Photoelectrochemical Enhancement of Graphene@WS2 Nanosheets for Water Splitting Reaction
Abstract
:1. Introduction
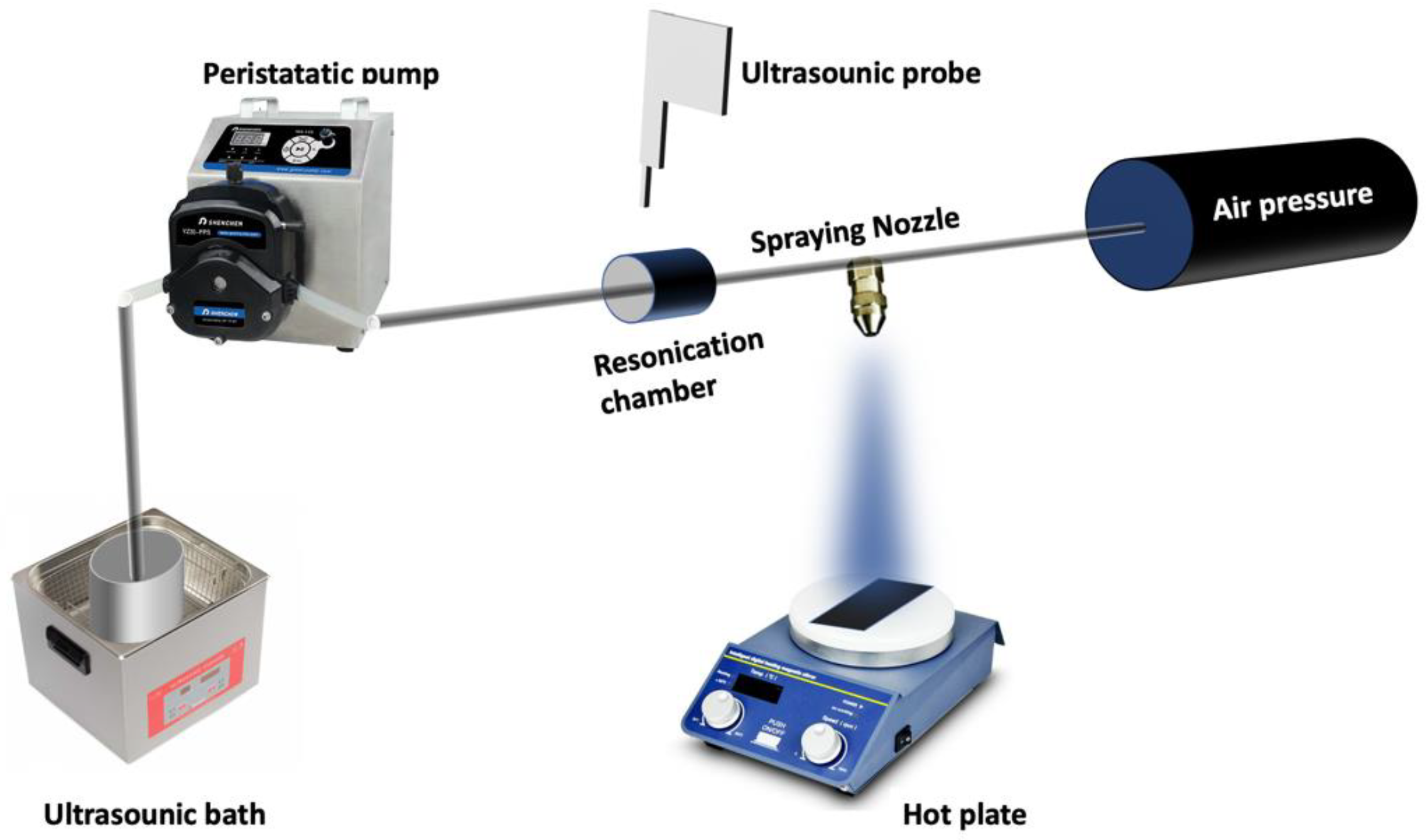
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
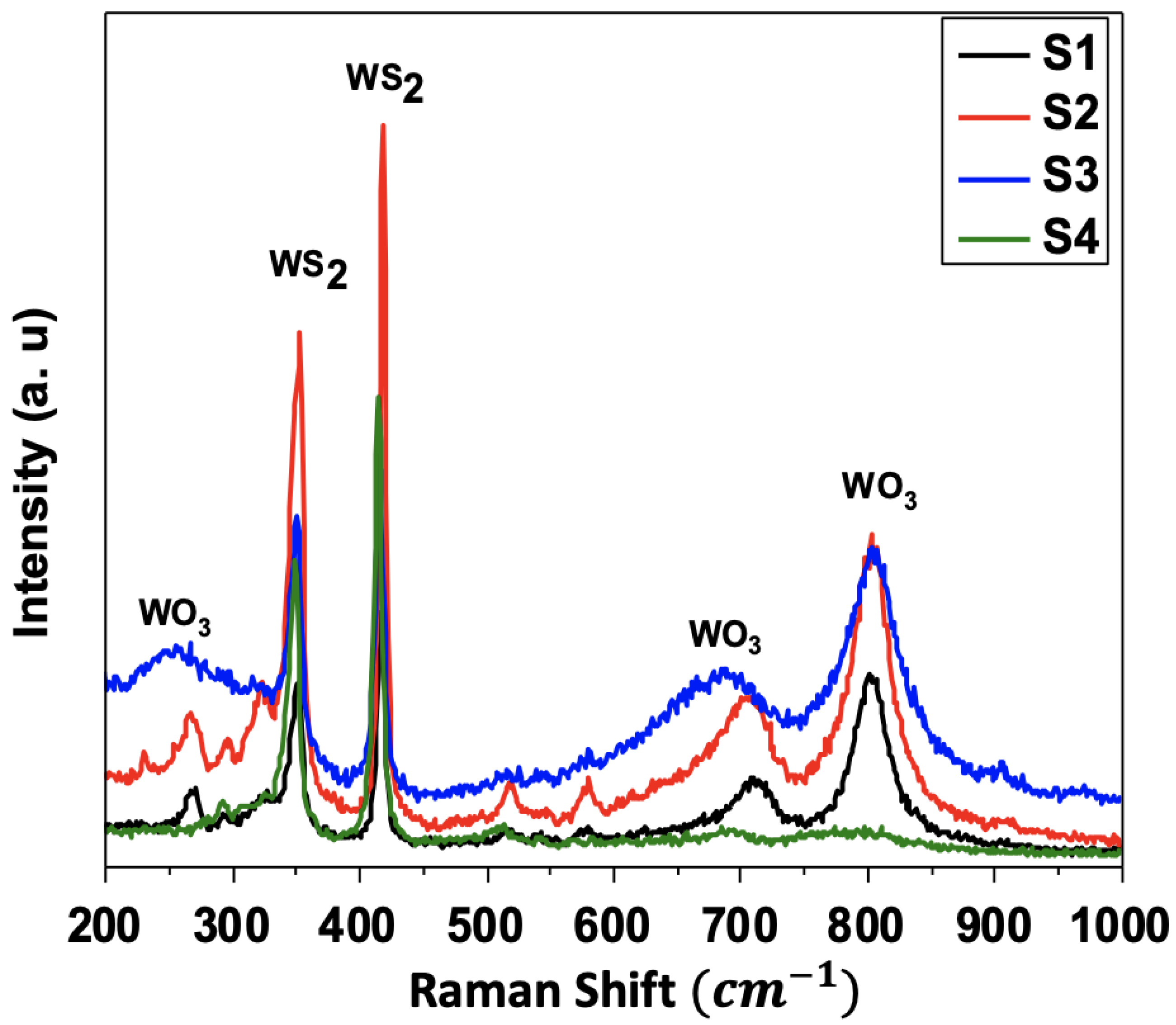
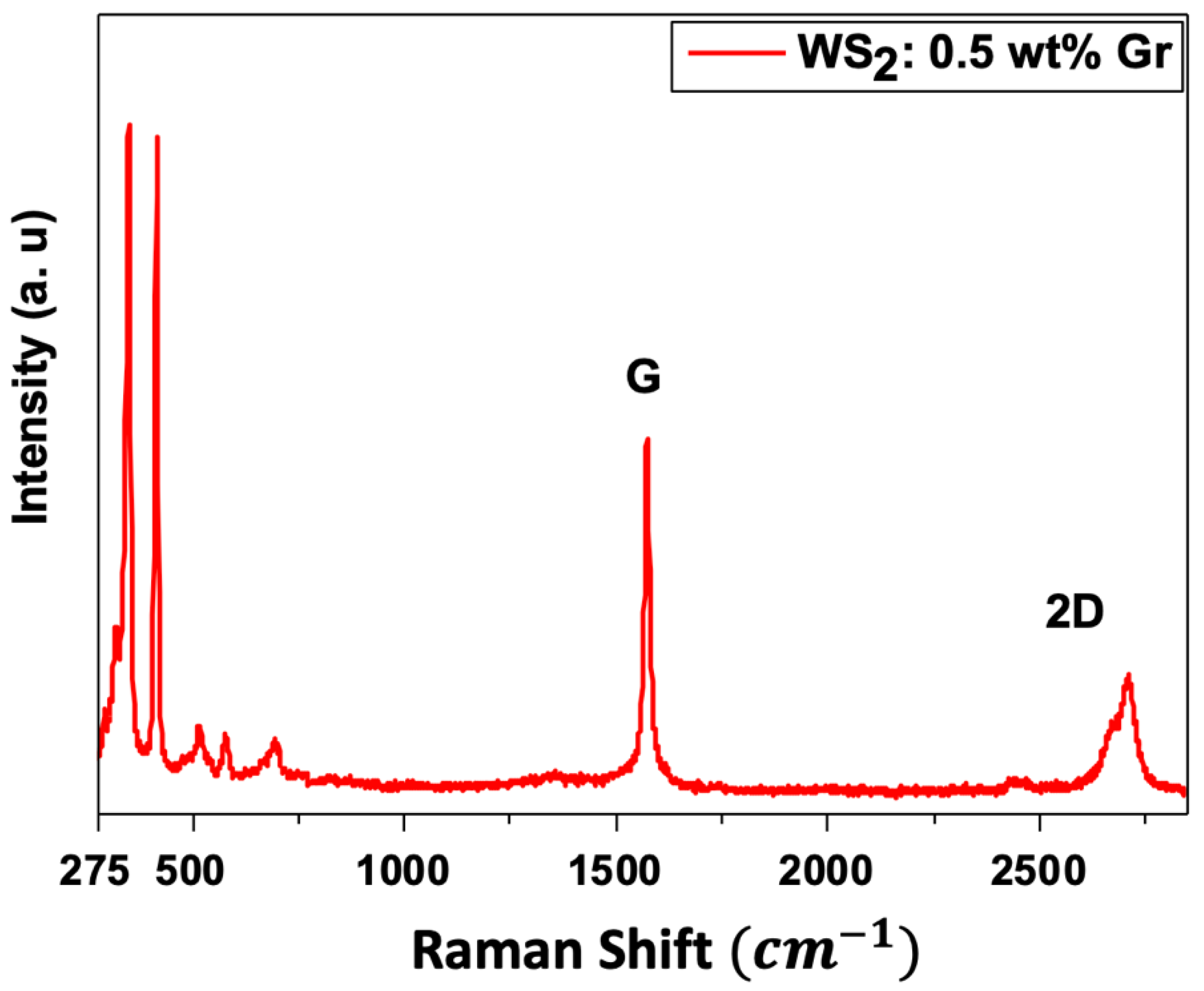
3.2. Raman Spectroscopy
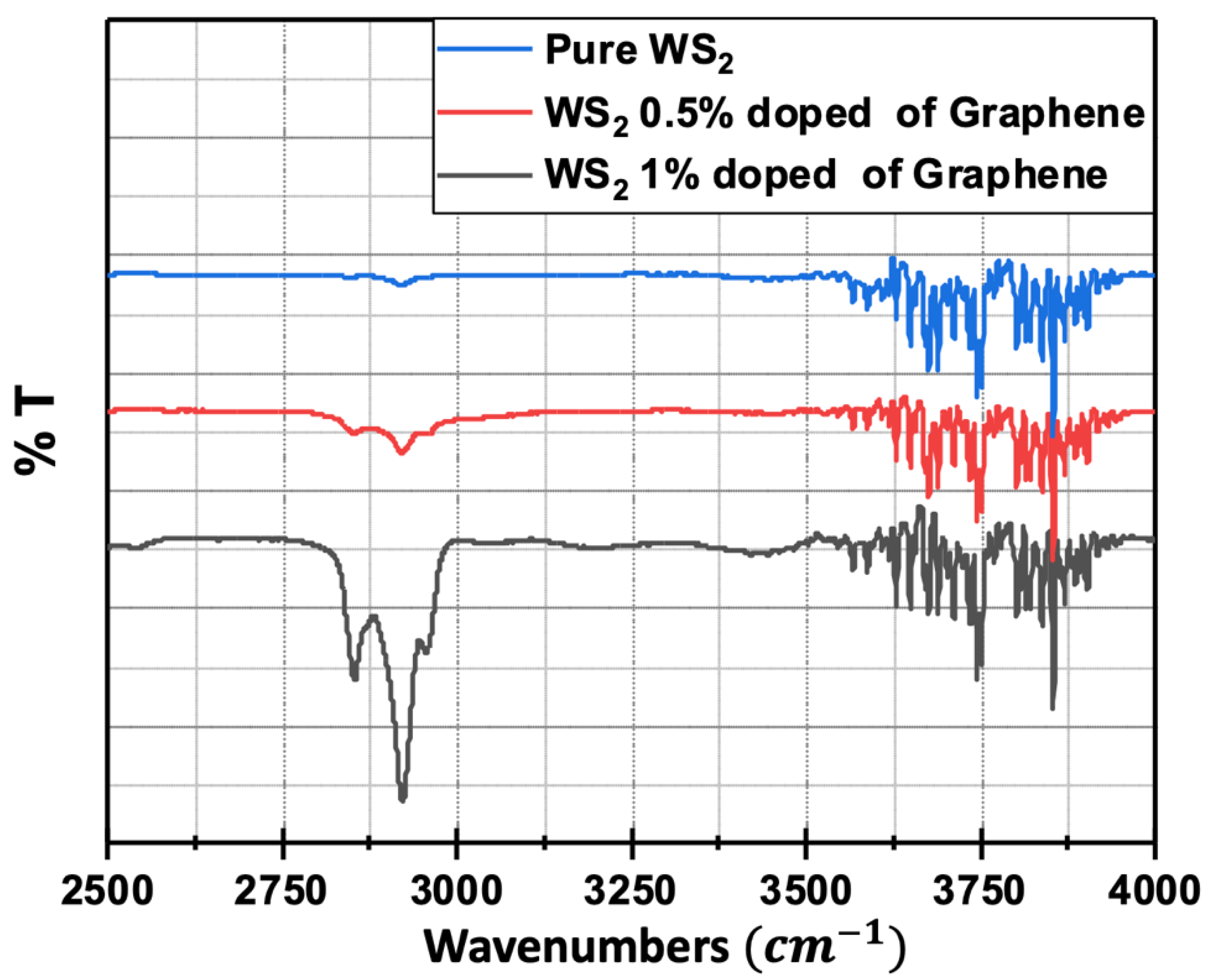
3.3. FTIR Spectroscopy
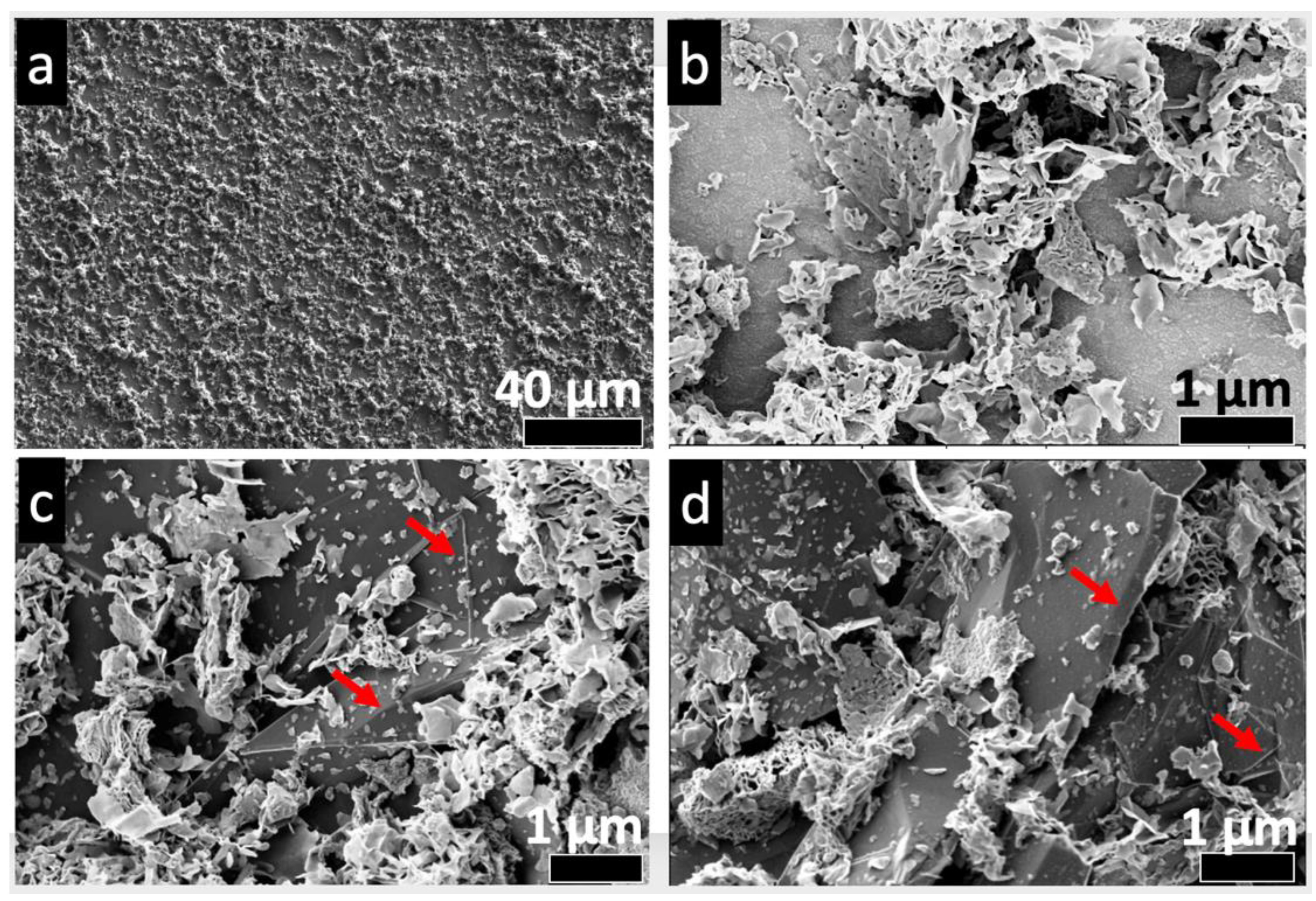
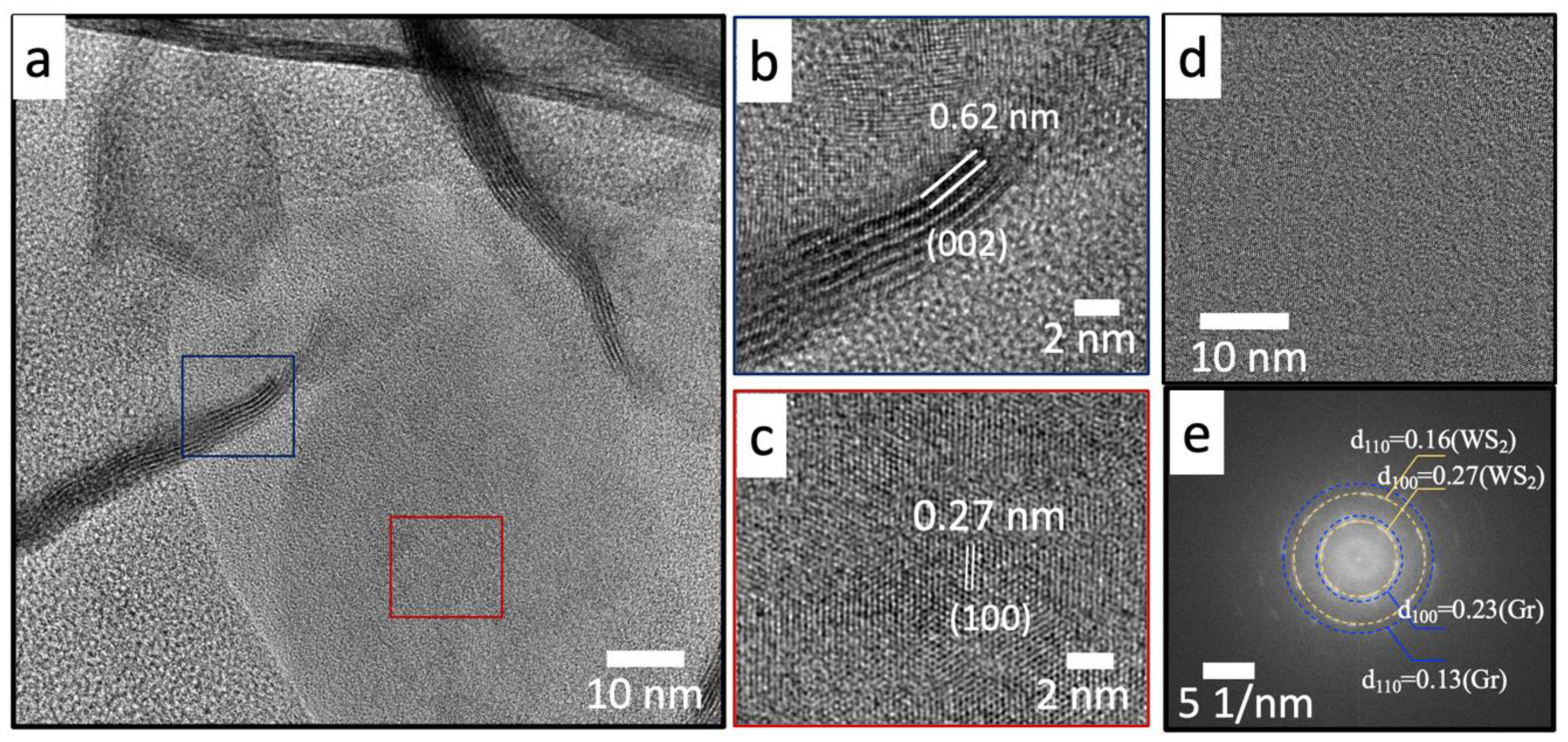
3.4. Microstructure Analysis
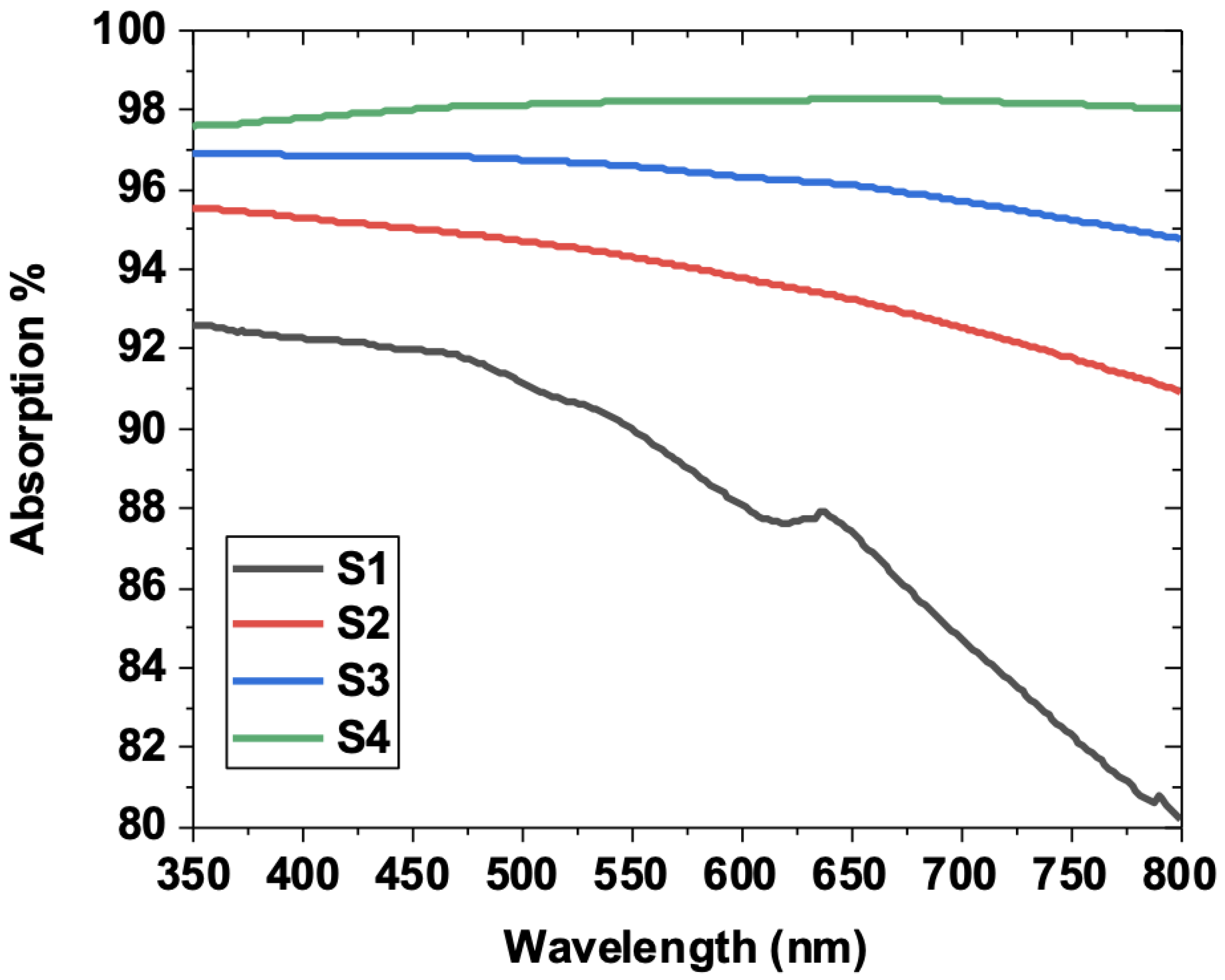
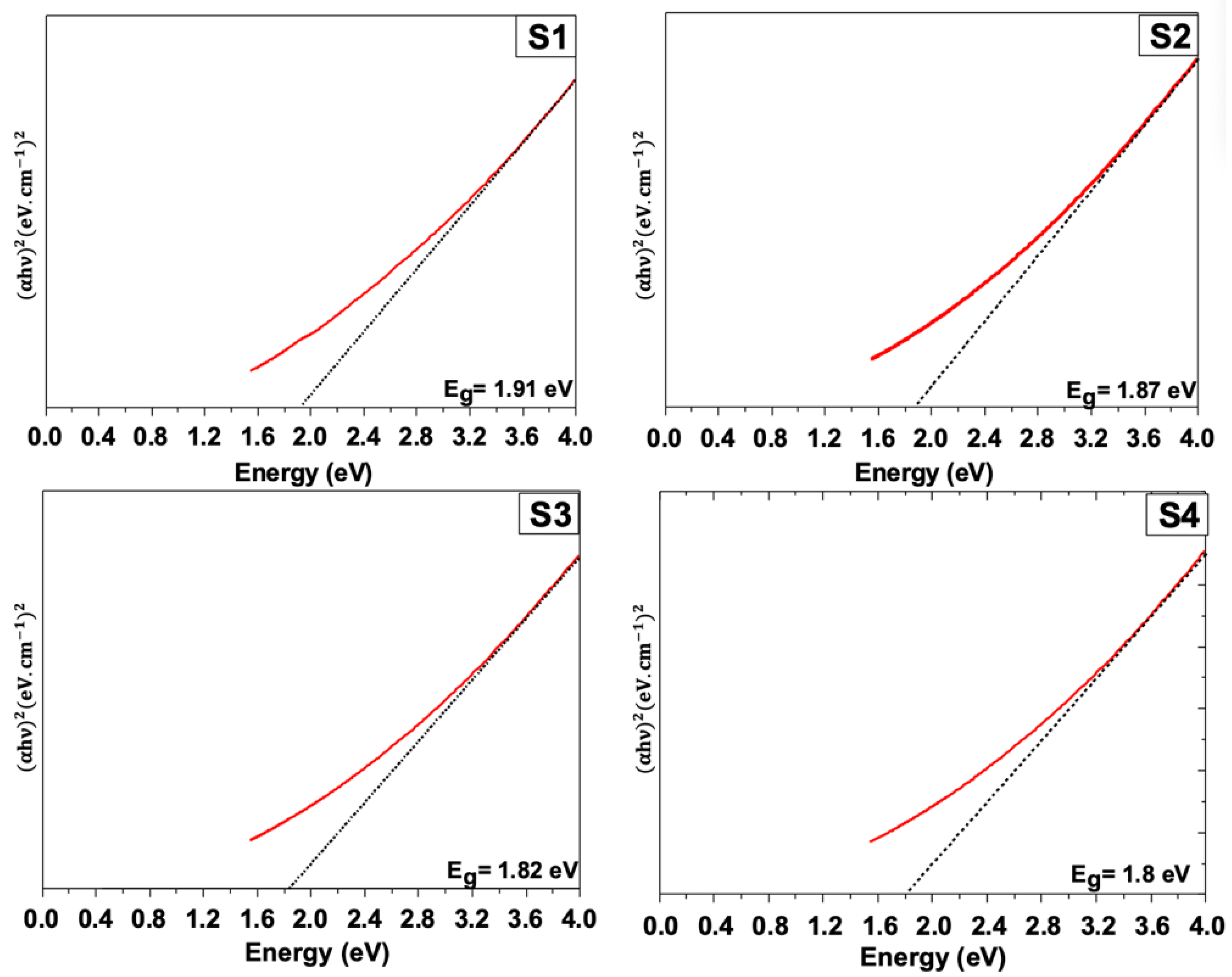
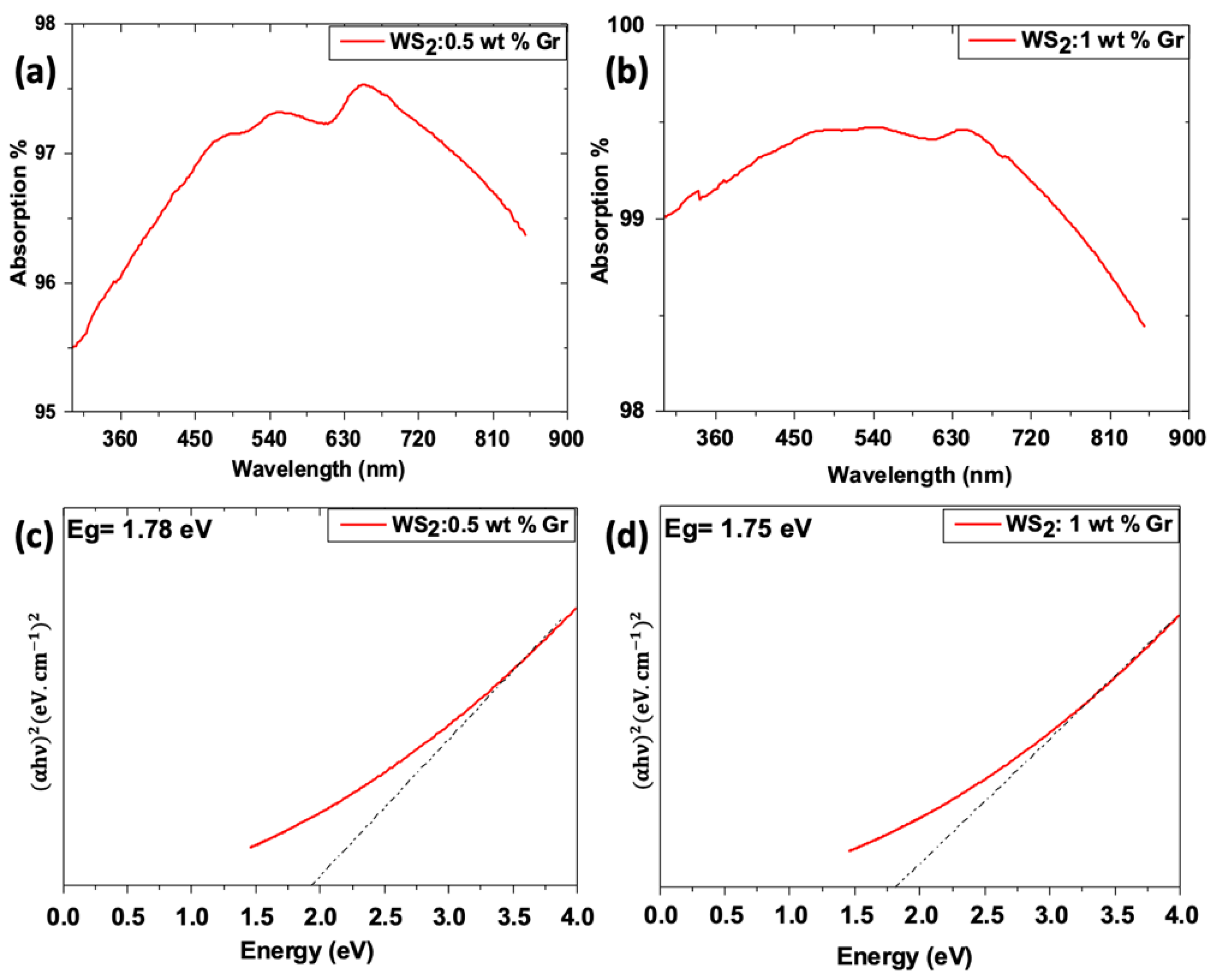
3.5. Optical Properties
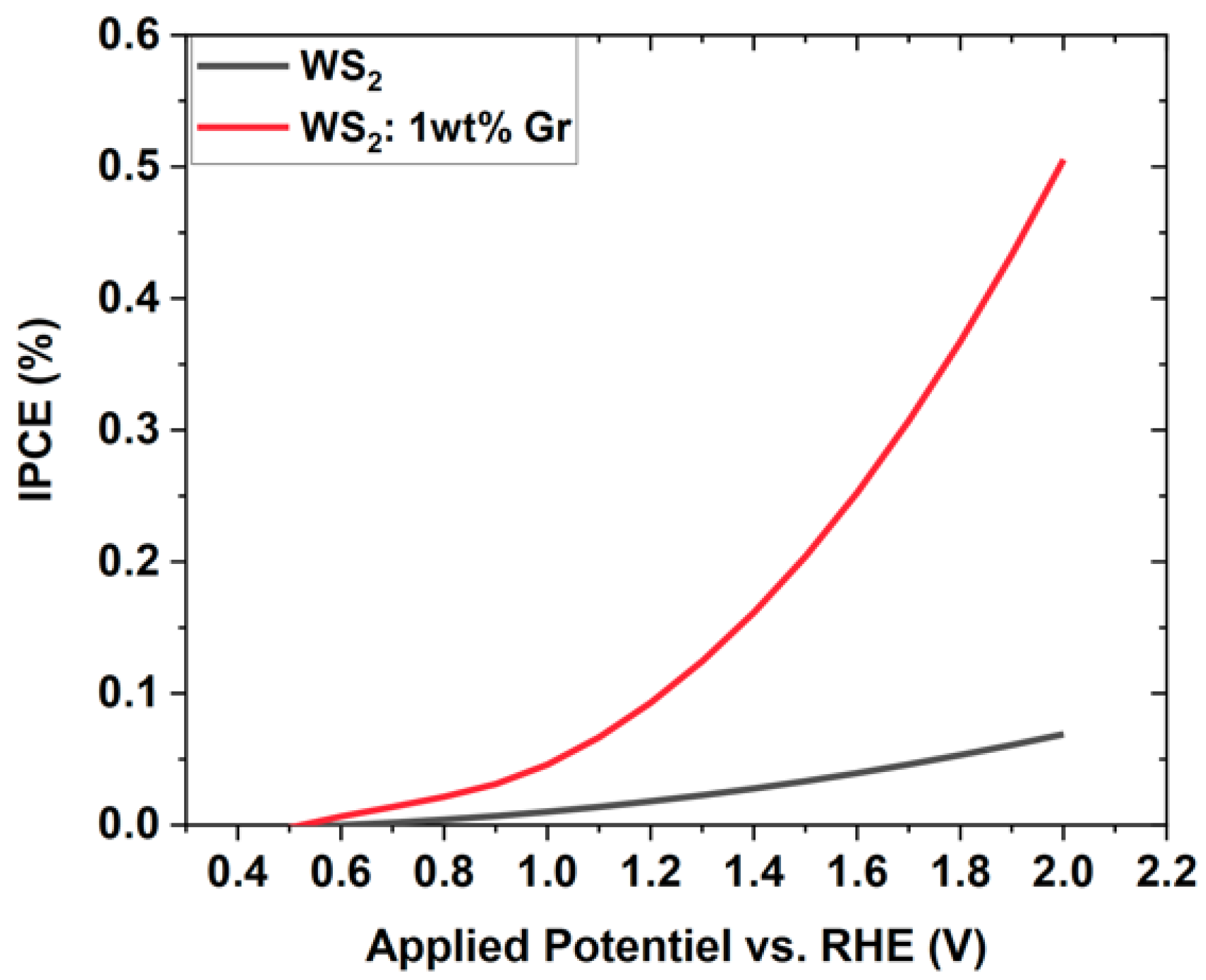
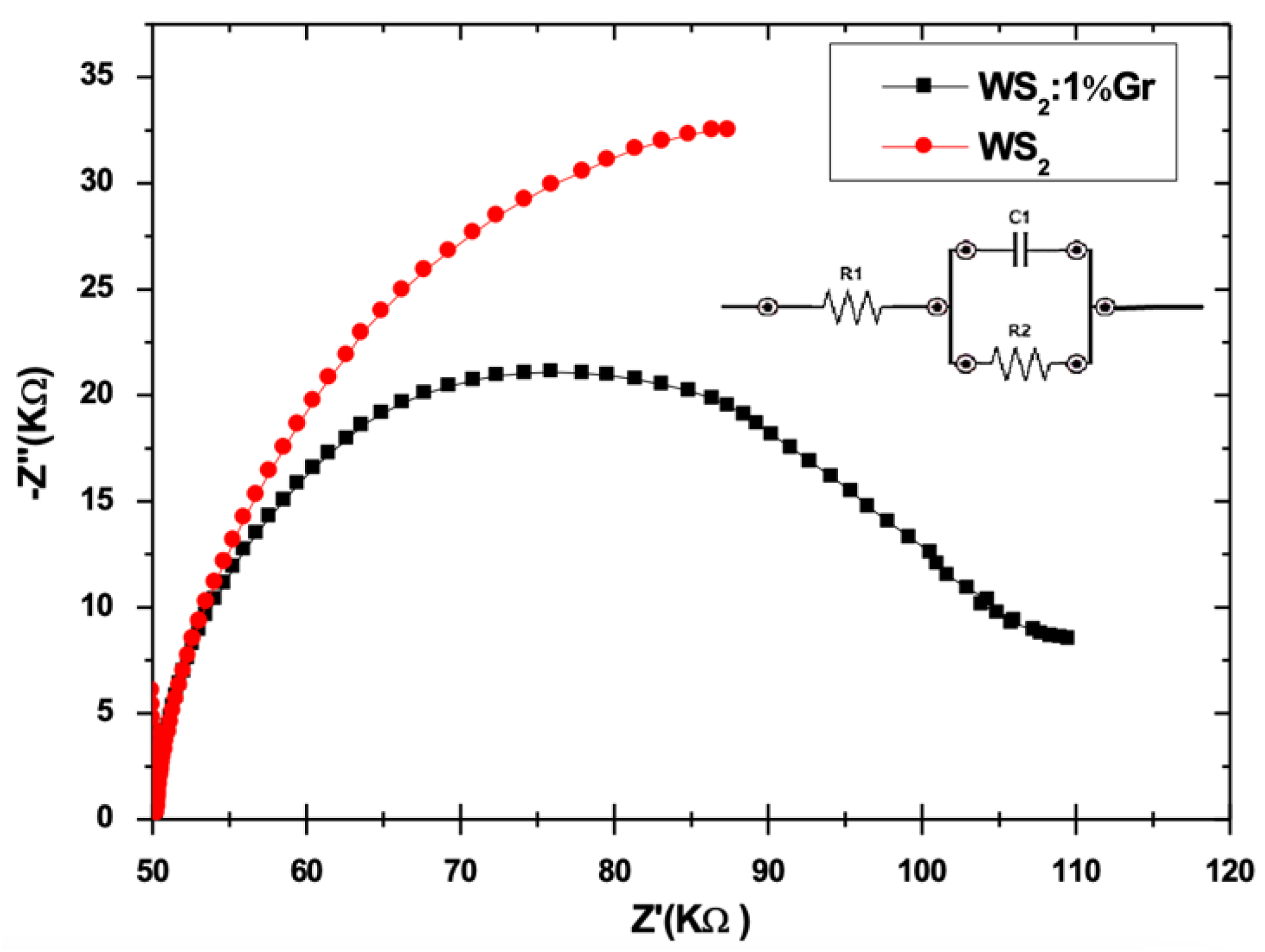
3.6. Photochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A Review on Solar-Hydrogen/Fuel Cell Hybrid Energy Systems for Stationary Applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Rajeshwar, K.; McConnell, R.; Licht, S. Solar Hydrogen Generation; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of Photocatalytic Activity by Metal Deposition: Characterisation and Photonic Efficiency of Pt, Au and Pd Deposited on TiO2 Catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Abed, J.; Rajput, N.S.; El Moutaouakil, A.; Jouiad, M. Recent Advances in the Design of Plasmonic Au/TiO2 Nanostructures for Enhanced Photocatalytic Water Splitting. Nanomaterials 2020, 10, 2260. [Google Scholar] [CrossRef]
- Wu, B.-H.; Liu, W.-T.; Chen, T.-Y.; Perng, T.-P.; Huang, J.-H.; Chen, L.-J. Plasmon-Enhanced Photocatalytic Hydrogen Production on Au/TiO2 Hybrid Nanocrystal Arrays. Nano Energy 2016, 27, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Li, D.; Wu, P. One-Pot, Facile, and Versatile Synthesis of Monolayer MoS2/WS2 Quantum Dots as Bioimaging Probes and Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shoushtari, M.Z.; Farbod, M. Photoelectrochemical Application of WS2 Nanosheets Prepared via a Low-Temperature CVD Method. J. Mater. Sci. Mater. Electron. 2019, 30, 6342–6349. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, M. Two-Dimensional WS2/MoS2 Heterostructures: Properties and Applications. Nanoscale 2021, 13, 5594–5619. [Google Scholar] [CrossRef] [PubMed]
- Thiehmed, Z.; Shakoor, A.; Altahtamouni, T. Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts 2021, 11, 1283. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, F.; Wang, X.; Qin, H.; Liu, E.; Sha, J.; Zhao, N.; Zhang, P.; Ma, L. Enhanced Electrochemical Hydrogen Evolution Performance of WS2 Nanosheets by Te Doping. J. Catal. 2020, 382, 204–211. [Google Scholar] [CrossRef]
- Han, A.; Zhou, X.; Wang, X.; Liu, S.; Xiong, Q.; Zhang, Q.; Gu, L.; Zhuang, Z.; Zhang, W.; Li, F.; et al. One-Step Synthesis of Single-Site Vanadium Substitution in 1T-WS2 Monolayers for Enhanced Hydrogen Evolution Catalysis. Nat. Commun. 2021, 12, 709. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zargar Shoushtari, M. Enhancing the Photoelectrochemical Water Splitting Performance of WS2 Nanosheets by Doping Titanium and Molybdenum via a Low Temperature CVD Method. J. Electroanal. Chem. 2019, 849, 113361. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 To Preferentially Expose Active Edge Sites For Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Deokar, G.; Rajput, N.S.; Vancsó, P.; Ravaux, F.; Jouiad, M.; Vignaud, D.; Cecchet, F.; Colomer, J.F. Large Area Growth of Vertically Aligned Luminescent MoS2nanosheets. Nanoscale 2017, 9, 277–287. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L. Eco-Friendly Synthesis of Metal Dichalcogenides Nanosheets and Their Environmental Remediation Potential Driven by Visible Light. Sci. Rep. 2015, 5, 15718. [Google Scholar] [CrossRef] [Green Version]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.; Shi, L.; Ye, J. Engineering the Edges of MoS2 (WS2) Crystals for Direct Exfoliation into Monolayers in Polar Micromolecular Solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Wen, Z.; Ci, S.; Chang, J.; Mao, S.; Chen, J. Superior Electrocatalysis for Hydrogen Evolution with Crumpled Graphene/Tungsten Disulfide/Tungsten Trioxide Ternary Nanohybrids. Nano Energy 2018, 47, 66–73. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.J.; Zhu, H. Engineering Graphene and TMDs Based van Der Waals Heterostructures for Photovoltaic and Photoelectrochemical Solar Energy Conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Shifa, T.A.; Wang, F.; Cheng, Z.; Zhan, X.; Wang, Z.; Liu, K.; Safdar, M.; Sun, L.; He, J. A Vertical-Oriented WS2 Nanosheet Sensitized by Graphene: An Advanced Electrocatalyst for Hydrogen Evolution Reaction. Nanoscale 2015, 7, 14760–14765. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Das Sarma, S.; Adam, S.; Hwang, E.H.; Rossi, E. Electronic Transport in Two-Dimensional Graphene. Rev. Mod. Phys. 2011, 83, 407–470. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yu, F.; Sun, J.; He, R.; Wang, Y.; Guo, C.F.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. Highly active and durable self-standing WS2/graphene hybrid catalysts for hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 9472–9476. [Google Scholar] [CrossRef]




| Sample | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Thiourea (g) | 4.5 | 5 | 5 | 7 |
| WO3 (g) | 0.23 | 0.23 | 0.22 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, M.; Benhamou, L.; Kotbi, A.; Rajput, N.S.; Campos, A.; Lahmar, A.-I.; Hoummada, K.; Kaja, K.; El Marssi, M.; Jouiad, M. Photoelectrochemical Enhancement of Graphene@WS2 Nanosheets for Water Splitting Reaction. Nanomaterials 2022, 12, 1914. https://doi.org/10.3390/nano12111914
Nasr M, Benhamou L, Kotbi A, Rajput NS, Campos A, Lahmar A-I, Hoummada K, Kaja K, El Marssi M, Jouiad M. Photoelectrochemical Enhancement of Graphene@WS2 Nanosheets for Water Splitting Reaction. Nanomaterials. 2022; 12(11):1914. https://doi.org/10.3390/nano12111914
Chicago/Turabian StyleNasr, Mahmoud, Lamyae Benhamou, Ahmed Kotbi, Nitul S. Rajput, Andrea Campos, Abdel-Ilah Lahmar, Khalid Hoummada, Khaled Kaja, Mimoun El Marssi, and Mustapha Jouiad. 2022. "Photoelectrochemical Enhancement of Graphene@WS2 Nanosheets for Water Splitting Reaction" Nanomaterials 12, no. 11: 1914. https://doi.org/10.3390/nano12111914
APA StyleNasr, M., Benhamou, L., Kotbi, A., Rajput, N. S., Campos, A., Lahmar, A.-I., Hoummada, K., Kaja, K., El Marssi, M., & Jouiad, M. (2022). Photoelectrochemical Enhancement of Graphene@WS2 Nanosheets for Water Splitting Reaction. Nanomaterials, 12(11), 1914. https://doi.org/10.3390/nano12111914









