Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production
Abstract
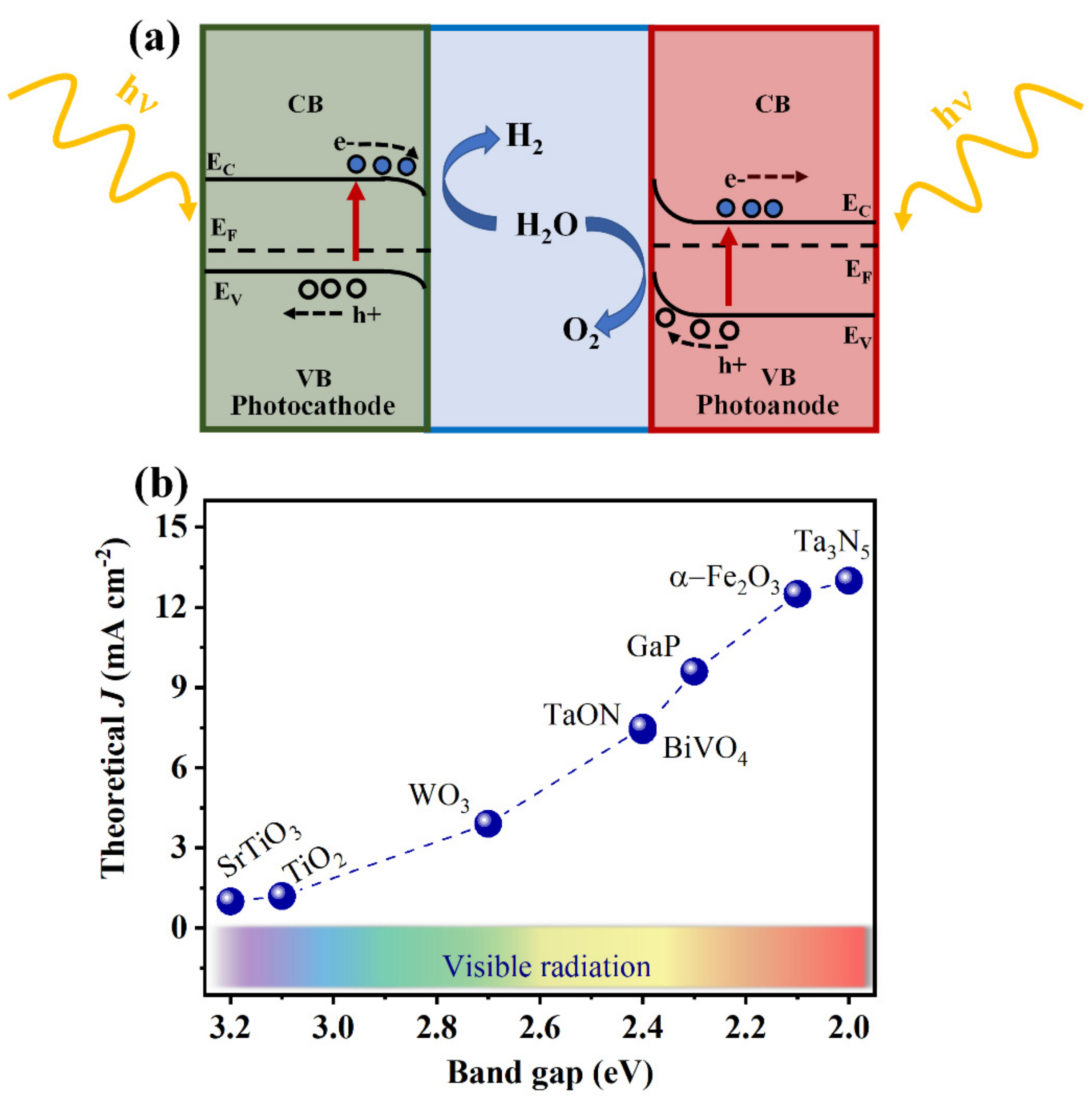
:1. Introduction
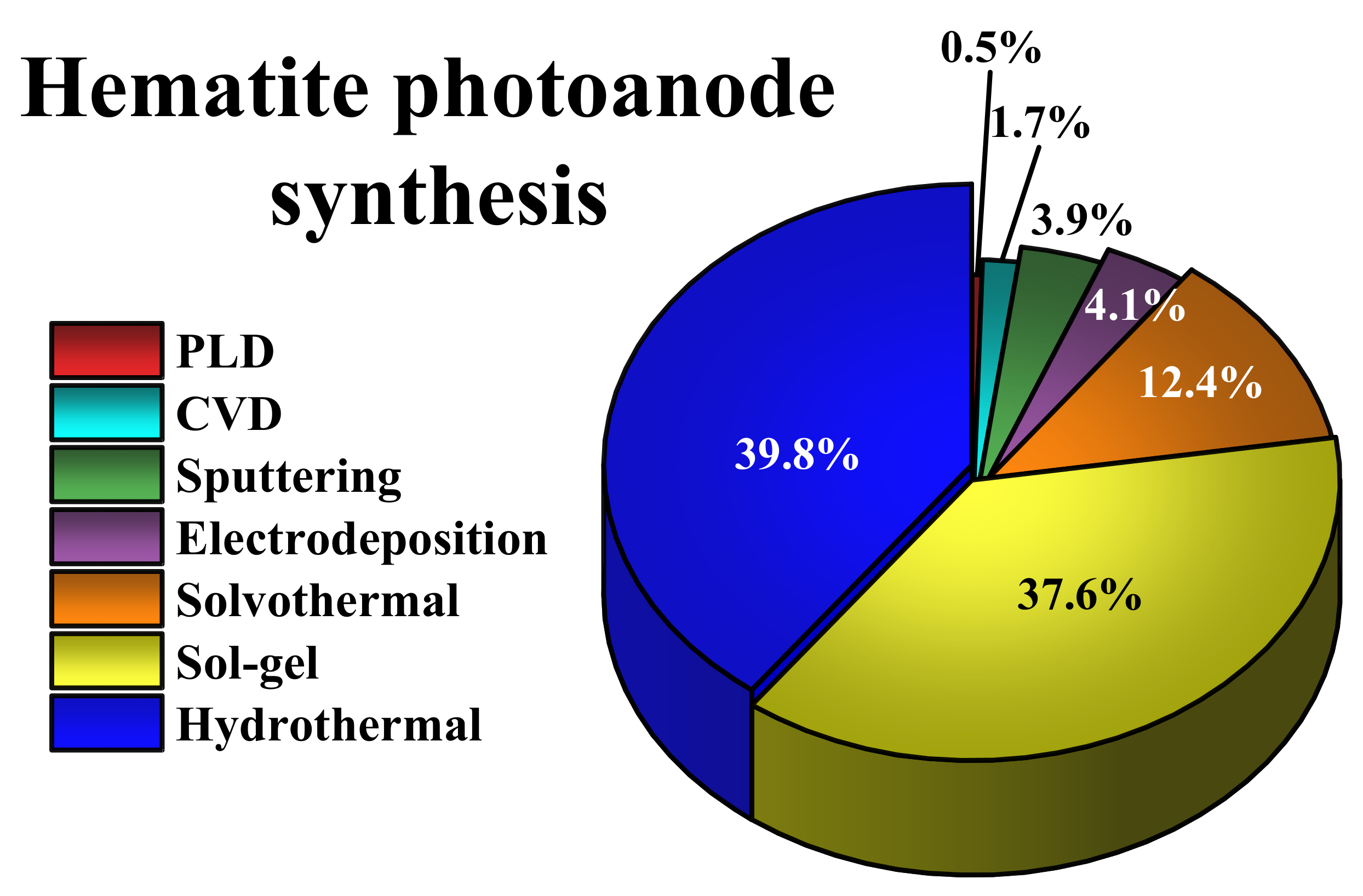
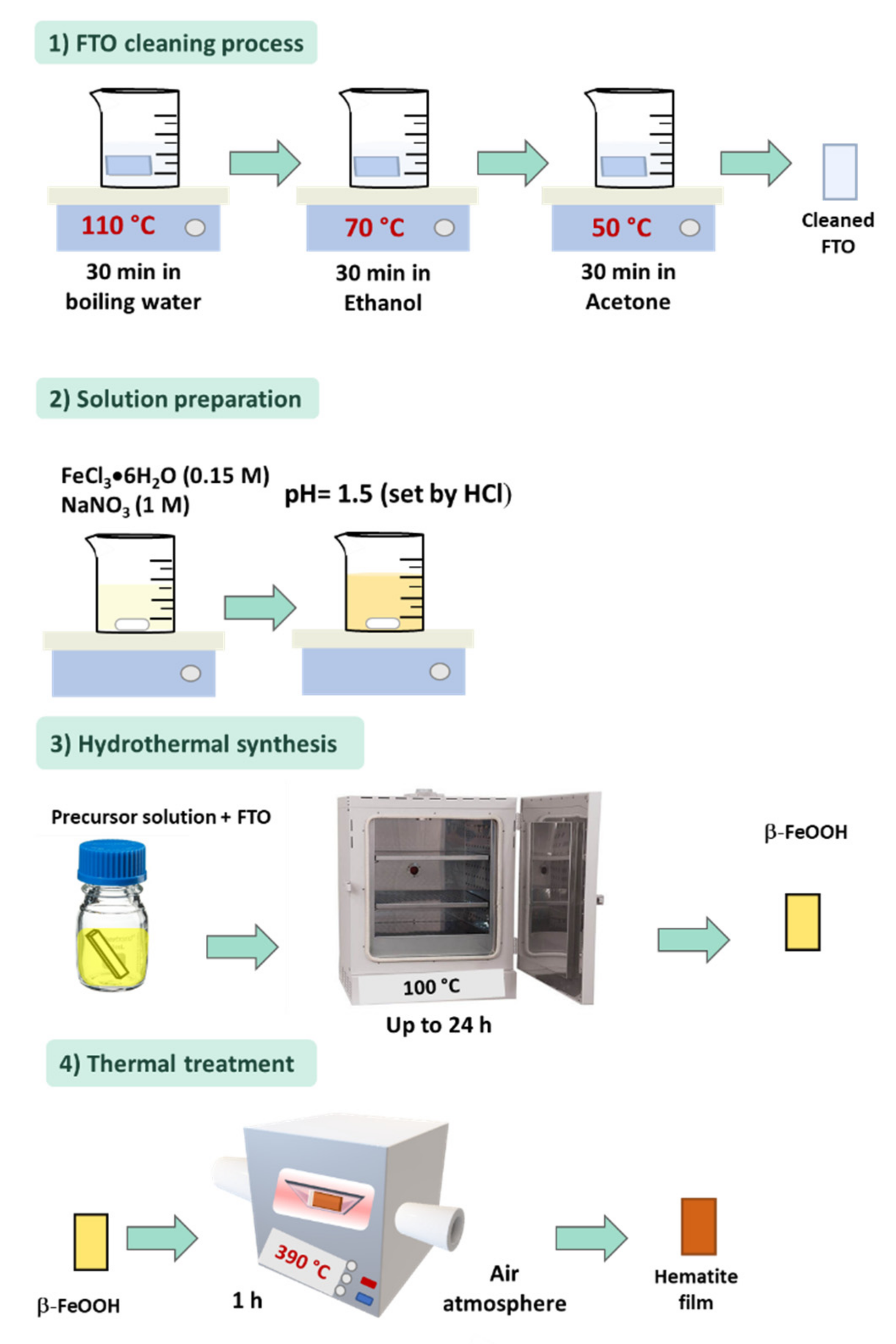
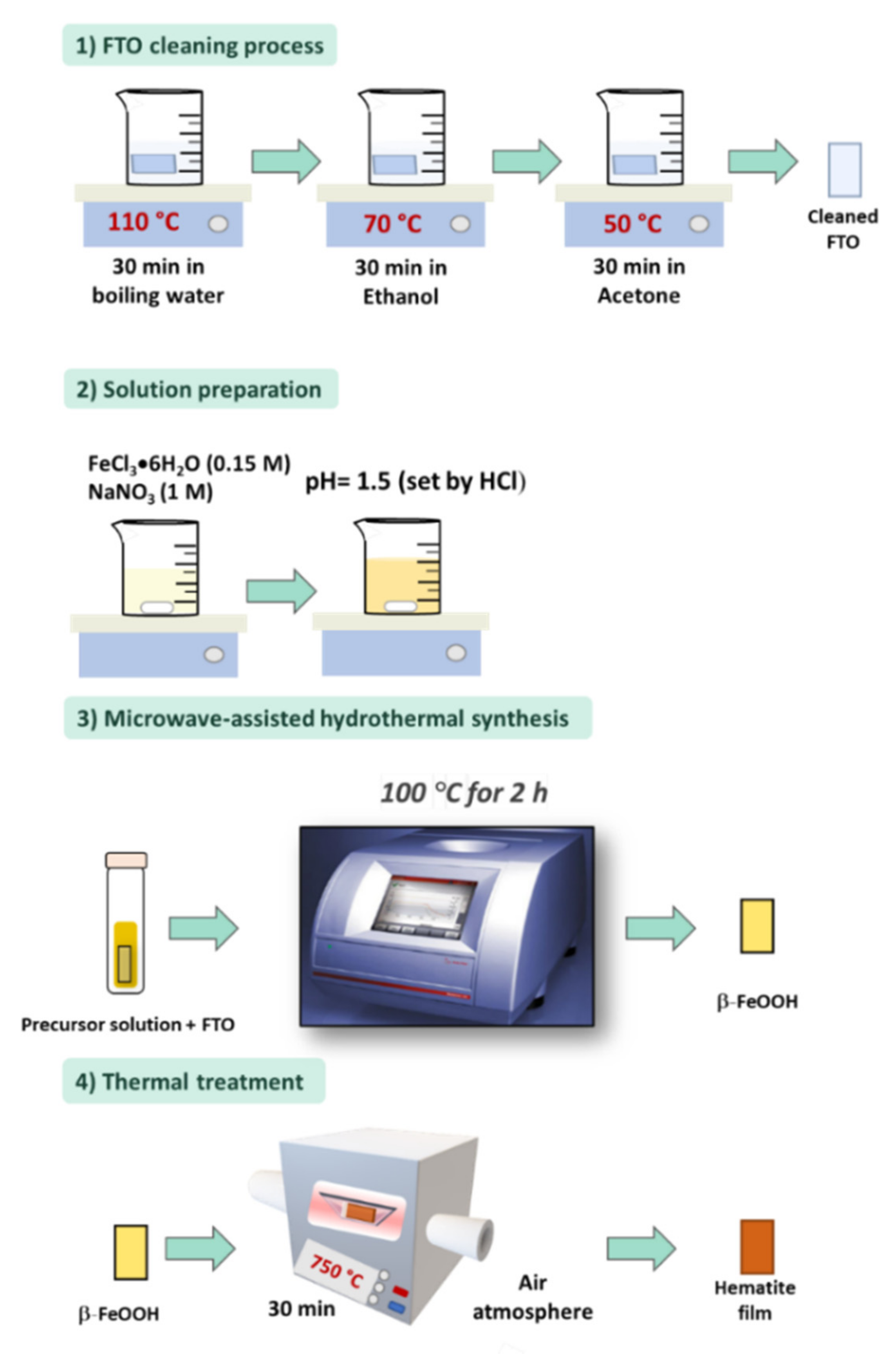
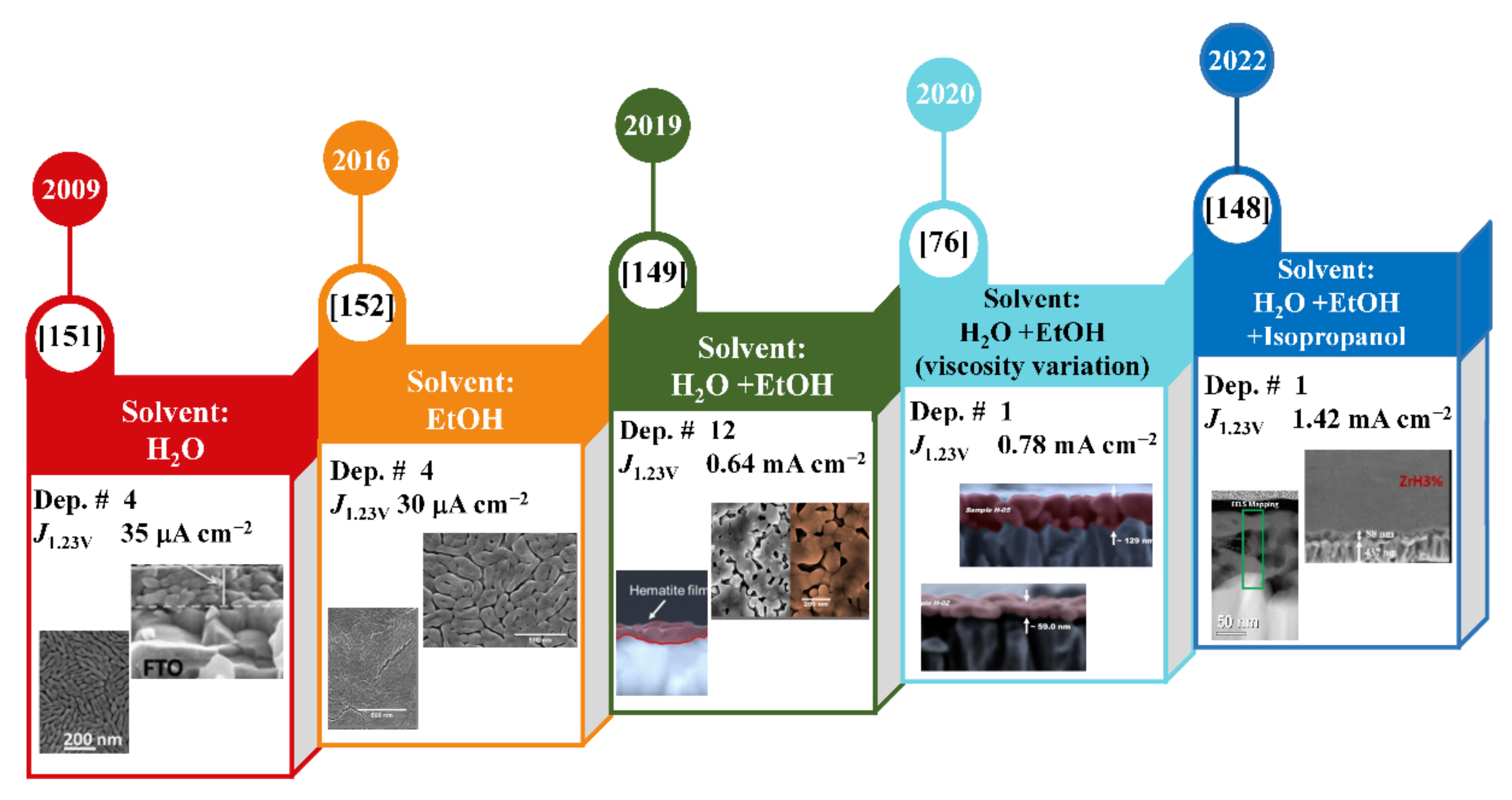
2. Hydrothermal Synthesis
3. Polymeric-Precursor Solution-Based Method
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Framework Convention on Climate Change the Paris Agreement|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 19 January 2022).
- COP26 The Glasgow Climate Pact. Available online: https://ukcop26.org/wp-content/uploads/2021/11/COP26-Presidency-Outcomes-The-Climate-Pact.pdf (accessed on 21 January 2022).
- Kerstine, A.; Julian, W. COP26—Glasgow Climate Pact Decided in Overtime with Weakened Coal Pledge. Available online: https://www.cleanenergywire.org/news/cop26-glasgow-climate-pact-decided-overtime-weakened-coal-pledge (accessed on 20 January 2022).
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Sundaram, M.M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the nanoparticle interfacial properties and stability of the core–shell structure in Zn-doped NiMoO4 @AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Lai, S.Y.; Cheng, C.K.; Cheng, Y.W.; Chong, C.C. Photocatalytic water splitting for solving energy crisis: Myth, fact or busted? Chem. Eng. J. 2021, 417, 128847. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Von Zuben, T.; Moreira, D.; Germscheidt, R.; Yoshimura, R.; Dorretto, D.; de Araujo, A.; Salles, A., Jr.; Bonacin, J. Is hydrogen indispensable for a sustainable world? A review of H2 applications and perspectives for the next years. J. Braz. Chem. Soc. 2022, 1–20. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2021. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 20 April 2022).
- Gondal, I.A.; Masood, S.A.; Khan, R. Green hydrogen production potential for developing a hydrogen economy in Pakistan. Int. J. Hydrogen Energy 2018, 43, 6011–6039. [Google Scholar] [CrossRef]
- Skjanes, K. H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. Int. J. Hydrogen Energy 2008, 33, 511–521. [Google Scholar] [CrossRef]
- Miller, E.; Randolph, K.; Peterson, D. The HydroGEN Consortium: Foundational early stage water-splitting research supporting diversification of the domestic hydrogen supply chain. Curr. Opin. Electrochem. 2018, 12, 196–201. [Google Scholar] [CrossRef]
- Dinh, H.N.; Weber, A.; Mcdaniel, A.; Boardman, R. HydroGEN: A Consortium on Advanced Water Splitting Materials; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2017; pp. 1–14. [Google Scholar]
- Ziani, A.; Al-Shankiti, I.; Khan, M.A.; Idriss, H. Integrated photo-electrocatalytic (PEC) systems for water splitting to hydrogen and oxygen under concentrated sunlight: Effect of internal parameters on performance. Energy Fuels 2020, 34, 13179–13185. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Thongthep, P.; Moonmangmee, S.; Ponchio, C. Solar/photoelectrocatalytic cell development for H2 production and simultaneous organic dye degradation. Mater. Sci. Semicond. Process. 2021, 124, 105597. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting: Standards, Experimental Methods, and Protocols, 1st ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-8297-0. [Google Scholar]
- Bosserez, T.; Rongé, J.; van Humbeeck, J.; Haussener, S.; Martens, J. Design of compact photoelectrochemical cells for water splitting. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2015, 70, 877–889. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [Green Version]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.S. Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells. J. Mater. Chem. A 2015, 3, 10632–10659. [Google Scholar] [CrossRef]
- Kou, S.; Yu, Q.; Meng, L.; Zhang, F.; Li, G.; Yi, Z. Photocatalytic activity and photocorrosion of oriented BiVO4 single crystal thin films. Catal. Sci. Technol. 2020, 10, 5091–5099. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Pei, L.; Yuan, Y.; Zhong, J.; Li, T.; Yang, T.; Yan, S.; Ji, Z.; Zou, Z. Ta3N5 nanorods encapsulated into 3D hydrangea-like MoS2 for enhanced photocatalytic hydrogen evolution under visible light irradiation. Dalt. Trans. 2019, 48, 13176–13183. [Google Scholar] [CrossRef]
- He, Y.; Thorne, J.E.; Wu, C.H.; Ma, P.; Du, C.; Dong, Q.; Guo, J.; Wang, D. What limits the performance of Ta3N5 for solar water splitting? Chem 2016, 1, 640–655. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Sun, K.; Sukrittanon, S.; Takabayashi, K.; Kamiya, I.; Lewis, N.S.; Tu, C.W. Enhancement of the performance of GaP solar cells by embedded In(N)P quantum dots. Nano Energy 2015, 15, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, N.C.; Turner-Evans, D.B.; Tamboli, A.C.; Chen, C.T.; Atwater, H.A.; Lewis, N.S. Photoelectrochemical behavior of planar and microwire-array Si|GaP electrodes. Adv. Energy Mater. 2012, 2, 1109–1116. [Google Scholar] [CrossRef]
- Tournet, J.; Lee, Y.; Karuturi, S.K.; Tan, H.H.; Jagadish, C. III–V semiconductor materials for solar hydrogen production: Status and prospects. ACS Energy Lett. 2020, 5, 611–622. [Google Scholar] [CrossRef]
- Siddiqi, G.; Pan, Z.; Hu, S. III–V semiconductor photoelectrodes. In Semiconductors and Semimetals; Elsevier Inc.: Cambridge, MA, USA, 2017; Volume 97, pp. 81–138. [Google Scholar]
- Guan, X.; Chowdhury, F.A.; Pant, N.; Guo, L.; Vayssieres, L.; Mi, Z. Efficient unassisted overall photocatalytic seawater splitting on GaN-based nanowire arrays. J. Phys. Chem. C 2018, 122, 13797–13802. [Google Scholar] [CrossRef]
- Hu, S.; Shaner, M.R.; Beardslee, J.A.; Lichterman, M.; Brunschwig, B.S.; Lewis, N.S. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 2014, 344, 1005–1009. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Young, J.L.; Deutsch, T.G.; Lewis, N.S. Understanding the stability of etched or platinized p-GaInP photocathodes for solar-driven H2 evolution. ACS Appl. Mater. Interfaces 2021, 13, 57350–57361. [Google Scholar] [CrossRef]
- Saxena, S.; Verma, A.; Biswas, N.K.; Khan, S.A.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Zr–W Co-doping in BiVO4—Synergistic effect in photoelectrochemical water splitting. Mater. Chem. Phys. 2021, 267, 124675. [Google Scholar] [CrossRef]
- Seabold, J.A.; Zhu, K.; Neale, N.R. Efficient solar photoelectrolysis by nanoporous Mo:BiVO4 through controlled electron transport. Phys. Chem. Chem. Phys. 2014, 16, 1121–1131. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.-K. The effects of W/Mo-co-doped BiVO4 photoanodes for improving photoelectrochemical water splitting performance. Catal. Today 2021, 361, 183–190. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Liu, Q.; Li, H.; Yang, S.; Zhong, J. Photoelectrochemical water oxidation in α-Fe2O3 thin films enhanced by a controllable wet-chemical Ti-doping strategy and Co–Pi co-catalyst modification. J. Mater. Sci. Mater. Electron. 2019, 30, 21444–21453. [Google Scholar] [CrossRef]
- Klahr, B.; Hamann, T. Water oxidation on hematite photoelectrodes: Insight into the nature of surface states through in situ spectroelectrochemistry. J. Phys. Chem. C 2014, 118, 10393–10399. [Google Scholar] [CrossRef]
- Le Formal, F.; Sivula, K.; Grätzel, M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C 2012, 116, 26707–26720. [Google Scholar] [CrossRef]
- Barroso, M.; Mesa, C.A.; Pendlebury, S.R.; Cowan, A.J.; Hisatomi, T.; Sivula, K.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl. Acad. Sci. USA 2012, 109, 15640–15645. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, H.; Triana, C.A.; Patzke, G.R. Hematite photoanodes for water oxidation: Electronic transitions, carrier dynamics, and surface energetics. Angew. Chem. Int. Ed. 2021, 60, 18380–18396. [Google Scholar] [CrossRef]
- Grave, D.A.; Ellis, D.S.; Piekner, Y.; Kölbach, M.; Dotan, H.; Kay, A.; Schnell, P.; van de Krol, R.; Abdi, F.F.; Friedrich, D.; et al. Extraction of mobile charge carrier photogeneration yield spectrum of ultrathin-film metal oxide photoanodes for solar water splitting. Nat. Mater. 2021, 20, 833–840. [Google Scholar] [CrossRef]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentová, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zbořil, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Ferraz, L.C.C.; Carvalho, W.M.; Criado, D.; Souza, F.L. Vertically oriented iron oxide films produced by hydrothermal process: Effect of thermal treatment on the physical chemical properties. ACS Appl. Mater. Interfaces 2012, 4, 5515–5523. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Gu, J.; Liu, Y.; Zhang, X. Promoting photoelectrochemical water oxidation on Ti-doped Fe2O3 nanowires photoanode by O2 plasma treatment. Catalysts 2021, 11, 82. [Google Scholar] [CrossRef]
- Guijarro, N.; Prévot, M.S.; Sivula, K. Surface modification of semiconductor photoelectrodes. Phys. Chem. Chem. Phys. 2015, 17, 15655–15674. [Google Scholar] [CrossRef] [PubMed]
- Bedin, K.C.; Muche, D.N.F.; Melo, M.A.; Freitas, A.L.M.; Gonçalves, R.V.; Souza, F.L. Role of cocatalysts on hematite photoanodes in photoelectrocatalytic water splitting: Challenges and future perspectives. ChemCatChem 2020, 12, 3156–3169. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Y. Review of Sn-doped hematite nanostructures for photoelectrochemical water splitting. Part. Part. Syst. Charact. 2014, 31, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-promoted solar water oxidation on hematite photoanodes. Molecules 2016, 21, 868. [Google Scholar] [CrossRef] [Green Version]
- Junior, J.B.S.; Souza, F.L.; Vayssieres, L.; Varghese, O.K. On the relevance of understanding and controlling the locations of dopants in hematite photoanodes for low-cost water splitting. Appl. Phys. Lett. 2021, 119, 200501. [Google Scholar] [CrossRef]
- Malviya, K.D.; Klotz, D.; Dotan, H.; Shlenkevich, D.; Tsyganok, A.; Mor, H.; Rothschild, A. Influence of Ti doping levels on the photoelectrochemical properties of thin-film hematite (α-Fe2O3) photoanodes. J. Phys. Chem. C 2017, 121, 4206–4213. [Google Scholar] [CrossRef]
- Scherrer, B.; Li, T.; Tsyganok, A.; Döbeli, M.; Gupta, B.; Malviya, K.D.; Kasian, O.; Maman, N.; Gault, B.; Grave, D.A.; et al. Defect segregation and its effect on the photoelectrochemical properties of Ti-doped hematite photoanodes for solar water splitting. Chem. Mater. 2020, 32, 1031–1040. [Google Scholar] [CrossRef]
- Uribe, J.D.; Osorio, J.; Barrero, C.A.; Giratá, D.; Morales, A.L.; Devia, A.; Gómez, M.E.; Ramirez, J.G.; Gancedo, J.R. Hematite thin films: Growth and characterization. Hyperfine Interact. 2007, 169, 1355–1362. [Google Scholar] [CrossRef]
- Orlandi, M.; Mazzi, A.; Arban, G.; Bazzanella, N.; Rudatis, P.; Caramori, S.; Patel, N.; Fernandes, R.; Bignozzi, C.A.; Miotello, A. On the effect of Sn-doping in hematite anodes for oxygen evolution. Electrochim. Acta 2016, 214, 345–353. [Google Scholar] [CrossRef]
- Yan, D.; Tao, J.; Kisslinger, K.; Cen, J.; Wu, Q.; Orlov, A.; Liu, M. The role of the domain size and titanium dopant in nanocrystalline hematite thin films for water photolysis. Nanoscale 2015, 7, 18515–18523. [Google Scholar] [CrossRef] [PubMed]
- Warwick, M.E.A.; Kaunisto, K.; Barreca, D.; Carraro, G.; Gasparotto, A.; Maccato, C.; Bontempi, E.; Sada, C.; Ruoko, T.-P.; Turner, S.; et al. Vapor phase processing of α-Fe2O3 photoelectrodes for water splitting: An insight into the structure/property interplay. ACS Appl. Mater. Interfaces 2015, 7, 8667–8676. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, M.; Ruoko, T.-P.; Leduc, J.; Gönüllü, Y.; Deo, M.; Tkachenko, N.V.; Mathur, S. Critical role and modification of surface states in hematite films for enhancing oxygen evolution activity. J. Mater. Res. 2018, 33, 455–466. [Google Scholar] [CrossRef]
- Shadabipour, P.; Hamann, T.W. Interface passivation to overcome shunting in semiconductor–catalyst junctions. Chem. Commun. 2020, 56, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chu, H.; Xiang, X.; Luo, R.; He, J.; Chen, A. Fabricating of Fe2O3/BiVO4 heterojunction based photoanode modified with NiFe-LDH nanosheets for efficient solar water splitting. Chem. Eng. J. 2018, 350, 148–156. [Google Scholar] [CrossRef]
- Cai, J.; Li, S.; Li, Z.; Wang, J.; Ren, Y.; Qin, G. Electrodeposition of Sn-doped hollow α-Fe2O3 nanostructures for photoelectrochemical water splitting. J. Alloys Compd. 2013, 574, 421–426. [Google Scholar] [CrossRef]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.-S. Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Cao, R. Facile fabrication of pure α-Fe2O3 nanoparticles via forced hydrolysis using microwave-assisted esterification and their sensing property. J. Am. Ceram. Soc. 2009, 92, 2188–2191. [Google Scholar] [CrossRef]
- Zhang, Z.; Nagashima, H.; Tachikawa, T. Ultra-narrow depletion layers in a hematite mesocrystal-based photoanode for boosting multihole water oxidation. Angew. Chem. Int. Ed. 2020, 59, 9047–9054. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimata, I.; Nagashima, H.; Muto, S.; Ohara, K.; Sugimoto, K.; Tachikawa, T. Interfacial oxygen vacancies yielding long-lived holes in hematite mesocrystal-based photoanodes. Nat. Commun. 2019, 10, 4832. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.-M.; Lin, L.-Y. Design of efficient Mn-doped α-Fe2O3/Ti-doped α-Fe2O3 homojunction for catalyzing photoelectrochemical water splitting. Int. J. Hydrogen Energy 2020, 45, 6487–6499. [Google Scholar] [CrossRef]
- Freitas, A.L.M.; Souza, F.L. Synergetic effect of Sn addition and oxygen-deficient atmosphere to fabricate active hematite photoelectrodes for light-induced water splitting. Nanotechnology 2017, 28, 454002. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, A.; Shinde, P.S.; Jeon, T.H.; Lee, H.H.; Kim, H.G.; Choi, W.; Jang, J.S. Fabrication of superior α-Fe2O3 nanorod photoanodes through ex-situ Sn-doping for solar water splitting. Sol. Energy Mater. Sol. Cells 2016, 144, 247–255. [Google Scholar] [CrossRef]
- Vayssieres, L.; Beermann, N.; Lindquist, S.-E.; Hagfeldt, A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: Application to iron(III) oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar] [CrossRef]
- Jang, J.-W.; Du, C.; Ye, Y.; Lin, Y.; Yao, X.; Thorne, J.; Liu, E.; McMahon, G.; Zhu, J.; Javey, A.; et al. Enabling unassisted solar water splitting by iron oxide and silicon. Nat. Commun. 2015, 6, 7447. [Google Scholar] [CrossRef]
- Li, Y.; Guijarro, N.; Zhang, X.; Prévot, M.S.; Jeanbourquin, X.A.; Sivula, K.; Chen, H.; Li, Y. Templating sol–gel hematite films with sacrificial copper oxide: Enhancing photoanode performance with nanostructure and oxygen vacancies. ACS Appl. Mater. Interfaces 2015, 7, 16999–17007. [Google Scholar] [CrossRef]
- Ianasi, C.; Costisor, O.; Putz, A.-M.; Lazau, R.; Negrea, A.; Niznansky, D.; Sacarescu, L.; Savii, C. Low temperature superparamagnetic nanocomposites obtained by Fe(acac)3-SiO2-PVA hybrid xerogel thermolysis. Process. Appl. Ceram. 2016, 10, 265–275. [Google Scholar] [CrossRef]
- Hamd, W.; Cobo, S.; Fize, J.; Baldinozzi, G.; Schwartz, W.; Reymermier, M.; Pereira, A.; Fontecave, M.; Artero, V.; Laberty-Robert, C.; et al. Mesoporous α-Fe2O3 thin films synthesized via the sol–gel process for light-driven water oxidation. Phys. Chem. Chem. Phys. 2012, 14, 13224. [Google Scholar] [CrossRef]
- Muche, D.N.F.; Carminati, S.A.; Nogueira, A.F.; Souza, F.L. Engineering interfacial modification on nanocrystalline hematite photoanodes: A close look into the efficiency parameters. Sol. Energy Mater. Sol. Cells 2020, 208, 110377. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable hydrothermal and solvothermal synthesis of advanced carbon materials in multidimensional applications: A review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Modan, E.M.; Plaiasu, A.G. Advantages and disadvantages of chemical methods in the elaboration of nanomaterials. Ann. Dunarea Jos Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Guo, L. High aspect ratio TiO2 nanowires tailored in concentrated HCl hydrothermal condition for photoelectrochemical water splitting. RSC Adv. 2015, 5, 53012–53018. [Google Scholar] [CrossRef]
- Kong, H.; Kim, H.; Hwang, S.; Mun, J.; Yeo, J. Laser-induced hydrothermal growth of iron oxide nanoparticles on diverse substrates for flexible micro-supercapacitors. ACS Appl. Nano Mater. 2022, 5, 4102–4111. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Cheng, C.; Zong, S.; Geng, J.; Guan, X.; Guo, L. Hydrothermal growth of Co3(OH)2(HPO4)2 nano-needles on LaTiO2N for enhanced water oxidation under visible-light irradiation. Appl. Catal. B Environ. 2018, 232, 268–274. [Google Scholar] [CrossRef]
- Vayssieres, L. On the aqueous stabilisation of crystalline metastable nanostructures. Int. J. Nanotechnol. 2007, 4, 750. [Google Scholar] [CrossRef]
- Jianlin, L.; Qingliu, W.; Ji, W. Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN ISBN 978-3-319-15337-7. [Google Scholar]
- Macera, L.; Daniele, V.; Mondelli, C.; Capron, M.; Taglieri, G. New Sustainable, Scalable and One-Step Synthesis of Iron Oxide Nanoparticles by Ion Exchange Process. Nanomaterials 2021, 11, 798. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci. 1989, 130, 157–164. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Vayssieres, L. On aqueous interfacial thermodynamics and the design of metal-oxide nanostructures. In Synthesis, Properties, and Applications of Oxide Nanomaterials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 49–78. ISBN 9780471724056. [Google Scholar]
- Vayssieres, L.; Sathe, C.; Butorin, S.M.; Shuh, D.K.; Nordgren, J.; Guo, J. One-dimensional quantum-confinement effect in α-Fe2O3 ultrafine nanorod arrays. Adv. Mater. 2005, 17, 2320–2323. [Google Scholar] [CrossRef]
- Vayssieres, L.; Graetzel, M. Highly ordered SnO2 nanorod arrays from controlled aqueous growth. Angew. Chem. Int. Ed. 2004, 43, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, L. Metal oxide rods and dots-based structures and devices: Cost-effective fabrication and surface chemistry control. MRS Proc. 2009, 1209, 107. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, M.; Zheng, J.; Rui, Z. Morphology control of the hematite photoanodes for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2020, 45, 31667–31677. [Google Scholar] [CrossRef]
- Bedin, K.C.; Freitas, A.L.M.; Tofanello, A.; Rodríguez-Gutiérrez, I.; Souza, F.L. Revealing the synergy of Sn insertion in hematite for next-generation solar water splitting nanoceramics. Int. J. Ceram. Eng. Sci. 2020, 2, 204–227. [Google Scholar] [CrossRef]
- Wang, J.; Perry, N.H.; Guo, L.; Vayssieres, L.; Tuller, H.L. On the theoretical and experimental control of defect chemistry and electrical and photoelectrochemical properties of hematite nanostructures. ACS Appl. Mater. Interfaces 2019, 11, 2031–2041. [Google Scholar] [CrossRef]
- Li, C.; Luo, Z.; Wang, T.; Gong, J. Surface, bulk, and interface: Rational design of hematite architecture toward efficient photo-electrochemical water splitting. Adv. Mater. 2018, 30, 1707502. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Zegkinoglou, I.; Rogero, C.; Guo, J.-H.; Mao, S.S.; Himpsel, F.J.; Vayssieres, L. On the interfacial electronic structure origin of efficiency enhancement in hematite photoanodes. J. Phys. Chem. C 2012, 116, 22780–22785. [Google Scholar] [CrossRef]
- Kim, S.; Mahadik, M.A.; Anushkkaran, P.; Chae, W.-S.; Choi, S.H.; Jang, J.S. A systematic study of post-activation temperature dependence on photoelectrochemical water splitting of one-step synthesized FeOOH CF photoanodes with erratically loaded ZrO2. Sustain. Energy Fuels 2021, 5, 3414–3427. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Tong, X.; Liu, M. Temperature effect on photoelectrochemical water splitting: A model study based on BiVO4 photoanodes. ACS Appl. Mater. Interfaces 2021, 13, 61227–61236. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.; Peracchi, I.; Gerke, J.L.; Irani, R.; Abdi, F.F.; van de Krol, R. Shining a hot light on emerging photoabsorber materials: The Power of rapid radiative heating in developing oxide thin-film photoelectrodes. ACS Energy Lett. 2022, 7, 514–522. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, I.; Mouriño, B.; Freitas, A.L.M.; Costa, C.A.R.; Pires, E.L.; Gonçalves, R.V.; Vayssieres, L.; Souza, F.L. On the effect of thermal processing on Sn diffusion and efficiency enhancement in hematite/FTO photoanodes. ECS J. Solid State Sci. Technol. 2022, 11, 043001. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Zegkinoglou, I.; Shen, S.-H.; Liao, P.; Cho, I.S.; Zandi, O.; Liu, Y.-S.; Lashgari, K.; Westin, G.; Guo, J.-H.; et al. Titanium incorporation into hematite photoelectrodes: Theoretical considerations and experimental observations. Energy Environ. Sci. 2014, 7, 3100–3121. [Google Scholar] [CrossRef]
- Engel, J.; Tuller, H.L. The electrical conductivity of thin film donor doped hematite: From insulator to semiconductor by defect modulation. Phys. Chem. Chem. Phys. 2014, 16, 11374. [Google Scholar] [CrossRef]
- Ito, N.M.; Carvalho, W.M.; Muche, D.N.F.; Castro, R.H.R.; Dalpian, G.M.; Souza, F.L. High temperature activation of hematite nanorods for sunlight driven water oxidation reaction. Phys. Chem. Chem. Phys. 2017, 19, 25025–25032. [Google Scholar] [CrossRef]
- Freitas, A.L.M.; Carvalho, W.M.; Souza, F.L. Enhanced water oxidation efficiency of hematite thin films by oxygen-deficient atmosphere. J. Mater. Res. 2015, 30, 3595–3604. [Google Scholar] [CrossRef]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Anushkkaran, P.; Hwang, J.B.; Chae, W.-S.; Kumar, M.; Lee, H.-H.; Choi, S.H.; Jang, J.S.; Lee, J.S. Microwave-assisted metal-ion attachment for ex-situ zirconium doping into hematite for enhanced photoelectrochemical water splitting. Renew. Energy 2022, 189, 694–703. [Google Scholar] [CrossRef]
- Wang, C.-T.; Lin, H.-S.; Wang, W.-P. Hydrothermal synthesis of Fe and Nb-doped titania nanobelts and their tunable electronic structure toward photovoltaic application. Mater. Sci. Semicond. Process. 2019, 99, 85–91. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, H.; Yang, Z.; Long, X.; Wang, Z.; Zhu, Z.; Qu, Y.; Yang, S. Origin of the different photoelectrochemical performance of mesoporous BiVO4 photoanodes between the BiVO4 and the FTO side illumination. J. Phys. Chem. C 2015, 119, 23350–23357. [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, I.; Souza Junior, J.B.; Leite, E.R.; Vayssieres, L.; Souza, F.L. An intensity modulated photocurrent spectroscopy study of the role of titanium in thick hematite photoanodes. Appl. Phys. Lett. 2021, 119, 071602. [Google Scholar] [CrossRef]
- Baldovi, H.G. Optimization of α-Fe2O3 nanopillars diameters for photoelectrochemical enhancement of α-Fe2O3-TiO2 heterojunction. Nanomaterials 2021, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, J.; Liu, C.; Feng, B.; Chen, Y.; Guo, L. On the role of metal atom doping in hematite for improved photoelectrochemical properties: A comparison study. RSC Adv. 2016, 6, 101745–101751. [Google Scholar] [CrossRef]
- Kay, A.; Cesar, I.; Grätzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef]
- Song, J.; Kim, T.L.; Lee, J.; Cho, S.Y.; Cha, J.; Jeong, S.Y.; An, H.; Kim, W.S.; Jung, Y.-S.; Park, J.; et al. Domain-engineered BiFeO3 thin-film photoanodes for highly enhanced ferroelectric solar water splitting. Nano Res. 2018, 11, 642–655. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Zhang, T.; Wang, Z.; Guo, P.; Su, J.; Guo, L. Facile synthesis of ultrafine hematite nanowire arrays in mixed water–ethanol–acetic acid solution for enhanced charge transport and separation. ACS Appl. Mater. Interfaces 2018, 10, 12594–12602. [Google Scholar] [CrossRef]
- Jeong, I.K.; Mahadik, M.A.; Hwang, J.B.; Chae, W.-S.; Choi, S.H.; Jang, J.S. Lowering the onset potential of Zr-doped hematite nanocoral photoanodes by Al co-doping and surface modification with electrodeposited Co–Pi. J. Colloid Interface Sci. 2021, 581, 751–763. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.Y.; Tian, Z.F.; Ruan, G.S.; Ye, Y.X.; Liang, C.H.; Shao, G.S. Highly oriented Ge-doped hematite nanosheet arrays for photoelectrochemical water oxidation. Nano Energy 2014, 9, 282–290. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Wheeler, D.A.; Jiang, J.; Lindley, S.A.; Kronawitter, C.X.; Zhang, J.Z.; Guo, L.; Mao, S.S. Physical and photoelectrochemical properties of Zr-doped hematite nanorod arrays. Nanoscale 2013, 5, 9867. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, J.; Guo, L. Controlled aqueous growth of hematite nanoplate arrays directly on transparent conductive substrates and their photoelectrochemical properties. Chem. Asian J. 2016, 11, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dong, C.-L.; Lee, W.-Y.; Chen, J.; Guo, P.; Zhao, L.; Shen, S. Nb-doped hematite nanorods for efficient solar water splitting: Electronic structure evolution versus morphology alteration. ChemNanoMat 2016, 2, 704–711. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, C.-L.; Zhou, Z.; Lee, W.-Y.; Chen, J.; Guo, P.; Zhao, L.; Shen, S. Solution growth of Ta-doped hematite nanorods for efficient photoelectrochemical water splitting: A tradeoff between electronic structure and nanostructure evolution. Phys. Chem. Chem. Phys. 2016, 18, 3846–3853. [Google Scholar] [CrossRef]
- Subramanian, A.; Gracia-Espino, E.; Annamalai, A.; Lee, H.H.; Lee, S.Y.; Choi, S.H.; Jang, J.S. Effect of tetravalent dopants on hematite nanostructure for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 427, 1203–1212. [Google Scholar] [CrossRef]
- Dunn, H.K.; Feckl, J.M.; Müller, A.; Fattakhova-Rohlfing, D.; Morehead, S.G.; Roos, J.; Peter, L.M.; Scheu, C.; Bein, T. Tin doping speeds up hole transfer during light-driven water oxidation at hematite photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 24610–24620. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, A.; Sandström, R.; Gracia-Espino, E.; Boulanger, N.; Boily, J.-F.; Mühlbacher, I.; Shchukarev, A.; Wågberg, T. Influence of Sb5+ as a double donor on hematite (Fe3+) photoanodes for surface-enhanced photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2018, 10, 16467–16473. [Google Scholar] [CrossRef]
- Regue, M.; Ahmet, I.Y.; Bassi, P.S.; Johnson, A.L.; Fiechter, S.; van de Krol, R.; Abdi, F.F.; Eslava, S. Zn-doped Fe2TiO5 pseudobrookite-based photoanodes grown by aerosol-assisted chemical vapor deposition. ACS Appl. Energy Mater. 2020, 3, 12066–12077. [Google Scholar] [CrossRef]
- Shen, S.; Kronawitter, C.X.; Wheeler, D.A.; Guo, P.; Lindley, S.A.; Jiang, J.; Zhang, J.Z.; Guo, L.; Mao, S.S. Physical and photoelectrochemical characterization of Ti-doped hematite photoanodes prepared by solution growth. J. Mater. Chem. A 2013, 1, 14498. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Y.-R.; Ren, F.; Xing, Z.; Chen, J.; Guo, P.; Pong, W.-F.; Dong, C.-L.; Zhao, L.; Shen, S. Surface electronic structure reconfiguration of hematite nanorods for efficient photoanodic water oxidation. Sol. RRL 2020, 4, 1900349. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, J.; Dong, C.-L.; Hu, Y.; Tseng, E.N.; Guo, P.; Guo, L.; Mao, S.S. Surface engineered doping of hematite nanorod arrays for improved photoelectrochemical water splitting. Sci. Rep. 2015, 4, 6627. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Jiang, J.; Guo, P.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Effect of Cr doping on the photoelectrochemical performance of hematite nanorod photoanodes. Nano Energy 2012, 1, 732–741. [Google Scholar] [CrossRef]
- Dalmaschio, C.J.; Mastelaro, V.R.; Nascente, P.; Bettini, J.; Zotin, J.L.; Longo, E.; Leite, E.R. Oxide surface modification: Synthesis and characterization of zirconia-coated alumina. J. Colloid Interface Sci. 2010, 343, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, Y.; Kong, T.; Shang, Y.; Niu, F.; Diao, Z.; Shen, S. Protected hematite nanorod arrays with molecular complex co-catalyst for efficient and stable photoelectrochemical water oxidation. Eur. J. Inorg. Chem. 2019, 2019, 2078–2085. [Google Scholar] [CrossRef]
- Mao, L.; Huang, Y.-C.; Fu, Y.; Dong, C.-L.; Shen, S. Surface sulfurization activating hematite nanorods for efficient photoelectrochemical water splitting. Sci. Bull. 2019, 64, 1262–1271. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Li, M.; Guo, L.; Jiang, J.; Mao, S.S. Surface passivation of undoped hematite nanorod arrays via aqueous solution growth for improved photoelectrochemical water splitting. J. Colloid Interface Sci. 2014, 427, 20–24. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Fu, Y.; Shen, S. Cobalt oxide and carbon modified hematite nanorod arrays for improved photoelectrochemical water splitting. Chin. Chem. Lett. 2017, 28, 2207–2211. [Google Scholar] [CrossRef]
- Jeon, T.H.; Moon, G.; Park, H.; Choi, W. Ultra-efficient and durable photoelectrochemical water oxidation using elaborately designed hematite nanorod arrays. Nano Energy 2017, 39, 211–218. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere 2020, 244, 125474. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Kakihana, M.; Domen, K. The synthesis of photocatalysts using the polymerizable-complex method. MRS Bull. 2000, 25, 27–31. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to form a Capacitor. U.S. Patent 3330697A, 11 July 1967. [Google Scholar]
- Sunde, T.O.L.; Grande, T.; Einarsrud, M.-A. Modified pechini synthesis of oxide powders and thin films. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–30. [Google Scholar]
- Dimesso, L. Pechini processes: An alternate approach of the sol–gel method, preparation, properties, and applications. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.K.; Muto, H.; Kawamura, G.; Lockman, Z.; Matsuda, A. Nanomaterial fabrication through the modification of sol–gel derived coatings. Nanomaterials 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Prévot, M.S.; Jeanbourquin, X.A.; Bourée, W.S.; Abdi, F.; Friedrich, D.; van de Krol, R.; Guijarro, N.; Le Formal, F.; Sivula, K. Evaluating charge carrier transport and surface states in CuFeO2 photocathodes. Chem. Mater. 2017, 29, 4952–4962. [Google Scholar] [CrossRef]
- Fernandes, J.D.; Melo, D.M.; Zinner, L.; Salustiano, C.; Silva, Z.; Martinelli, A.; Cerqueira, M.; Alves Júnior, C.; Longo, E.; Bernardi, M.I. Low-temperature synthesis of single-phase crystalline LaNiO3 perovskite via Pechini method. Mater. Lett. 2002, 53, 122–125. [Google Scholar] [CrossRef]
- Bedin, K.C.; Mouriño, B.; Rodríguez-Gutiérrez, I.; Junior, J.B.S.; dos Santos, G.T.; Bettini, J.; Costa, C.A.R.; Vayssieres, L.; Souza, F.L. Solution chemistry back-contact FTO/hematite interface engineering for efficient photocatalytic water oxidation. Chin. J. Catal. 2022, 43, 1247–1257. [Google Scholar] [CrossRef]
- Muche, D.N.F.; dos Santos, T.M.G.; Leite, G.P.; Melo, M.A.; Gonçalves, R.V.; Souza, F.L. Tailoring hematite/FTO interfaces: New horizons for spin-coated hematite photoanodes targeting water splitting. Mater. Lett. 2019, 254, 218–221. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Longo, E.; Leite, E.R. The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 2009, 11, 1215. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Nascente, P.A.P.; Leite, E.R. Nanostructured hematite thin films produced by spin-coating deposition solution: Application in water splitting. Sol. Energy Mater. Sol. Cells 2009, 93, 362–368. [Google Scholar] [CrossRef]
- Bellido-Aguilar, D.A.; Tofanello, A.; Souza, F.L.; Furini, L.N.; Constantino, C.J.L. Effect of thermal treatment on solid–solid interface of hematite thin film synthesized by spin-coating deposition solution. Thin Solid Film. 2016, 604, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Kakihana, M. Invited review “sol-gel” preparation of high temperature superconducting oxides. J. Sol-Gel Sci. Technol. 1996, 6, 7–55. [Google Scholar] [CrossRef]
- Selim, S.; Francàs, L.; García-Tecedor, M.; Corby, S.; Blackman, C.; Gimenez, S.; Durrant, J.R.; Kafizas, A. WO3/BiVO4: Impact of charge separation at the timescale of water oxidation. Chem. Sci. 2019, 10, 2643–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Yoon, K.-Y.; Kim, T.; Jang, H.; Kwak, M.-J.; Kim, J.Y.; Jang, J.-H. A highly transparent thin film hematite with multi-element dopability for an efficient unassisted water splitting system. Nano Energy 2020, 76, 105089. [Google Scholar] [CrossRef]
- Soares, M.R.S.S.; Costa, C.A.R.R.; Lanzoni, E.M.; Bettini, J.; Ramirez, C.A.O.O.; Souza, F.L.; Longo, E.; Leite, E.R. Unraveling the role of Sn segregation in the electronic transport of polycrystalline hematite: Raising the electronic conductivity by lowering the grain-boundary blocking effect. Adv. Electron. Mater. 2019, 5, 1900065. [Google Scholar] [CrossRef]
- Zandi, O.; Beardslee, J.A.; Hamann, T. Substrate dependent water splitting with ultrathin α-Fe2O3 electrodes. J. Phys. Chem. C 2014, 118, 16494–16503. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, A.; Shinde, P.S.; Subramanian, A.; Kim, J.Y.; Kim, J.H.; Choi, S.H.; Lee, J.S.; Jang, J.S. Bifunctional TiO2 underlayer for α-Fe2O3 nanorod based photoelectrochemical cells: Enhanced interface and Ti4+ doping. J. Mater. Chem. A 2015, 3, 5007–5013. [Google Scholar] [CrossRef]
- Hu, Y.; Boudoire, F.; Mayer, M.T.; Yoon, S.; Graetzel, M.; Braun, A. Function and electronic structure of the SnO2 buffer layer between the α-Fe2O3 water oxidation photoelectrode and the transparent conducting oxide current collector. J. Phys. Chem. C 2021, 125, 9158–9168. [Google Scholar] [CrossRef]
- Bedin, K.C.; Rodríguez-Gutiérrez, I.; Peregrino, L.R.P.; Vayssieres, L.; Souza, F.L. On electron loss lowering at hematite photoelectrode interfaces. J. Am. Ceram. Soc. 2022; accepted manuscript. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Gutiérrez, I.; Bedin, K.C.; Mouriño, B.; Souza Junior, J.B.; Souza, F.L. Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production. Nanomaterials 2022, 12, 1957. https://doi.org/10.3390/nano12121957
Rodríguez-Gutiérrez I, Bedin KC, Mouriño B, Souza Junior JB, Souza FL. Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production. Nanomaterials. 2022; 12(12):1957. https://doi.org/10.3390/nano12121957
Chicago/Turabian StyleRodríguez-Gutiérrez, Ingrid, Karen Cristina Bedin, Beatriz Mouriño, João Batista Souza Junior, and Flavio Leandro Souza. 2022. "Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production" Nanomaterials 12, no. 12: 1957. https://doi.org/10.3390/nano12121957
APA StyleRodríguez-Gutiérrez, I., Bedin, K. C., Mouriño, B., Souza Junior, J. B., & Souza, F. L. (2022). Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production. Nanomaterials, 12(12), 1957. https://doi.org/10.3390/nano12121957





