Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Sample Characterization
2.3. Antibacterial Activity Test
2.4. Statistical Data Analysis
3. Results and Discussion
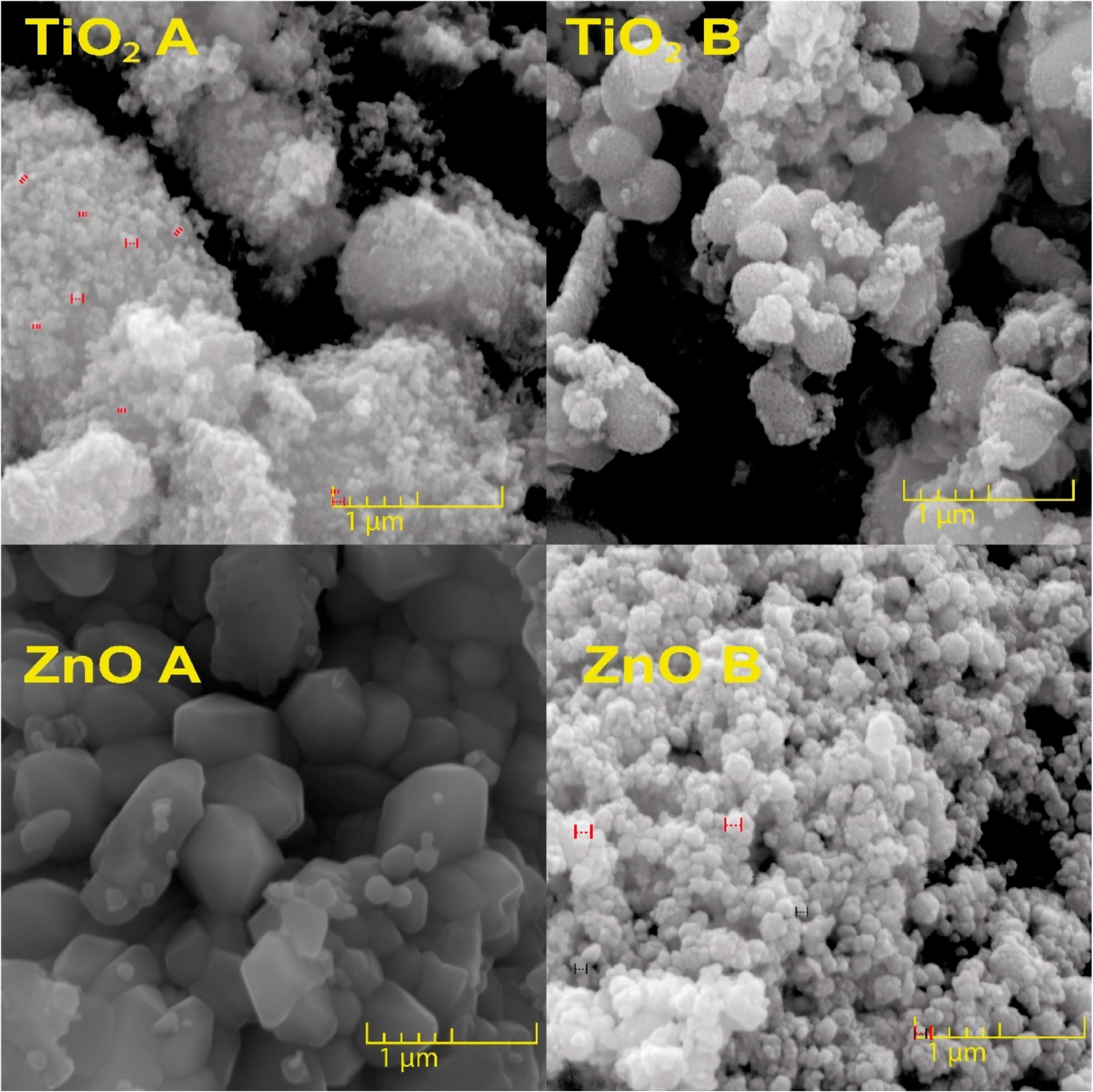
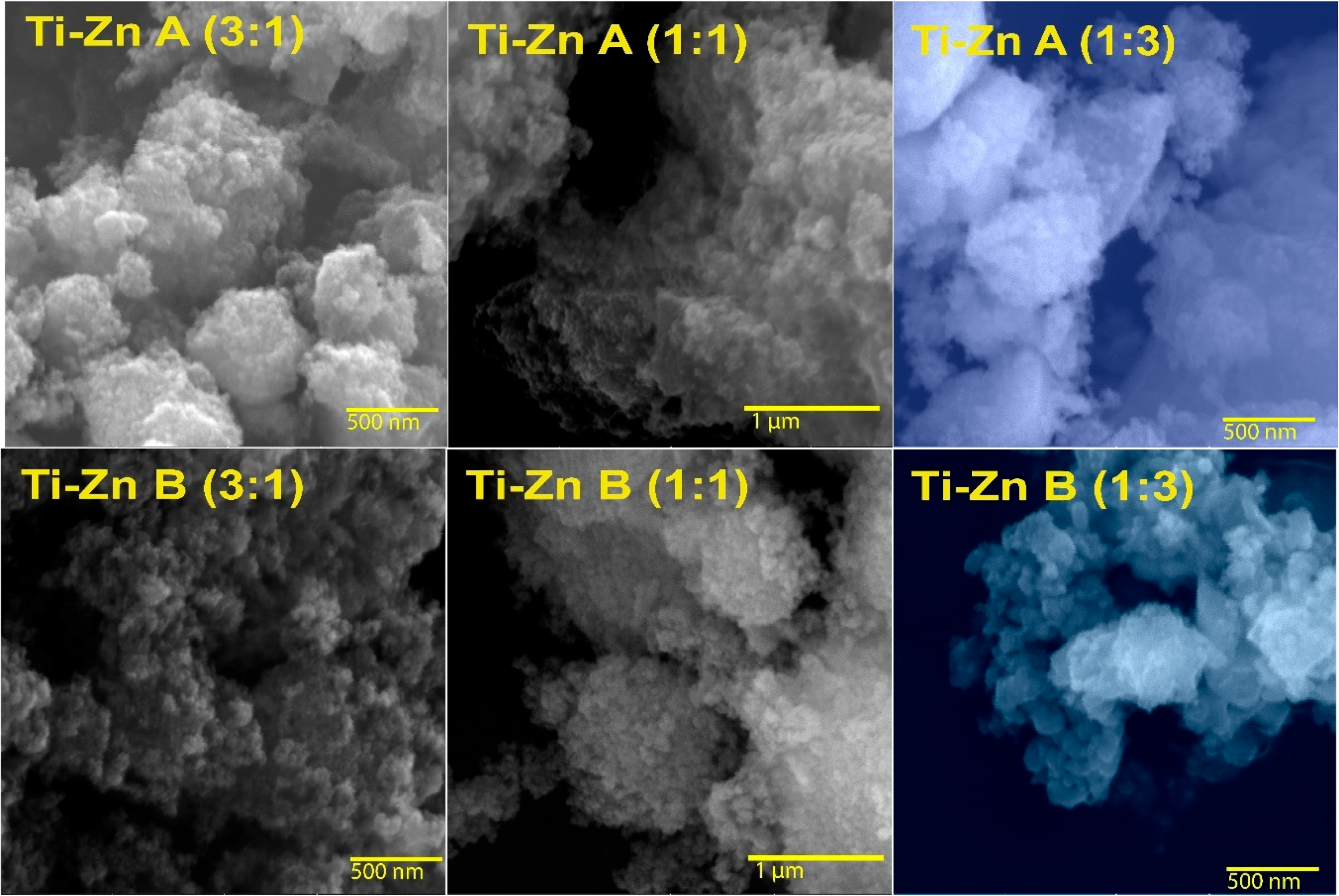
3.1. Morphological Characteristics
3.2. UV-Vis Analysis
3.3. Infrared Analysis
3.4. X-ray Diffraction
3.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijayalakshmi, K.; Sivaraj, D. Synergistic antibacterial activity of barium doped TiO2 nanoclusters synthesized by microwave processing. RSC Adv. 2016, 6, 9663–9671. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; González-Silva, N.; Yahia, E.M.; González-Vargas, O.A.; Montalvo-González, E.; Pérez-Larios, A. Effect of TiO2-ZnO-MgO mixed oxide on microbial growth and toxicity against Artemia salina. Nanomaterials 2019, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Zhukova, L.V. Evidence for Compression of Escherichia coli K12 Cells under the Effect of TiO2 Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 27197–27205. [Google Scholar] [CrossRef]
- Carré, G.; Benhamida, D.; Peluso, J.; Muller, C.D.; Lett, M.C.; Gies, J.P.; Keller, V.; Keller, N.; André, P. On the use of capillary cytometry for assessing the bactericidal effect of TiO2. Identification and involvement of reactive oxygen species. Photochem. Photobiol. Sci. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Vladkova, T.; Staneva, A.; Albu-Kaya, M.; Martinov, B.; Ivanova, I. Collagen/(ZnTiO3 /SiO2) composites of an wide spectrum antimicrobial activity Todorka. J. Chem. Technol. Metall. 2020, 55, 60–72. [Google Scholar]
- Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-fouling and anti-bacterial modification of poly(vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubramanian, R.; Srivastava, R.S.; Misra, R.D.K. Comparative study of antimicrobial and photocatalytic activity in titania encapsulated composite nanoparticles with different dopants. Mater. Sci. Technol. 2008, 24, 589–595. [Google Scholar] [CrossRef]
- Nikpasand, A.; Parvizi, M.R. Evaluation of the Effect of Titatnium Dioxide Nanoparticles/Gelatin Composite on Infected Skin Wound Healing; An Animal Model Study. Bull. Emerg. Trauma 2019, 7, 366–372. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin-TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Suo, H.; Peng, C.; Jing, F.; Yu, S.; Cui, S.; Shen, X. Facile preparation of TiO2/ZnO composite aerogel with excellent antibacterial activities. Mater. Lett. 2019, 234, 253–256. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, W.J.; Jassim, H.A. A novel study of pH influence on Ag nanoparticles size with antibacterial and antifungal activity using green synthesis. World Sci. News 2018, 97, 139–152. [Google Scholar]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Review—Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [Green Version]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Datteo, M.; Ferraro, L.; Seifert, G.; Di Valentin, C. TETT-functionalized TiO2 nanoparticles for DOX loading: A quantum mechanical study at the atomic scale. Nanoscale Adv. 2020, 2, 2774–2784. [Google Scholar] [CrossRef]
- Li, Z.; Ou-Yang, Y.; Liu, Y.; Wang, Y.Q.; Zhu, X.L.; Zhang, Z.Z. Folic acid-conjugated TiO2-doped mesoporous carbonaceous nanocomposites loaded with Mitoxantrone HCl for chemo-photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 1197–1206. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.S.; Cho, Y.K. Significantly enhanced antibacterial activity of TiO2 nanofibers with hierarchical nanostructures and controlled crystallinity. Analyst 2015, 140, 616–622. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [Green Version]
- Faraji, M.; Mohaghegh, N.; Abedini, A. TiO2 nanotubes/Ti plates modified by silver-benzene with enhanced photocatalytic antibacterial properties. New J. Chem. 2018, 42, 2058–2066. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Dobrowolska, A.; Czaczyk, K.; Motylenko, M.; Rafaja, D.; Ehrlich, H.; Jesionowski, T. Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 2019, 463, 791–801. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, W.; Yao, L.; Wang, J.; Han, H.; Zhu, T.; Liang, Y.; Fu, J.; Wang, Y. 3D Ag/ZnO microsphere SERS substrate with ultra-sensitive, recyclable and self-cleaning performances: Application for rapid in site monitoring catalytic dye degradation and insight into the mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125507. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kaur, S.; Singh, J.; Rawat, M.; Kumar, S. Expanding horizon: Green synthesis of TiO2 nanoparticles using Carica papaya leaves for photocatalysis application. Mater. Res. Express 2019, 6, 095034. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Jose, M. Streptomycin loaded TiO2 nanoparticles: Preparation, characterization and antibacterial applications. J. Nanostruct. Chem. 2017, 7, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, G.; Thangavel, S.; Vasudevan, V.; Zoltán, K. Efficient visible-light piezophototronic activity of ZnO-Ag8S hybrid for degradation of organic dye molecule. J. Phys. Chem. Solids 2020, 143, 109473. [Google Scholar] [CrossRef]
- Rani, S.; Suri, P.; Shishodia, P.K.; Mehra, R.M. Synthesis of nanocrystalline ZnO powder via sol-gel route for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1639–1645. [Google Scholar] [CrossRef]
- Jing, F.; Suo, H.; Cui, S.; Tang, X.; Zhang, M.; Shen, X.; Lin, B.; Jiang, G.; Wu, X. Facile synthesis of TiO2/Ag composite aerogel with excellent antibacterial properties. J. Sol-Gel Sci. Technol. 2018, 86, 590–598. [Google Scholar] [CrossRef]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2018, 25, 10328–10339. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.H.; Van Le, Q.; Peng, W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Baruah, R.; Yadav, A.; Das, A.M. Livistona jekinsiana fabricated ZnO nanoparticles and their detrimental effect towards anthropogenic organic pollutants and human pathogenic bacteria. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 251, 119459. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Lopez, R.; Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2–ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Hu, S.; Li, J. Preparation and enhanced photoelectrochemical performance of coupled bicomponent ZnO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.U.; Tahir, M.B.; Nadeem Riaz, K.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2020, 102, 434–442. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnO nanocomposites for solar energy driven photocatalysis. Mater. Sci. Semicond. Process. 2017, 61, 114–124. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Morales-Luna, M. Enhanced photocatalytic activity of TiO2-ZnO thin films deposited by dc reactive magnetron sputtering. Ceram. Int. 2017, 43, 8831–8838. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Taha, S.Y. Synthesis and Characterization of TiO2 Nanoparticles via Sol-Gel Method by Pulse Laser Ablation Synthesis and Characterization of TiO2 Nanoparticles via Sol-Gel Method by Pulse Laser Ablation. Eng. Tech. J. 2015, 33, 761–771. [Google Scholar]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO nanoparticles-modified dressings to inhibit wound pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Baldeón-Apaestegui, S.; Hernández-Gorritti, W.R. Identification of capsaicin and dihydrocapsaicin in the oleoresin extract obtained from panca chili (Capsicum chinense). Ing. Ind. 2017, 35, 223. [Google Scholar] [CrossRef] [Green Version]
- Borcan, L.C.; Dudas, Z.; Len, A.; Fuzi, J.; Borcan, F.; Tomescu, M.C. Synthesis and characterization of a polyurethane carrier used for a prolonged transmembrane transfer of a chili pepper extract. Int. J. Nanomed. 2018, 13, 7155–7166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Arreguin, A.; Carriles, R.; Ochoa-Alejo, N.; López, M.G.; Sánchez-Segura, L. Generation of BSA-capsaicin Nanoparticles and Their Hormesis Effect on the Rhodotorula mucilaginosa Yeast. Molecules 2019, 24, 2800. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.L.; Wei, M.M.; Song, C.; Ma, Y.F.; Zheng, X.J.; Xiong, D.C.; Ye, X.S. Chemical synthesis and biological evaluation of penta- to octa-saccharide fragments of Vi polysaccharide from: Salmonella typhi. Org. Chem. Front. 2018, 5, 2179–2188. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 nanoparticles via sol-gel method. Adv. Mater. Res. 2011, 173, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, Y.; Ghazali, M.J.; Otsuka, Y.; Ohnuma, K.; Morakul, S.; Nakamura, S.; Abdollah, M.F. Antibacterial properties of laser surface-textured TiO2/ZnO ceramic coatings. Ceram. Int. 2020, 46, 3949–3959. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B. Influence of acidic pH on the formulation of TiO2 nanocrystalline powders with enhanced photoluminescence property. Heliyon 2017, 3, e00246. [Google Scholar] [CrossRef] [Green Version]
- Stoyanova, A.; Hitkova, H.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Ivanova, N.; Sredkova, M. Synthesis and antibacterial activity of TiO2/ZnO nanocomposites prepared via nonhydrolytic route. J. Chem. Technol. Metall. 2013, 48, 154–161. [Google Scholar]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wei, D.; Du, M.; Ming, S.; Ding, Q.; Tan, R. Targeting Antibacterial Effect and Promoting of Skin Wound Healing after Infected with Methicillin-Resistant Staphylococcus aureus for the Novel Polyvinyl Alcohol Nanoparticles. Int. J. Nanomed. 2021, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [Green Version]
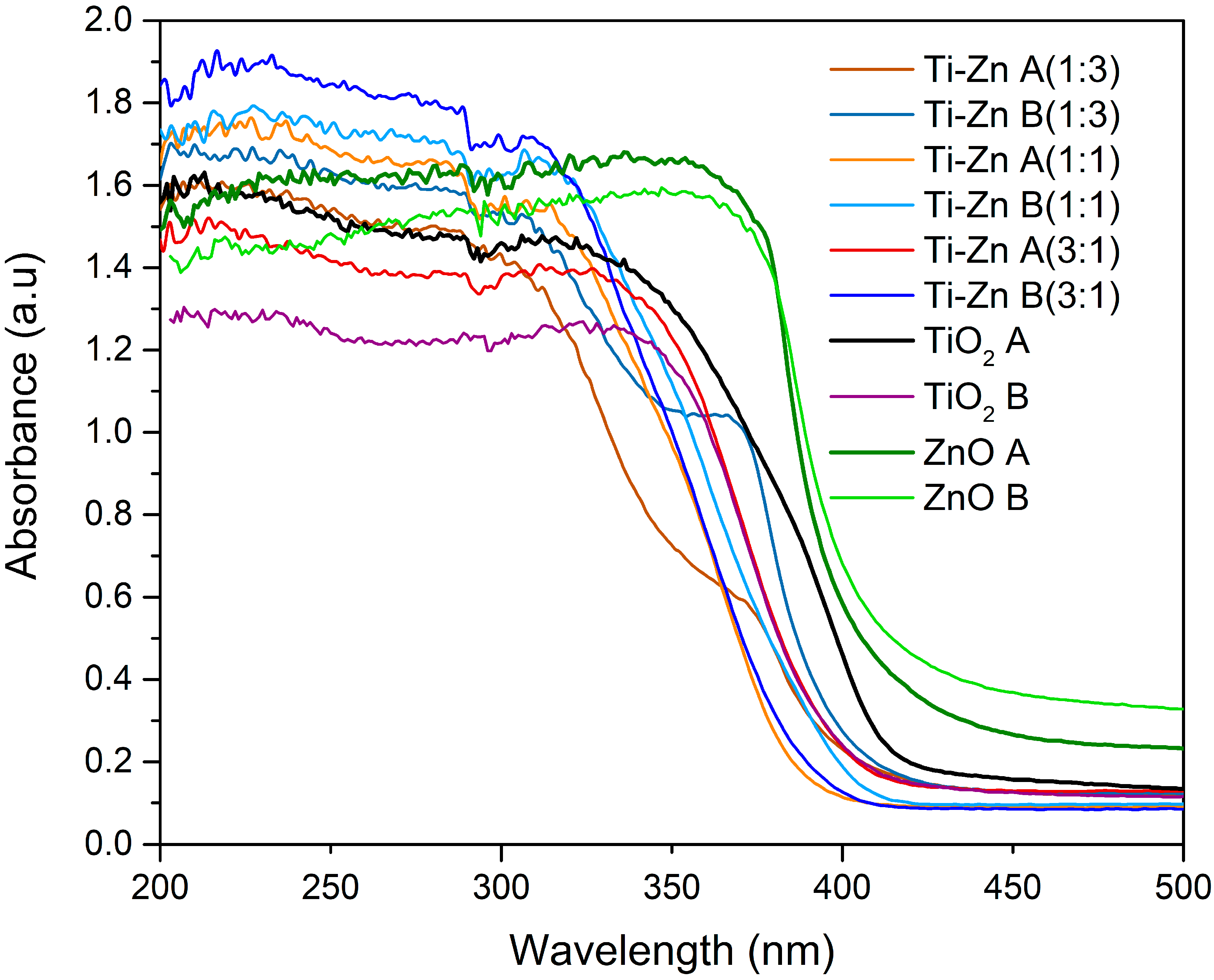

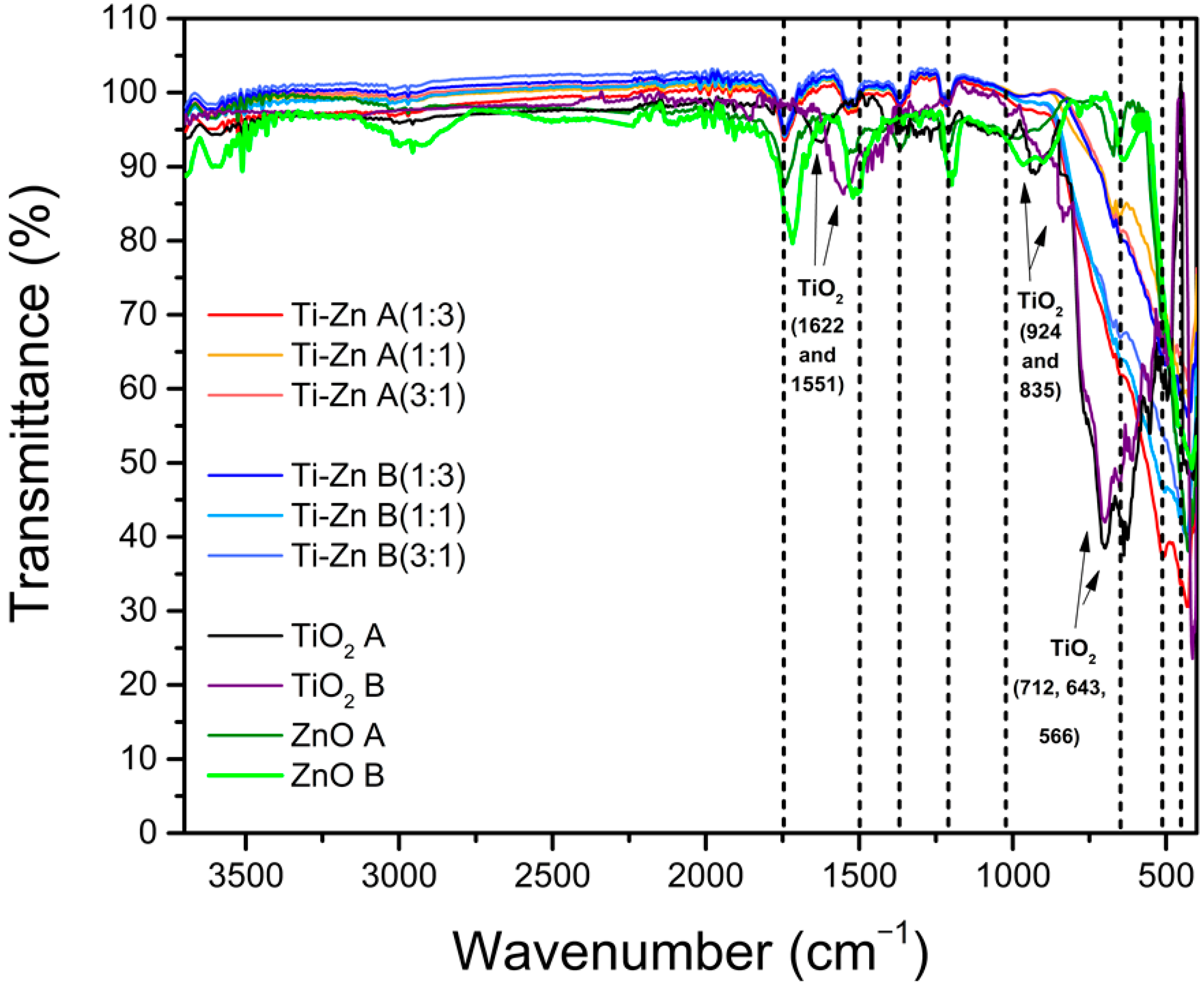



| Code | Relation Molar | pH (HNO3) | pH (NH3·H2O) | Direct Bandgap (Eg in eV) | Indirect Bandgap (Eg in eV) | ||
|---|---|---|---|---|---|---|---|
| TiO2 | 1 | 2.56 | 8.37 | 2.77 a | 2.82 b | 3.02 a | 3.20 b |
| Ti–Zn3 | 3:1 | 2.51 | 8.26 | 2.83 a | 2.92 b | 3.22 a | 3.31 b |
| Ti–Zn2 | 1:1 | 2.27 | 8.28 | 2.86 a | 3.03 b | 3.29 a | 3.30 b |
| Ti–Zn1 | 1:3 | 2.68 | 8.18 | 2.87 a | 2.94 b | 3.16 a | 3.19 b |
| ZnO c | 1 | 2.38 c | 8.34 | 2.96 | 2.90 | 3.17 | 3.15 |
| Material | Crystal Size (nm) | Principal Peak Position (°2θ) | d-Spacing (Å) | (hkl) | a (Å) | c (Å) |
|---|---|---|---|---|---|---|
| TiO2A | 12.26 ± 0.22 | 25.153 | 3.5376 | [101] | 3.85 | 10.50 |
| TiO2B | 23.87 ± 0.78 | 25.215 | 3.5290 | [101] | 3.78 | 9.52 |
| ZnOA | 69.05 ± 5.81 | 36.216 | 2.4780 | [101] | 3.25 | 5.20 |
| ZnOB | 70.82 ± 5.50 | 36.178 | 2.4808 | [101] | 3.14 | 5.21 |
| Ti–Zn A (3:1) | 17.19 ± 0.51 | 25.308, 35.288 | 3.5163, 2.5413 | [101] [212] | 7.95 | 6.151 |
| Ti–Zn B (3:1) | 41.30 ± 3.60 | 25.278, 35.829 | 3.5204, 2.5413 | [101] [021] | 9.93 | 8.19 |
| Ti–Zn A (1:1) | 36.34 ± 2.20 | 25.163, 35.258 | 3.5362, 2.5434 | [101] [300] | 7.91 | 10.93 |
| Ti–Zn B (1:1) | 34.55 ± 1.57 | 25.223, 35.265 | 3.5279, 2.5429 | [101] [205] | 9.62 | 5.93 |
| Ti–Zn A (1:3) | 21.57 ± 0.68 | 25.156, 36.637 | 3.5371, 2.4508 | [101] [004] | 5.94 | 6.39 |
| Ti–Zn B (1:3) | 29.71 ± 1.30 | 29.930, 36.204 | 2.9829, 2.6039 | [220] [302] | 5.99 | 8.42 |
| Treatment | E. coli (mm) | S. paratyphi (mm) | S. aureus (mm) | L. monocytogenes (mm) |
|---|---|---|---|---|
| Ampicillin (C+) a | 19.78 ± 1.09 | 28.56 ± 1.24 | 24.44 ± 0.88 | 26.22 ± 1.09 |
| Distilled water (C−) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| TiO2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| ZnO | 0 ± 0 | 0 ± 0 | 7.89 ± 0.60 b | 9.22 ± 1.09 b |
| Ti–Zn A (3:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn B (3:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn A (1:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn B (1:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn A (1:3) | 0 ± 0 | 0 ± 0 | 7.67 ± 0.58 b | 10.17 ± 1.04 b |
| Ti–Zn B (1:3) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Barajas, N.; Becerra-Solano, L.; Gutiérrez-Mercado, Y.K.; Macías-Carballo, M.; M. Gómez, C.; Pérez-Larios, A. Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials 2022, 12, 1948. https://doi.org/10.3390/nano12121948
Rodríguez-Barajas N, Becerra-Solano L, Gutiérrez-Mercado YK, Macías-Carballo M, M. Gómez C, Pérez-Larios A. Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials. 2022; 12(12):1948. https://doi.org/10.3390/nano12121948
Chicago/Turabian StyleRodríguez-Barajas, Noé, Luis Becerra-Solano, Yanet Karina Gutiérrez-Mercado, Monserrat Macías-Carballo, Claudia M. Gómez, and Alejandro Pérez-Larios. 2022. "Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties" Nanomaterials 12, no. 12: 1948. https://doi.org/10.3390/nano12121948
APA StyleRodríguez-Barajas, N., Becerra-Solano, L., Gutiérrez-Mercado, Y. K., Macías-Carballo, M., M. Gómez, C., & Pérez-Larios, A. (2022). Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials, 12(12), 1948. https://doi.org/10.3390/nano12121948








