Domnich, V.; Haber, R.; Karpets, M.V.; Samus, E.V.; et al. Lightweight ceramics based on aluminum dodecaboride, boron carbide and self-bonded silicon carbide. Ceram. Int. 2019, 45, 9580–9588. [Google Scholar] [CrossRef]
- Murakami, T.; Inui, H. Friction and wear properties of α-AlB12-and SiB6-based ceramics in water. Tribol. Int. 2014, 74, 38–45. [Google Scholar] [CrossRef]
- Ramos, G.; Manzano, R.; Suarez, O.M. A comparative hardness study of Al-Si/AlB2 and Al-Si/AlB12 composites. Sci. Eng. Compos. 2012, 19, 67–73. [Google Scholar] [CrossRef]
- Adil, S.; Karati, A.; Murty, B.S. Mechanochemical synthesis of nanocrystalline aluminium boride (AlB12). Ceram. Int. 2018, 44, 20105–20110. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Škapin, S.D.; Jelen, M.; Krnel, K.; Meden, A. Cost-effective synthesis of AlMgB14–xTiB2. J. Eur. Ceram. Soc. 2007, 27, 493–497. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Matveev, A.E.; Sokolov, S.D.; Boldin, M.S.; Vorozhtsov, A.B. AlMgB14–TiB2 composite materials obtained by self-propagating high-temperature synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 22733–22737. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Matveev, A.E.; Zhukov, I.A. Energy-effective AlMgB14 production by self-propagating high-temperature synthesis (SHS) using the chemical furnace as a source of heat energy. Ceram. Int. 2021, 47, 21698–21704. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, J.; Li, F.; Zhu, S.; Li, W.; Yang, J.; Liu, W. Tribological study on a novel wear-resistant AlMgB14-Si composite. Ceram. Int. 2017, 43, 12362–12371. [Google Scholar] [CrossRef]
- Cook, B.A.; Harringa, J.L.; Lewis, T.L.; Russel, A.M. A new class of ultra-hard materials based on AlMgB14. Scr. Mater. 2000, 42, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Evseev, N.S.; Matveev, A.E.; Nikitin, P.Y.; Abzaev, Y.A.; Zhukov, I.A. A theoretical and experimental investigation on the SHS synthesis of (HfTiCN)-TiB2 high-entropy composite. Ceram. Int. 2022, 48, 16010–16014. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Ziatdinov, M.K.; Dubkova, Y.A.; Nikitin, P.Y. Synthesis of AlMgB14: Influence of Mechanical Activation of Al–Mg–B Powder Mixture on Phase Composition of Sintered Materials. Russ. Phys. J. 2018, 61, 1466–1471. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Nikitin, P.Y.; Vorozhtsov, A.B.; Perevislov, S.N.; Sokolov, S.D.; Ziatdinov, M.H. The use of intermetallic AlxMgy powder to obtain AlMgB14-based materials. Mater. Today Commun. 2020, 22, 100848. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Vorozhtsov, A.B. Decomposition mechanism of AlMgB14 during the spark plasma sintering. J. Mater. Res. Technol. 2020, 11, 687–692. [Google Scholar] [CrossRef]
- Nečina, V.; Pabst, W. Transparent MgAl2O4 spinel ceramics prepared via sinter-forging. J. Eur. Ceram. Soc. 2021, 41, 4313–4318. [Google Scholar] [CrossRef]
- Hříbalová, S.; Pabst, W. Theoretical study of the influence of carbon contamination on the transparency of spinel ceramics prepared by spark plasma sintering (SPS). J. Eur. Ceram. Soc. 2021, 41, 4337–4342. [Google Scholar] [CrossRef]
- Schnurre, S.M.; Gröbner, J.; Schmid-Fetzer, R. Thermodynamics and phase stability in the Si–O system. J. Non-Cryst. Solids 2004, 336, 1–25. [Google Scholar] [CrossRef]
- Imam, M.A.; Reddy, R.G. A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials. High Temp. Mater. Process 2019, 38, 411–424. [Google Scholar] [CrossRef]
- Roberts, D.J.; Zhao, J.; Munir, Z.A. Mechanism of reactive sintering of MgAlB14 by pulse electric current. Int. J. Refract. Hard Met. 2009, 27, 556–563. [Google Scholar] [CrossRef]
- Pant, H.K.; Debnath, D.; Chakraborty, S.; Wani, M.F.; Das, P.K. Mechanical and tribological properties of spark plasma sintered SiC–TiB2 and SiC–TiB2–TaC composites: Effects of sintering temperatures (2000 °C and 2100 °C). J. Tribol. 2018, 140, 011608. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Zhu, D.; Cook, B.A.; Elmoursi, A.A. Tribological characteristics of AlMgB14 and nanocomposite AlMgB14-TiB2 superhard coatings. In Proceedings of the International Joint Tribology Conference, Miami, FL, USA, 20–22 October 2008; Volume 43369, pp. 757–759. [Google Scholar] [CrossRef]


| Powder | Average Particle Size | Purity, % |
|---|---|---|
| Al12Mg17 | 15 μm | ≥99.2 |
| Amorphous B | 600 nm | ≥98.7 |
| MA-Al12Mg17-B | 400 nm | ≥98.8 |
| Si (OJSC «Polema») | 60 μm | ≥99.2 |
| Designation | Composition (wt.%) | Tsintering, °C | Vheating, °C/min | P, MPa |
|---|---|---|---|---|
| 70Si-BAM | 70% Si + 30% Al12Mg17-B | 1115 | 50 | 70 |
| 50Si-BAM | 50% Si + 50% Al12Mg17-B | 1240 | 50 | |
| 30Si-BAM | 30% Si + 70% Al12Mg17-B | 1410 | 50 |
| Phase | State | a, Å | α | V, Å3 | E, eV |
|---|---|---|---|---|---|
| Al12Mg17 | Reference | 10.549 | 90 | 1174.0 | −34,471.7 |
| Refined | 10.405 | 90 | 1126.4 | −34,469.3 |
| Designation | Tsintering, °C | Friction Coefficient | Wear Rate, 10−5 mm−3/(N/m) |
|---|---|---|---|
| 70Si-BAM | 1115 | 0.56 | 13.60 |
| 50Si-BAM | 1240 | 0.36 | 9.40 |
| 30Si-BAM | 1410 | 0.32 | 5.60 |
| Designation | Density, g/cm3 | Hardness (HV), GPa |
|---|---|---|
| 70Si-BAM | 2.37 ± 0.01 | 16.55 ± 0.83 |
| 50Si-BAM | 2.16 ± 0.04 | 18.4 ± 1.35 |
| 30Si-BAM | 2.30 ± 0.01 | 21.24 ± 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitin, P.; Zhukov, I.; Matveev, A.; Sokolov, S.; Sachkov, V.; Vorozhtsov, A. Phase Composition, Structure and Properties of the Spark Plasma Sintered Ceramics Obtained from the Al12Mg17-B-Si Powder Mixtures. Nanomaterials 2022, 12, 1895. https://doi.org/10.3390/nano12111895
Nikitin P, Zhukov I, Matveev A, Sokolov S, Sachkov V, Vorozhtsov A. Phase Composition, Structure and Properties of the Spark Plasma Sintered Ceramics Obtained from the Al12Mg17-B-Si Powder Mixtures. Nanomaterials. 2022; 12(11):1895. https://doi.org/10.3390/nano12111895
Chicago/Turabian StyleNikitin, Pavel, Ilya Zhukov, Alexey Matveev, Sergei Sokolov, Victor Sachkov, and Alexander Vorozhtsov. 2022. "Phase Composition, Structure and Properties of the Spark Plasma Sintered Ceramics Obtained from the Al12Mg17-B-Si Powder Mixtures" Nanomaterials 12, no. 11: 1895. https://doi.org/10.3390/nano12111895
APA StyleNikitin, P., Zhukov, I., Matveev, A., Sokolov, S., Sachkov, V., & Vorozhtsov, A. (2022). Phase Composition, Structure and Properties of the Spark Plasma Sintered Ceramics Obtained from the Al12Mg17-B-Si Powder Mixtures. Nanomaterials, 12(11), 1895. https://doi.org/10.3390/nano12111895






