Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. The Andean Blueberry Extract Preparation
2.3. Preparation of the Sugarcane Bagasse Ash
2.4. Characterization of Sugar Cane Bagasse Ash
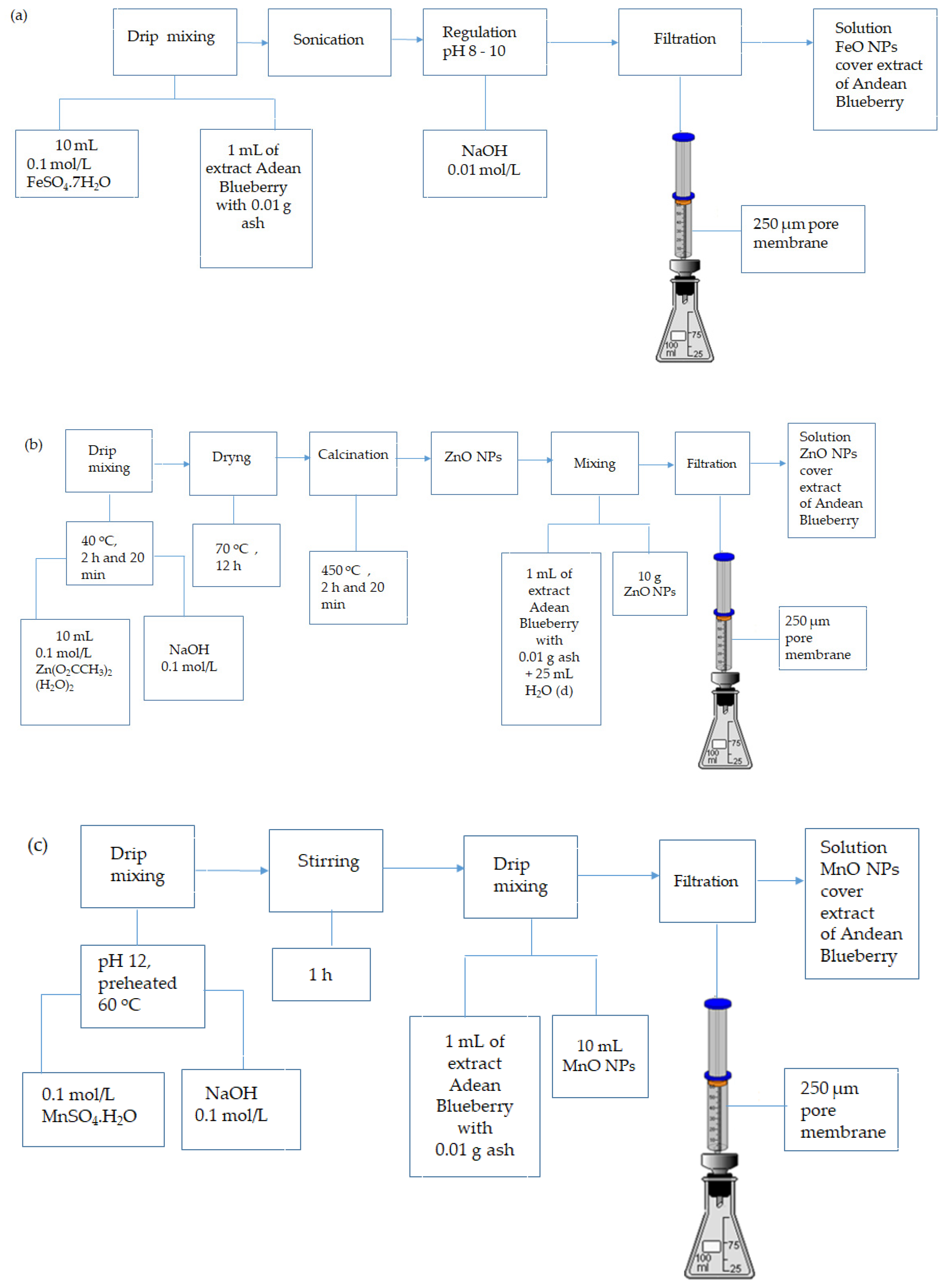
2.5. Synthesis of Nanoparticles (NPs)
2.6. Materials and Equipment Used in the Characterization of NPs
2.7. Growth of Cabbage and Andean Lupin Plants
2.8. Spray of Nanofertilizers to Crops
3. Results and Discussion
3.1. Characterization of Sugar Cane Bagasse Ash
3.2. Characterization of Andean Blue Berry Extract
3.3. Synthesis of the Nanoparticles
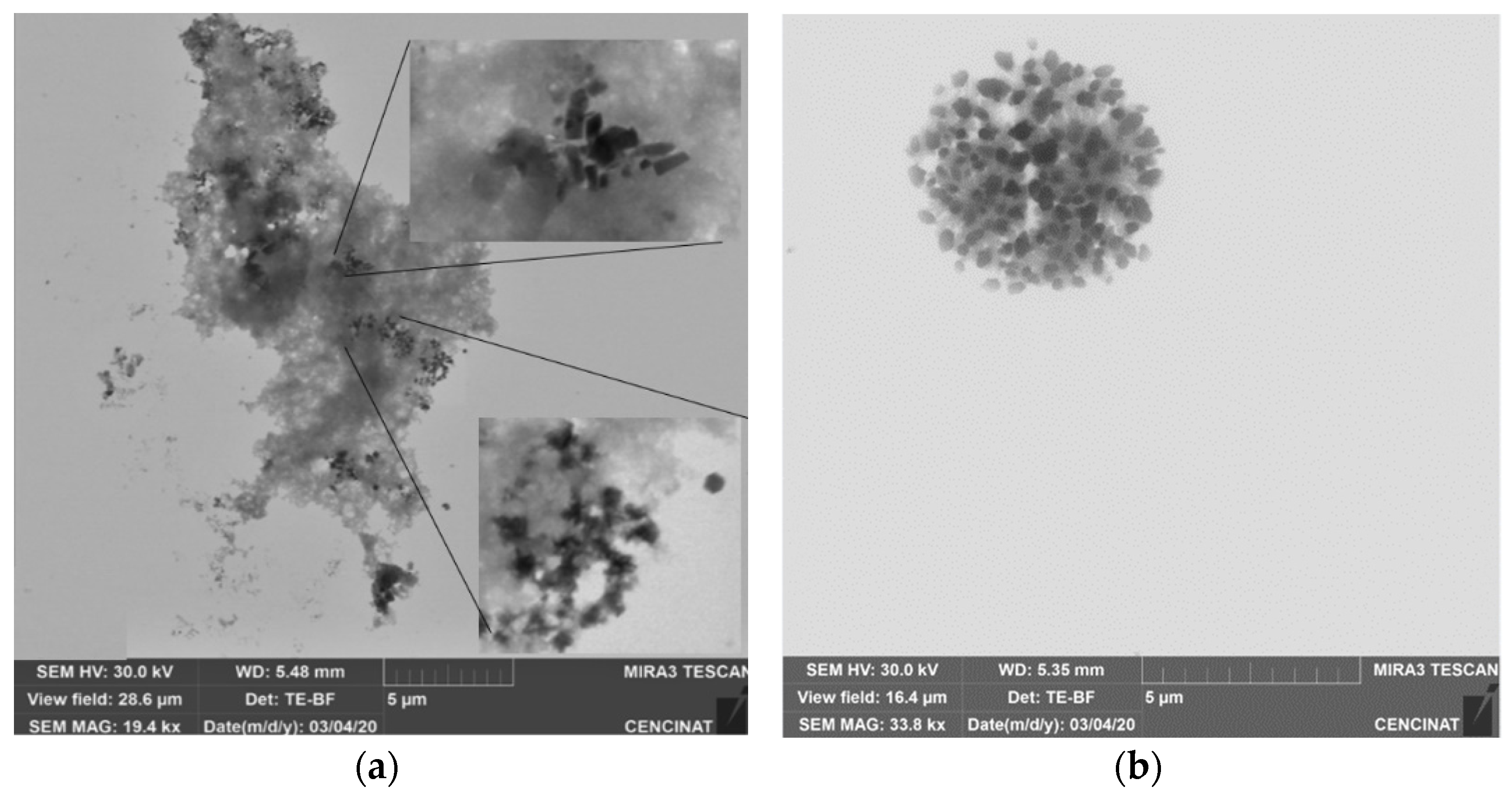
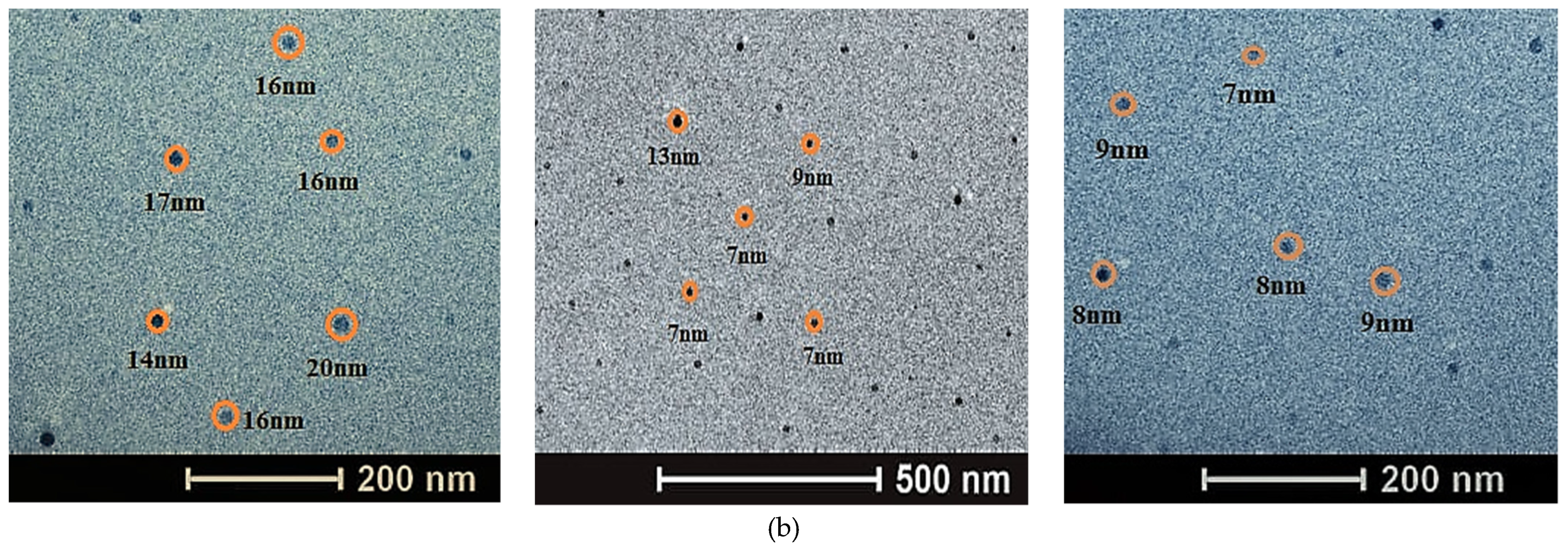
3.4. Characterization of NPs
3.5. Growth of Cabbage and Andean Lupin Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindblom, J.; Lundström, C.; Ljung, M.; Jonsson, A. Promoting sustainable intensification in precision agriculture: Review of decision support systems development and strategies. Precis. Agric. 2017, 18, 309–331. [Google Scholar] [CrossRef] [Green Version]
- Bucci, G.; Bentivoglio, D.; Finco, A. Precision agriculture as a driver for sustainable farming systems: State of art in literature and research. Calitatea 2018, 19, 114–121. [Google Scholar]
- McLennon, E.; Dari, B.; Jha, G.; Sihi, D.; Kankarla, V. Regenerative agriculture and integrative permaculture for sustainable and technology driven global food production and security. Agron. J. 2021, 113, 4541–4559. [Google Scholar] [CrossRef]
- Saatsaz, M. A historical investigation on water resources management in Iran. Environ. Dev. Sustain. 2019, 22, 1749–1785. [Google Scholar] [CrossRef]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Wallington, K.; Shafiee-Jood, M.; Marston, L. Understanding and managing the food-energy-water nexus–opportunities for water resources research. Adv. Water Resour. 2018, 111, 259–273. [Google Scholar] [CrossRef]
- Gosnell, H.; Gill, N.; Voyer, M. Transformational adaptation on the farm: Processes of change and persistence in transitions to ‘climate-smart’regenerative agriculture. Glob. Environ. Chang. 2019, 59, 101965. [Google Scholar] [CrossRef]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Chang. Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef]
- Carvalho, R.; de Aguiar, A.P.D.; Amaral, S. Diversity of cattle raising systems and its effects over forest regrowth in a core region of cattle production in the Brazilian Amazon. Reg. Environ. Chang. 2020, 20, 44. [Google Scholar] [CrossRef] [Green Version]
- Maja, M.M.; Ayano, S.F. The Impact of Population Growth on Natural Resources and Farmers’ Capacity to Adapt to Climate Change in Low-Income Countries. Earth Syst. Environ. 2021, 5, 271–283. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, L.; Zhang, G.; Meng, L.; Pan, Z.; Lun, F.; An, P. Green-depressing cropping system: A referential land use practice for fallow to ensure a harmonious human-land relationship in the farming-pastoral ecotone of northern China. Land Use Policy 2020, 100, 104917. [Google Scholar] [CrossRef]
- Ghosh, A.; Misra, S.; Bhattacharyya, R.; Sarkar, A.; Singh, A.K.; Tyagi, V.C.; Kumar, R.V.; Meena, V.S. Agriculture, dairy and fishery farming practices and greenhouse gas emission footprint: A strategic appraisal for mitigation. Environ. Sci. Pollut. Res. 2020, 27, 10160–10184. [Google Scholar] [CrossRef] [PubMed]
- Arden-Clarke, C.; Hodges, R.D. The environmental effects of conventional and organic/biological farming systems. II. Soil ecology, soil fertility and nutrient cycles. Biol. Agric. Hortic. 1988, 5, 223–287. [Google Scholar] [CrossRef]
- Tskhovrebov, V.S.; Faizova, V.I.; Lysenko, V.Y.; Novikov, A.A. Cycles of living and bio-inert systems in soil formation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 548, p. 052011. [Google Scholar]
- Sinha, M.; Dhillon, S.; Dhillon, K.; Dyanand, S. Solubility relationships of iron, manganese, copper and zinc in alkaline and calcareous soils. Soil Res. 1978, 16, 19–26. [Google Scholar] [CrossRef]
- Recena, R.; García-López, A.M.; Delgado, A. Zinc uptake by plants as affected by fertilization with Zn sulfate, phosphorus availability, and soil properties. Agronomy 2021, 11, 390. [Google Scholar] [CrossRef]
- Shrestha, J.; Kandel, M.; Subedi, S.; Shah, K.K. Role of nutrients in rice (Oryza sativa L.): A review. Agrica 2020, 9, 53–62. [Google Scholar] [CrossRef]
- Sanguansri, P.; Augustin, M.A. Nanoscale materials development–a food industry perspective. Trends Food Sci. Technol. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef]
- Naseer, B.; Srivastava, G.; Qadri, O.S.; Faridi, S.A.; Islam, R.U.; Younis, K. Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 2018, 7, 623–641. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2018, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, N.; Geetha, N.; Ghorbanpour, M.; Venkatachalam, P. Role of Engineered Zinc and Copper Oxide Nanoparticles in Promoting Plant Growth and Yield: Present Status and Future Prospects. In Advances in Phytonanotechnology: From Synthesis to Application; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–201. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Mungray, A.A.; Mungray, A.K. Fabricated nanoparticles: Current status and potential phytotoxic threats. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2014; Volume 230, pp. 83–110. [Google Scholar]
- Sengar, R.S.; Gautam, M.; Sengar, R.S.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead stress effects on physiobiochemical activities of higher plants. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2008; Volume 196, pp. 73–93. [Google Scholar]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S.; Tarafdar, J.C. Effect of nano-ZnO on growth of mung bean (Vigna radiata) and chickpea (Cicer arietinum) seedlings using plant agar method. Appl. Biol. Res. 2011, 13, 54–61. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of Iron and Silver Nanoparticles at Room Temperature Using Aqueous Sorghum Bran Extracts. Langmuir 2010, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1724, p. 020048. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mir, N.; Mousavi-Kamazani, M.; Bagheri, S.; Salavati-Niasari, M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016, 6, 32539. [Google Scholar] [CrossRef] [PubMed]
- Makarov, v.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. Green Nano-technologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 1, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Kuponiyi, A.; Kassama, L.; Kukhtareva, T. Physicochemical characterization of silver nanoparticles synthesize using Aloe Vera (Aloe barbadensis). In Nanobiosystems: Processing, Characterization, and Applications VII; SPIE: Bellingham, WA, USA, 2014; Volume 9171, pp. 103–114. [Google Scholar]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Manganese oxide nanoparticles as safer seed priming agent to improve chlorophyll and antioxidant profiles in watermelon seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.; Lal, R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: Nanotoxicants or nanonutrients? Water Air Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thoppey, U.U.G.; Hikku, G.S.; Selvakumar, N.; Subramania, A.; Krishnamoorthy, K. Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles. RSC Adv. 2016, 6, 15451–15459. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Kumar, G.D.; Natarajan, N.; Nakkeeran, S. Antifungal activity of nanofungicide Trifloxystrobin 25% + Tebuconazole 50% against Macrophomina phaseolina. Afr. J. Microbiol. Res. 2016, 10, 100–105. [Google Scholar]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Servin, A.D.; Hong, J.; Niu, G.; Peralta-Videa, J.R.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Influence of CeO2 and ZnO Nanoparticles on Cucumber Physiological Markers and Bioaccumulation of Ce and Zn: A Life Cycle Study. J. Agric. Food Chem. 2013, 61, 11945–11951. [Google Scholar] [CrossRef]
- Pandey, A.C.; Sanjay, S.S.; Yadav, R.S. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Blueberries and Their Anthocyanins: Factors Affecting Biosynthesis and Properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Franco, J.G.; Cefali, L.C.; Ataide, J.A.; Santini, A.; Souto, E.B.; Mazzola, P.G. Effect of nanoencapsulation of blueberry (Vaccinium myrtillus): A green source of flavonoids with antioxidant and photoprotective properties. Sustain. Chem. Pharm. 2021, 23, 100515. [Google Scholar] [CrossRef]
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green synthesis of iron nanoparticles: Application on the removal of petroleum oil from contaminated water and soils. J. Nanotechnol. 2018, 2018, 4184769. [Google Scholar] [CrossRef] [Green Version]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Benbi, D.K.; Thind, H.S.; Sharma, S.; Brar, K.; Toor, A.S. Bagasse Ash Application Stimulates Agricultural Soil C Sequestration Without Inhibiting Soil Enzyme Activity. Commun. Soil Sci. Plant Anal. 2017, 48, 1822–1833. [Google Scholar] [CrossRef]
- Wezel, A.; Bellon, S.; Doré, T.; Francis, C.; Vallod, D.; David, C. Agroecology as a science, a movement and a practice. A review. Agron. Sustain. Dev. 2009, 29, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Sales, A.; Lima, S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010, 30, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Sun, F.X.; Sun, R.C.; Su, Y.Q. Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; Suzane dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef]
- Melati, R.B.; Schmatz, A.A.; Pagnocca, F.C.; Contiero, J.; Brienzo, M. Sugarcane bagasse: Production, composition, properties, and feedstock potential. In Sugarcane: Production Systems, Uses and Economic Importance; Unesp: São Paulo, Brasil, 2017; pp. 1–38. [Google Scholar]
- Xu, Q.; Ji, T.; Gao, S.-J.; Yang, Z.; Wu, N. Characteristics and Applications of Sugar Cane Bagasse Ash Waste in Cementitious Materials. Materials 2018, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.S.; Shaarawy, H.H.; Hussien, N.H.; Hawash, S.I. Preparation of nano-fertilizer blend from banana peels. Bull. Natl. Res. Cent. 2019, 43, 26. [Google Scholar] [CrossRef] [Green Version]
- Falconí, C.E.; Yánez-Mendizábal, V. Available Strategies for the Management of Andean Lupin Anthracnose. Plants 2022, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Falconí, C. Lupinus mutabilis in Ecuador with Special Emphasis on Anthracnose Resistance; Wageningen University and Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Nagdeve, M. Twelve Proven Health Benefits & Uses of Cabbage. 2021. Available online: https://www.organicfacts.net/health-benefits/vegetable/health-benefits-of-cabbage.html#:~:text=Cabbage%20can%20be%20a%20very,choline%2C%20and%20beta%2Dcarotene (accessed on 1 February 2022).
- Sinde-González, I.; Gómez-López, J.P.; Tapia-Navarro, S.A.; Murgueitio, E.; Falconí, C.; Benítez, F.L.; Toulkeridis, T. Determining the Effects of Nanonutrient Application in Cabbage (Brassica oleracea var. capitate L.) Using Spectrometry and Biomass Estimation with UAV. Agronomy 2021, 12, 81. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods: For the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Manals-Cutiño, E.M.; Penedo-Medina, M.; Salas-Tort, D. Caracterización del bagazo de caña como biomasa vegetal. Tecnol. Química 2015, 35, 244–255. [Google Scholar]
- Carrera Villacrés, D.V. Salinidad en Suelos y Aguas Superficiales y Subterráneas de la Cuenca Evaporítica de Río Verde-Matehuala, San Luis Potosí. Ph.D. Thesis, Colegio de Postgraduados, Montecillo, Mexico, 2011. [Google Scholar]
- Aquino, P.; Osorio, A.M.; Ninán, E.; Torres, F. Caracterización de nanopartículas de ZnO sintetizadas por el método de precipitación y su evaluación en la incorporación en pinturas esmalte. Rev. Soc. Química Perú 2018, 84, 5–17. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ahmed, R.; Gao, A. Survival of S higa toxin-producing E scherichia coli in broth as influenced by p H, water activity and temperature. Lett. Appl. Microbiol. 2015, 60, 341–346. [Google Scholar] [CrossRef]
- Teixeira, G.; Katroschan, K.; Varennes, H.; Martins, J.; Stützel, A. The use of lupin seed as nitrogen fertilizer. In Lupin Crops: An Opportunity for Today, a Promise for the Future, Proceedings of the 13th International Lupin Conference, Poznań, Poland, 6–10 June 2011; International Lupin Association: Cochabamba, Bolivia; pp. 122–125.
- Vuong, L.D. Nanoparticles for the improved crop production. In Nanotechnology for Agriculture: Crop Production & Protection; Springer: Singapore, 2019; pp. 85–106. [Google Scholar]
- Timilsina, A.; Chen, H. The Emerging Applications of Zinc-Based Nanoparticles in Plant Growth Promotion. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Wiley: Hoboken, NJ, USA, 2021; pp. 45–62. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication. J. Saudi Chem. Soc. 2014, 18, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Çağlar, A.; Cengiz, U.; Yıldırım, M.; Kaya, I. Effect of deposition charges on the wettability performance of electrochromic polymers. Appl. Surf. Sci. 2015, 331, 262–270. [Google Scholar] [CrossRef]
- Petrenec, M.; Šmíd, M.; Kruml, T.; Obrtlík, K.; Polák, J. Low cycle fatigue of cast γ-TiAl based alloys at high temperature. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2011; Volume 452, pp. 421–424. [Google Scholar]
- Falconí, C.E.; Yánez–Mendizábal, V. Dry heat treatment of Andean lupin seed to reduce anthracnose infection. Crop Prot. 2016, 89, 178–183. [Google Scholar] [CrossRef]
- Gutiérrez Rodríguez, M.; Escalante Estrada, J.A.; Rodriguez Gonzalez, M.T.; Reynolds, M.P. Índices de reflectancia y rendimiento del frijol con aplicaciones de nitrógeno. In Terra Latinoamericana; Sociedad Mexicana de la Ciencia del Suelo, A.C.: Chapingo, México, 2004; Volume 22, pp. 40–416. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. In Geophysical Research Letters; Wiley: Hoboken, NJ, USA, 2003; Volume 30. [Google Scholar]
- Yánez-Mendizábal, V.; Falconí, C.E. Bacillus subtilis CtpxS2-1 induces systemic resistance against anthracnose in Andean lupin by lipopeptide production. Biotechnol. Lett. 2021, 43, 719–728. [Google Scholar] [CrossRef]
- Barrios, M.B.; Buján, A.; Debelis, S.P.; Sokolowski, A.C.; Blasón, Á.D.; Rodríguez, H.A.; López, S.C.; De Grazia, J.; Mazo, C.R.; Gagey, M.C. Relación de raíz/biomasa total de Soja (Glycine max) en dos sistemas de labranza. Terra Latinoam. 2014, 32, 221–230. [Google Scholar]
- Yánez-Mendizábal, V.; Falconi, C. Efficacy of Bacillus spp. to biocontrol of anthracnose and enhance plant growth on Andean lupin seeds by lipopeptide production. Biol. Control 2018, 122, 67–75. [Google Scholar] [CrossRef]
- López Bravo, E.; Rivera, A.; Javier, A.; Herrera Suárez, M.; Gonzalez Cueto, O.; García de la Figal Costales, A. Propiedades de un compost obtenido a partir de residuos de la producción de azúcar de caña. Cent. Agrícola 2017, 44, 49–55. [Google Scholar]
- Prieto García, J.O.; Quintana Puchol, R.; Rodríguez Suárez, E.; Mollineda Trujillo, Á. Ceniza de bagazo de caña de azúcar en la remoción de zinc en soluciones acuosas. Cent. Azúcar 2016, 43, 78–83. [Google Scholar]
- Agredo, J.T.; DE Gutierrez, R.M.; Giraldo, C.E.E.; Salcedo, L.O.G. Characterization of sugar cane bagasse ash as supplementary material for Portland cement. Ing. Investig. 2014, 34, 42787. [Google Scholar] [CrossRef]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceram. Int. 2013, 39, 9803–9808. [Google Scholar] [CrossRef]
- Austin, J.; Minelli, C.; Hamilton, D.; Wywijas, M.; Jones, H.J. Nanoparticle number concentration measurements by multi-angle dynamic light scattering. J. Nanopart. Res. 2020, 22, 108. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Mehr, F.P.; Pazoki, H.; Rahmani, B.M. Synthesis of ZnO Nanoparticles by Precipitation Method. Orient. J. Chem. 2015, 31, 1219–1221. [Google Scholar] [CrossRef]
- Moon, S.A.; Salunke, B.K.; Alkotaini, B.; Sathiyamoorthi, E.; Kim, B.S. Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract. IET Nanobiotechnol. 2015, 9, 220–225. [Google Scholar] [CrossRef]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green synthesis and characterization of Manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J. Appl. Pharm. Sci. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Barros, B.S.; Barbosa, R.; Dos Santos, N.R.; Barros, T.S.; Souza, M.A. Synthesis and x-ray diffraction characterization of nanocrystalline ZnO obtained by Pechini method. Inorg. Mater. 2006, 42, 1348–1351. [Google Scholar] [CrossRef]
- Devi, P.G.; Velu, A.S. Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J. Theor. Appl. Phys. 2016, 10, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Najjar, R.; Awad, R.; Abdel-Gaber, A.M. Physical Properties of Mn2O3 Nanoparticles Synthesized by Co-precipitation Method at Different pH Values. J. Supercond. Nov. Magn. 2018, 32, 885–892. [Google Scholar] [CrossRef]
- Cevallos, L.N.M.; García, J.L.R.; Suárez, B.I.A.; González, C.A.L.; González, I.S.; Campoverde, J.A.Y.; Guzmán, J.A.M.; Toulkeridis, T. A NDVI analysis contrasting different spectrum data methodologies applied in pasture crops previous grazing–A case study from Ecuador. In 2018 International Conference on eDemocracy & eGovernment (ICEDEG); IEEE: Piscataway, NJ, USA, 2018; pp. 126–135. [Google Scholar] [CrossRef]
- Gosens, I.; Post, J.A.; De La Fonteyne, L.J.J.; Jansen, E.H.J.M.; Geus, J.W.; Cassee, F.R.; De Jong, W.H. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part. Fibre Toxicol. 2010, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.-H.; Yeh, C.-H. Influence of hydrothermal conditions on the morphology and particle size of zinc oxide powder. Ceram. Int. 2000, 26, 351–357. [Google Scholar] [CrossRef]
- Chen, J.S.; Zhu, T.; Li, C.M.; Lou, X.W. Building Hematite Nanostructures by Oriented Attachment. Angew. Chem. Int. Ed. 2010, 50, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO Nanoparticles with High Dispersibility Based on Oriented Attachment (OA) Process. Nanoscale Res. Lett. 2019, 14, 210. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi-Majd, M.; Nejad, A.R.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346–360. [Google Scholar] [CrossRef]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of Magnetite Nanoparticles on Soybean Chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef] [Green Version]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical composition and phenolic compound profile of mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- Pappi, P.; Nikoloudakis, N.; Fanourakis, D.; Zambounis, A.; Delis, C.; Tsaniklidis, G. Differential triggering of the phenylpropanoid biosynthetic pathway key genes transcription upon cold stress and viral infection in tomato leaves. Horticulturae 2021, 7, 448. [Google Scholar] [CrossRef]
- Jamil, M.; Qasim, M.; Umar, M.; Subhan, A. Impact of organic wastes (bagasse ash) on the yield of wheat (Triticum aestivum L.) in a calcareous soil. Int. J. Agric. Biol. 2004, 6, 468–470. [Google Scholar]
- Scotti, I.A.; Silva, S.; Botteschi, G. Effect of fly ash on the availability of Zn, Cu, Ni and Cd to chicory. Agric. Ecosyst. Environ. 1999, 72, 159–163. [Google Scholar] [CrossRef]
- Carlson, C.L.; Adriano, D.C. Environmental Impacts of Coal Combustion Residues. J. Environ. Qual. 1993, 22, 227–247. [Google Scholar] [CrossRef]
- Mlynkowiak, W.; Snieg, M.; Tomaszewicz, T.; Dawidowski, J.B. Impact of fly-ashes from the “Dolna Odra” power plant on firmness and physicochemical properties of light silty loam. Inzynieria Rolnicza 2001, 237–247. [Google Scholar]
- Lal, R. Eco-intensification through soil carbon sequestration: Harnessing ecosystem services and advancing sustainable development goals. J. Soil Water Conserv. 2019, 74, 55A–61A. [Google Scholar] [CrossRef] [Green Version]





| Parameters | Values |
|---|---|
| Apparent density | 0.5 g/cm3 |
| pH | 11.2 |
| Electrical conductivity | 1427 µs/cm (24.9 °C). |
| Humidity percentage | 0.005% |
| Fe | 5.428 mg/g |
| Mn | 0.567 mg/g |
| Zn | 2.623 mg/g |
| Granulometric analysis | 58.03% ˂ a 75 µm 41.97% 63–1 µm |
| ZnO NPs (0.1 mol/L) | FeO NPs (0.1 mol/L) | MnO NPs (0.1 mol/L) | |
|---|---|---|---|
| S.P AREA | 6.4779 × 106 (cm2/cm3) | 8.298 × 106 (cm2/cm3) | 6.064 × 106 (cm2/cm3) |
| median | 9.5 (nm) | 7.8 (nm) | 10.1 (nm) |
| mean | 9.6 (nm) | 7.9 (nm) | 10.2 (nm) |
| Variance | 2.9218 (mm2) | 4.7152 (mm2) | 3.2688 (mm2) |
| S.D Standard Deviation | 1.7 (nm) | 2.2 (nm) | 1.8 (nm) |
| Diameter | ZnO NPs 0.1 mol/L (%) | MnO NPs 0.1 mol/L (%) |
|---|---|---|
| 5–10 nm | 12.32 | 47.73 |
| 10–15 nm | 43.40 | 30.57 |
| 15–20 nm | 32.55 | 18.93 |
| 20–25 nm | 10.85 | 2.76 |
| 25–30 nm | 0.59 | - |
| 30–35 nm | 0.29 | - |
| Diameter (nm) | FeO NPs 0.1 mol/L (%) |
|---|---|
| 12–22 | 64.62 |
| 22–32 | 9.23 |
| 32–42 | 12.31 |
| 42–52 | 3.08 |
| 52–62 | 7.69 |
| 62–70 | 3.08 |
| Variables | Treatments | ||
|---|---|---|---|
| Control | ZnO_MnO-NPs 270 ppm | ZnO_MnO-NPs 540 ppm | |
| Height (m) | 0.2767 | 0.2100 | 0.2133 |
| Root (m) | 0.1300 | 0.1433 | 0.1317 |
| Biomass (kg/m2) | 0.7900 | 1.2250 | 0.7440 |
| Chlorophyll Content Index | 0.1737 | 0.1860 | 0.1769 |
| Leaf area (cm2/plant) | 0.0380 | 0.0477 | 0.0304 |
| Variables | Treatments | ||
|---|---|---|---|
| Control (without nanoparticles) | FeO_ZnO-NPs 270 ppm | FeO_ZnO-NPs 540 ppm | |
| Plant Height (m) | 1.56 | 1.66 | 1.56 |
| Root size (m) | 0.47 | 0.56 | 0.47 |
| Biomass (g/m2) | 0.11 | 0.09 | 0.09 |
| Chlorophyll content Index | 4.20 | 4.35 | 4.19 |
| Leaf area | 0.240 | 0.964 | 1.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12, 1921. https://doi.org/10.3390/nano12111921
Murgueitio-Herrera E, Falconí CE, Cumbal L, Gómez J, Yanchatipán K, Tapia A, Martínez K, Sinde-Gonzalez I, Toulkeridis T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials. 2022; 12(11):1921. https://doi.org/10.3390/nano12111921
Chicago/Turabian StyleMurgueitio-Herrera, Erika, César E. Falconí, Luis Cumbal, Josselyn Gómez, Karina Yanchatipán, Alejandro Tapia, Kevin Martínez, Izar Sinde-Gonzalez, and Theofilos Toulkeridis. 2022. "Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants" Nanomaterials 12, no. 11: 1921. https://doi.org/10.3390/nano12111921
APA StyleMurgueitio-Herrera, E., Falconí, C. E., Cumbal, L., Gómez, J., Yanchatipán, K., Tapia, A., Martínez, K., Sinde-Gonzalez, I., & Toulkeridis, T. (2022). Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials, 12(11), 1921. https://doi.org/10.3390/nano12111921









