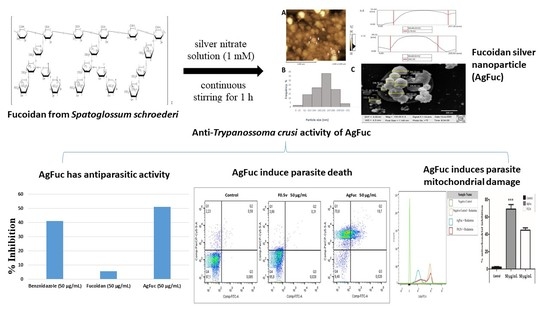
Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Seaweed Collection
2.3. Extraction of Fucoidans
2.4. Agarose Gel Electrophoresis
2.5. Chemical Analysis and Monosaccharide Composition
2.6. Nanoparticle Synthesis
2.7. Characterization of the Nanoparticles
2.7.1. UV-Visible Spectroscopy
2.7.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.7.3. Atomic Force Microscopy (AFM), X-Ray Diffraction (XRD), Dynamic Light Scattering (DLS), and Scanning Electron Microscopy (SEM) Analysis
2.7.4. Quantitative Determination of Silver in AgFuc Nanoparticles by ICP-OES
Digestion of AgFuc Nanoparticles
Determination of the Silver Content of the Digested AgFuc Nanoparticles
2.8. Evaluation of the Antiparasitic Activity of AgFuc Nanoparticles
2.9. Flow Cytometry
2.10. Analysis of Mitochondrial Damage in Parasites Treated with AgFuc Nanoparticles or F0.5v
2.11. Statistical Analysis
3. Results and Discussion
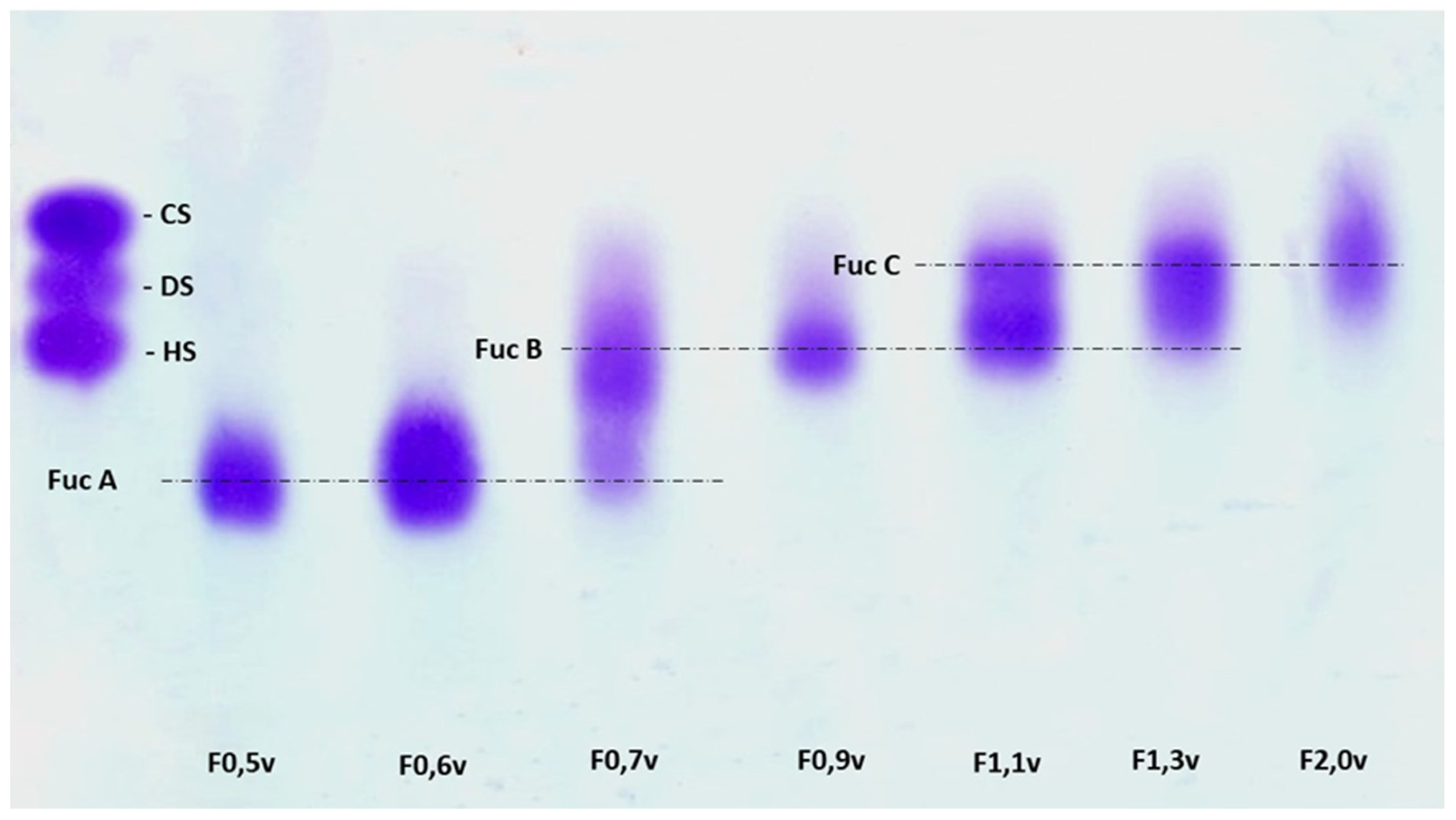
3.1. Obtaining Fucan A
3.2. Chemical Composition of Samples
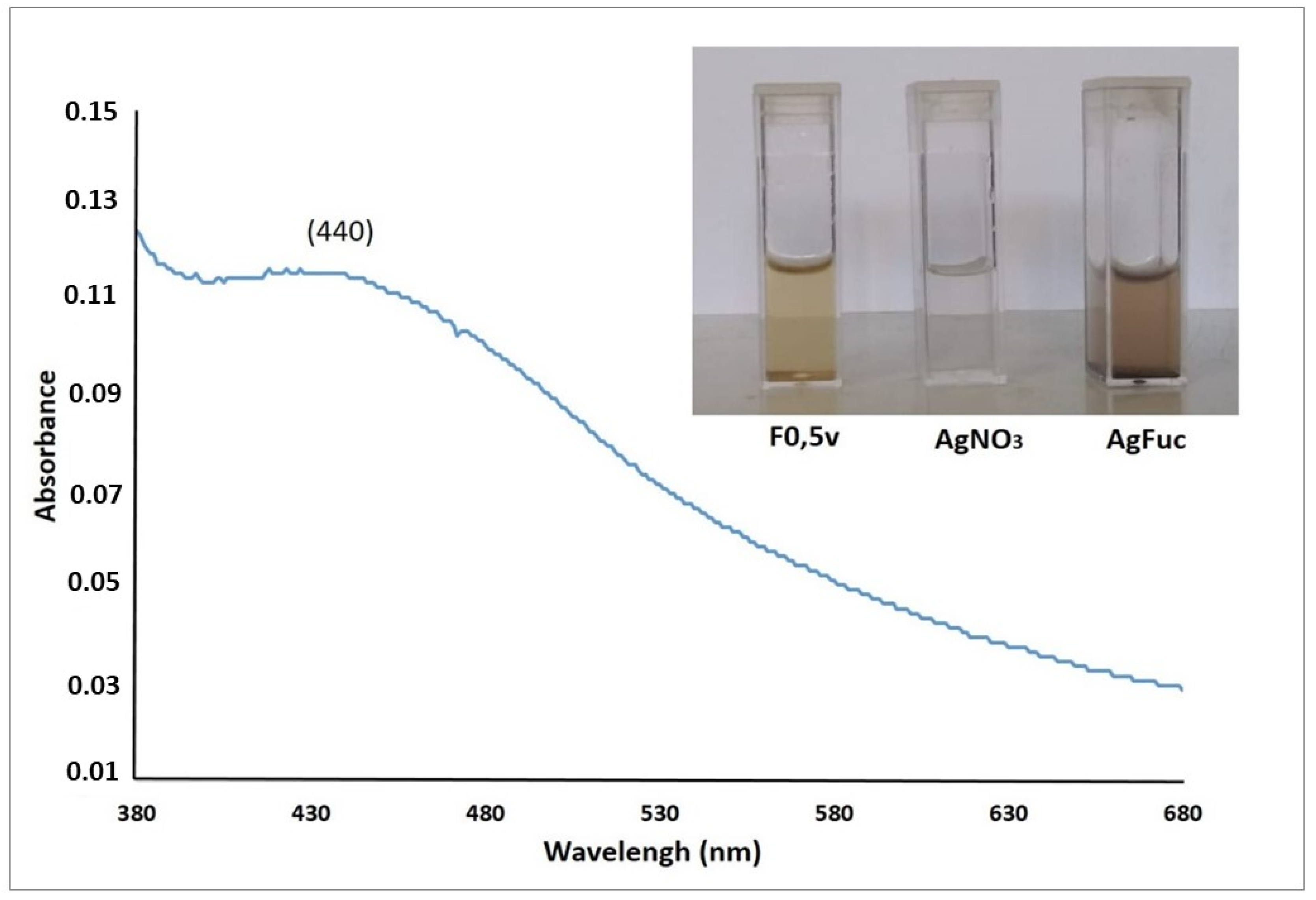
3.3. Synthesis of Silver Nanoparticle Containing Fucan A (AgFuc)
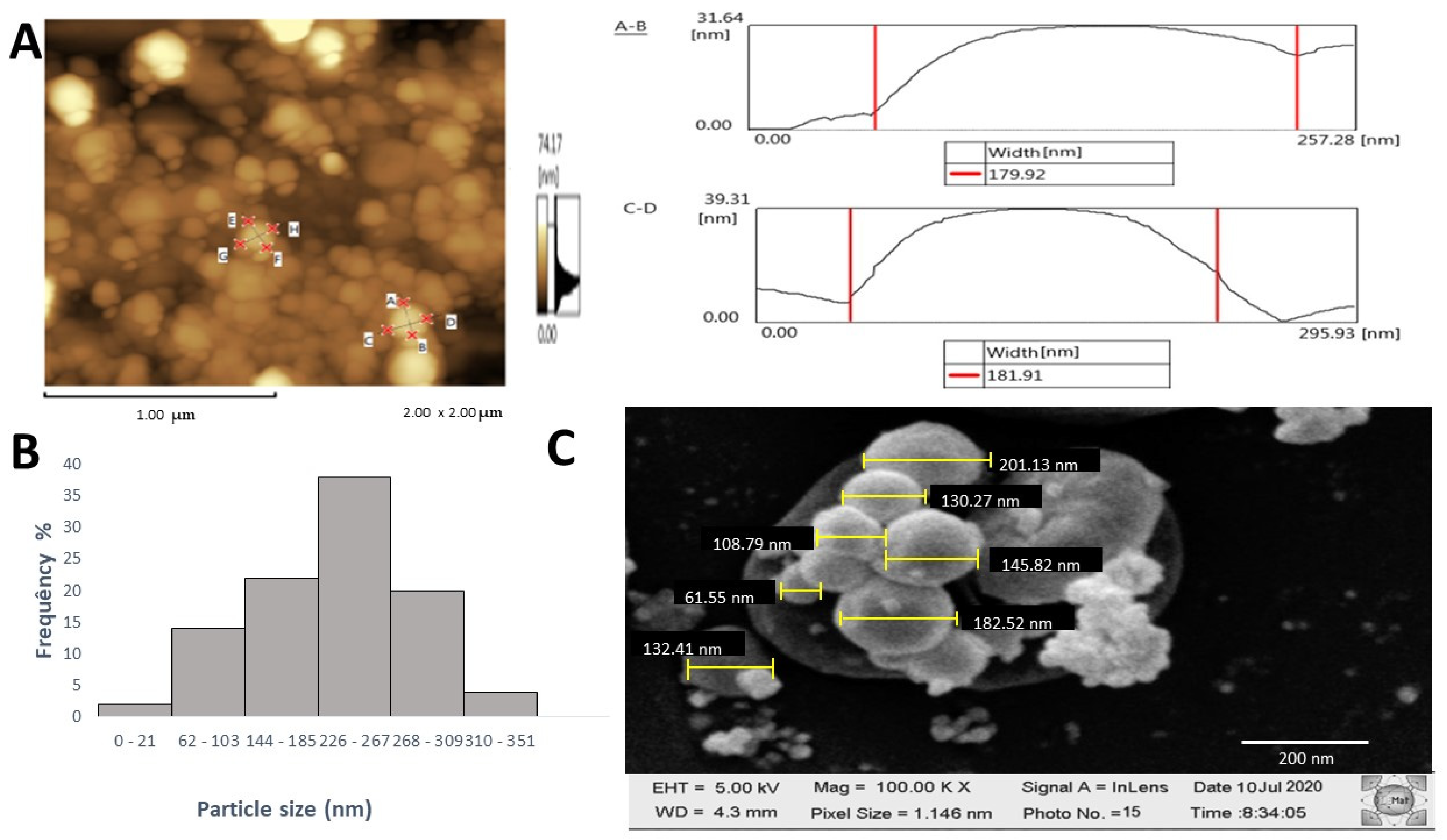
3.4. Characterization of AgFuc
3.5. Evaluation of the Antiparasitic Activity of AgFuc
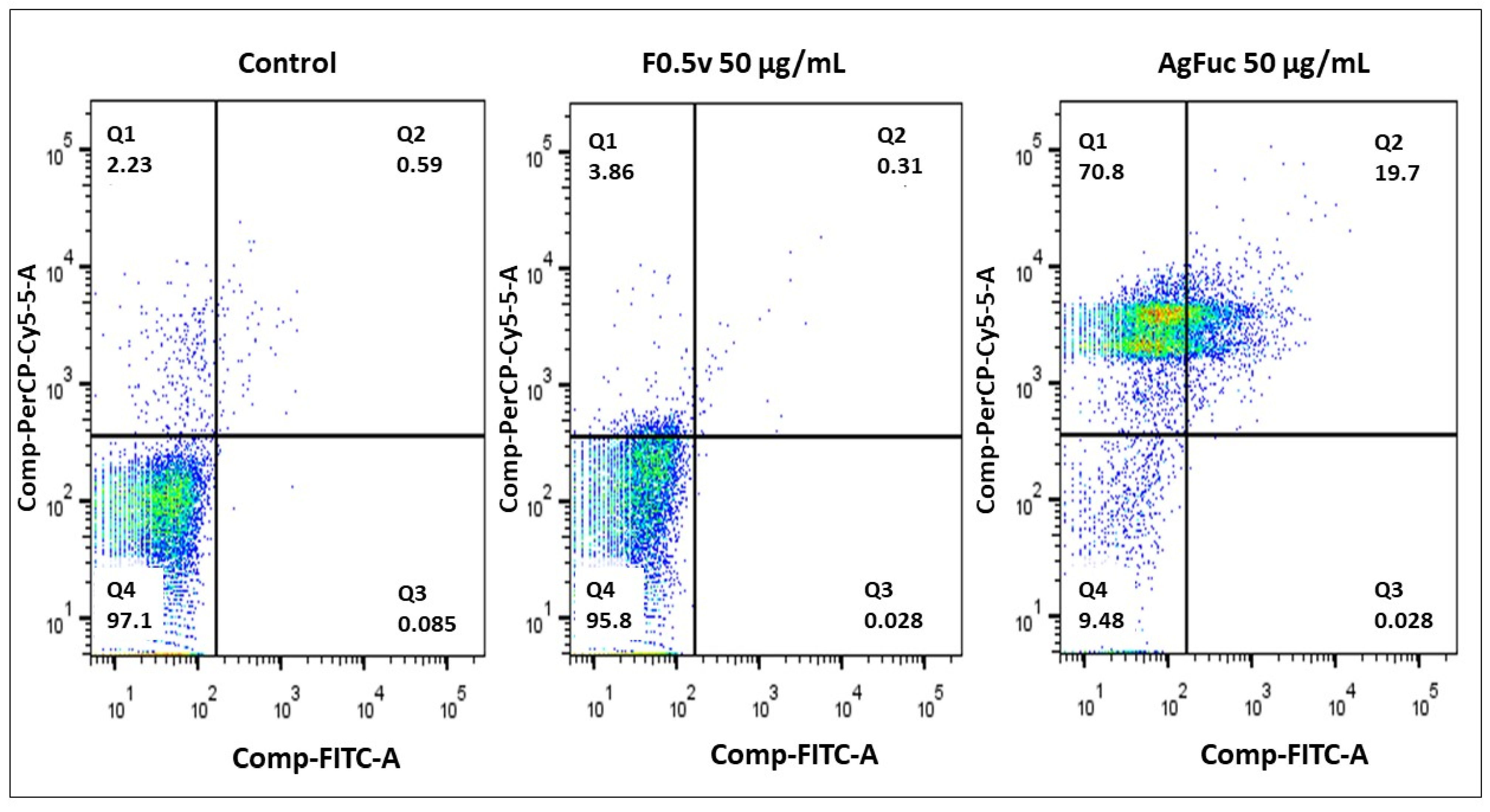
3.6. Evaluation of the Cell Death Process via Annexin V-FITC and Propidium Iodide (PI) Labeling
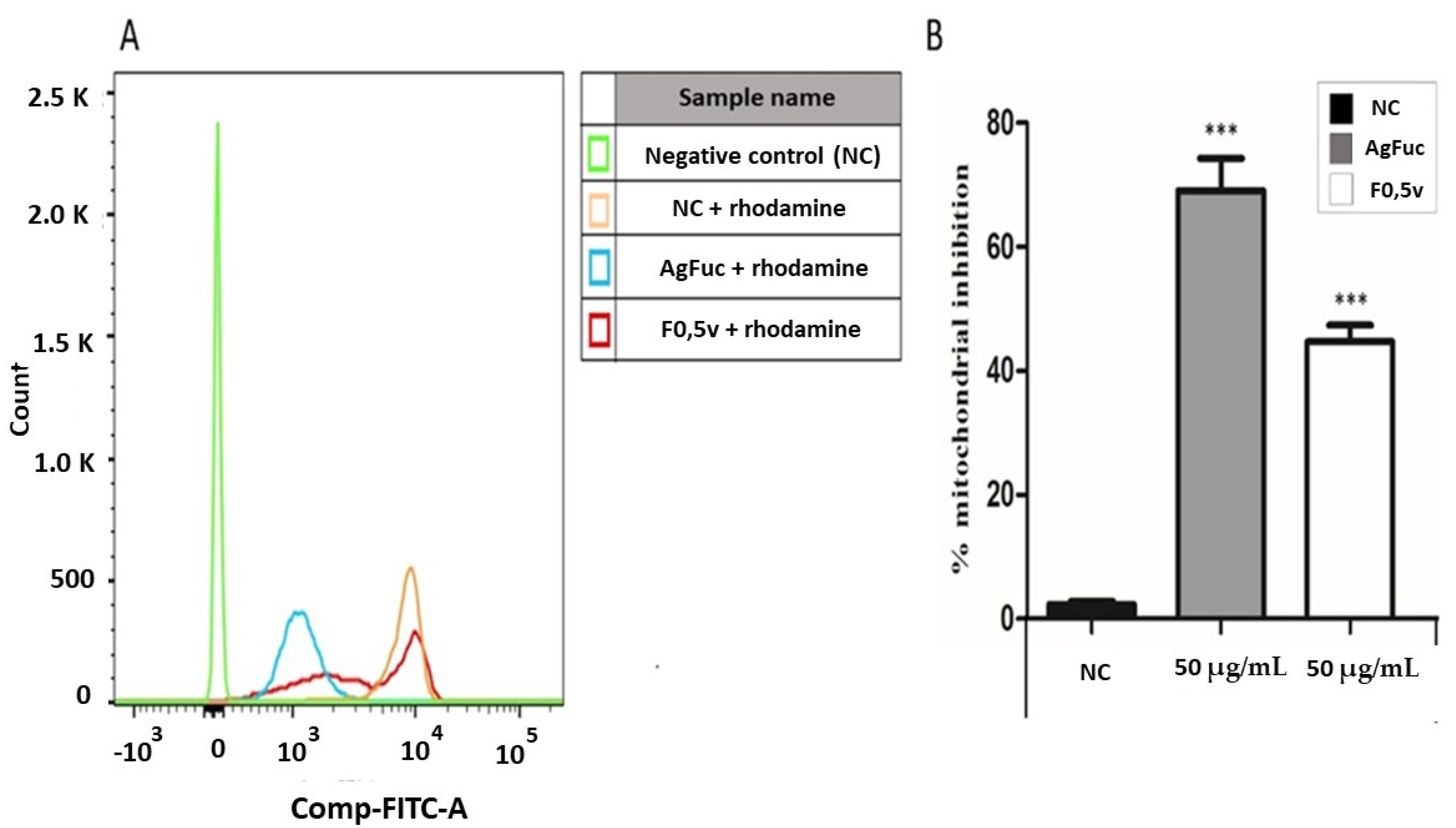
3.7. Analysis of the Damage in the Parasites’ Mitochondria after Treatment with F0.5v or AgFuc
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Souza, W.; Barrias, E.S. May the epimastigote form of Trypanosoma cruzi be infective? Acta Trop. 2020, 212, 105688. [Google Scholar] [CrossRef]
- Busselman, R.E.; Hamer, S.A. Chagas Disease Ecology in the United States: Recent Advances in Understanding Trypanosoma cruzi Transmission Among Triatomines, Wildlife, and Domestic Animals and a Quantitative Synthesis of Vector-Host Interactions. Annu. Rev. Anim. Biosci. 2022, 10, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.W.F.; Rocha, H.A.O.; de Medeiros, W.M.T.Q.; Silva, M.S. Application of dithiocarbamates as potential new antitrypanosomatids-drugs: Approach chemistry, functional and biological. Molecules 2019, 24, 2806. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 14 April 2022).
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Waseem, M. Chagas Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK459272/ (accessed on 17 April 2022).
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina; José, A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Zuma, A.A.; de Souza, W. Chagas Disease Chemotherapy: What Do We Know So Far? Curr. Pharm. Des. 2021, 27, 3963–3995. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.S.; da Rosa, R.; Zimmermann, L.A.; Rogério, K.R.; Kümmerle, A.E.; Bernardes, L.S.C.; Graebin, C.S. Antiprotozoal agents: How have they changed over a decade? Arch. Pharm. 2022, 355, e2100338. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Dasgupta, A.; Chopra, S. Update on nifurtimox for treatment of Chagas disease. Drugs Today 2021, 57, 251–263. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Avelis, C.M.; Asti, L.; Hertenstein, D.L.; Ndeffo-Mbah, M.; Galvani, A.; Lee, B.Y. The economic value of identifying and treating Chagas disease patients earlier and the impact on Trypanosoma cruzi transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006809. [Google Scholar] [CrossRef]
- Manlusoc, J.K.T.; Hsieh, C.L.; Hsieh, C.Y.; Salac, E.S.N.; Lee, Y.T.; Tsai, P.W. Pharmacologic Application Potentials of Sulfated Polysaccharide from Marine Algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Leal, D.; Mansilla, A.; Matsuhiro, B.; Moncada-Basualto, M.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; De Borggraeve, W.M. Chemical structure and biological properties of sulfated fucan from the sequential extraction of subAntarctic Lessonia sp. (Phaeophyceae). Carbohydr. Polym. 2018, 199, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.O.R.; Gomes, D.L.; Dantas, L.A.; Viana, R.L.S.; Chiquetti, S.C.; Almeida-Lima, J.; Costa, L.S.; Rocha, H.A.O. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int. J. Biol. Macromol. 2016, 93, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Negreiros, M.M.F.; Machado, R.I.A.; Bezerra, F.L.; Melo, M.C.N.; Alves, M.G.C.F.; Filgueira, L.G.A.; Morgano, M.A.; Trindade, E.S.; Costa, L.S.; Rocha, H.A.O. Antibacterial, antiproliferative, and immunomodulatory activity of silver nanoparticles synthesized with fucans from the alga Dictyota mertensii. Nanomaterials 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, N.S.; Araújo, N.K.; Daniele-Silva, A.; Oliveira, J.W.F.; Medeiros, J.M.; Araújo, R.M.; Ferreira, L.S.; Rocha, H.A.O.; Silva-Júnior, A.A.; Silva, M.S.; et al. Antimicrobial activity of Chitosan oligosaccharides with special attention to antiparasitic potential. Mar. Drugs 2021, 20, 110. [Google Scholar] [CrossRef]
- Wynne, M.J. A checklist of benthic marine algae of the tropical and subtropical western Atlantic. Can. J. Bot. 1986, 64, 2239–2281. [Google Scholar] [CrossRef]
- Rocha, H.A.O.; Moraes, F.A.; Trindade, E.S.; Franco, C.R.C.; Torquato, R.J.S.; Veiga, S.S.; Valente, A.P.; Mourão, P.A.S.; Leite, E.L.; Nader, H.B.; et al. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schröederi. An ideal antithrombotic agent? J. Biol. Chem. 2005, 280, 41278–41288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The protective role of sulfated polysaccharides from green seaweed Udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 20, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 250–256. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Spector, J. Refinement of the coomassie blue method of protein quantification. A simple and linear spectrophotometric assay of 0.5 to 50µg of protein. Anal. Biochem. 1978, 86, 142–143. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Leite, E.L.; Maria, G.L.; Rocha, H.A.O.; Farias, G.G.M.; Silva, L.F.; Chavante, S.F.; Abreu, L.D.; Dietrich, C.P.; Nader, H.B. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schröederi. Plant Sci. 1998, 132, 215–228. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Dantas, S.N.; Gomes, D.L.; Cordeiro, S.L.; Sabry, D.A.; Costa, L.S.; Freitas, M.L.; Silva, N.B.; Moura, C.E.B.; Lemos, T.M.A.M. Evaluation of acute and subchronic toxicity of a non-anticoagulant, but antithrombotic algal heterofucan from the Spatoglossum schröederi in Wistar rats. Rev. Bras. Farmacogn. 2011, 21, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Silveira, R.F.M.; Silva, F.V.; Felipe, M.B.M.C.; Batistuzzo, S.R.; Leite, E.L.; Rocha, H.A.O. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef]
- Nobre, L.T.; Vidal, A.A.; Almeida, A.J.; Oliveira, R.M.; Paredes-Gamero, E.J.; Medeiros, V.P.; Trindade, E.S.; Franco, C.R.; Nader, H.B.; Rocha, H.A. Fucan effect on CHO cell proliferation and migration. Carbohydr. Polym. 2013, 15, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.P.; Dietrich, S.M. Electrophoretic behaviour of acidic mucopolysaccharides in diamine buffers. Anal. Biochem. 1976, 70, 645–647. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using urtica dioica linn. Leaves and their synergistic effects with antibiotics. J. Radiat. Res. 2015, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, Z. Silver nanoparticles to self-assembled films: Green synthesis and characterization. Colloids Surf. B Biointerfaces 2012, 90, 48–52. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Lv, H. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013, 8, 1809. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, I.R.L.; Queiroz, K.C.S.; Alves, L.G.; Santos, E.A.; Leite, E.L.; Rocha, H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, D.; Mollick, M.M.; Mondal, D.; Bhowmick, B.; Bain, M.K.; Bankura, K.S.J.; Acharya, K.C.D. Synthesis of methylcellulose-silver nanocomposite and investigation of mechanical and antimicrobial properties. Carbohydr. Polym. 2012, 90, 1818–1825. [Google Scholar] [CrossRef]
- Brito, T.K.; Silva, V.R.L.; Gonçalves, M.C.J.; Silva, B.J.; Lopes, S.J.F.; Campos, M.M.J.; Melo-Silveira, R.F.; Almeida-Lima, J.; Lima, P.D.; Sousa, S.M.; et al. Synthesis of Silver Nanoparticle Employing Corn Cob Xylan as a Reducing Agent with Anti-Trypanosoma cruzi Activity. Int. J. Nanomed. 2020, 12, 965–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Suzuki, M.; Fujita, K.; Horie, M.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Nakamura, A.; Kinugasa, S. Reliable size determination of nanoparticles using dynamic light scattering method for in vitro toxicology assessment. Toxicol. In Vitr. 2009, 23, 927–934. [Google Scholar] [CrossRef]
- Venkatesan, J.; Singh, S.K.; Anil, S.; Kim, S.-K.; Shim, M.S. Preparation, Characterization and Biological Applications of Biosynthesized Silver Nanoparticles with Chitosan-Fucoidan Coating. Molecules 2018, 23, 1429. [Google Scholar] [CrossRef] [Green Version]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 30, 13608–13619. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- El-Rafie, H.M.; El-Rafie, M.H.; Zahran, M.K. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuma, A.A.; Barrias, E.S.; de Souza, W. Basic Biology of Trypanosoma cruzi. Curr. Pharm. Des. 2021, 27, 1671–1732. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.; de Castro, S.L. Clear Shot at Primary Aim: Susceptibility of Trypanosoma cruzi Organelles, Structures and Molecular Targets to Drug Treatment. Curr. Top. Med. Chem. 2017, 17, 1212–1234. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of metallic nanoparticles. Wires Nanomed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zou, P.; Tyner, K.; Lee, S. Physiologically based pharmacokinetic (PBPK) modeling of pharmaceutical nanoparticles. AAPS J. 2017, 19, 26–42. [Google Scholar] [CrossRef]
- Singh, S.P.; Bhargava, C.; Dubey, V.; Mishra, A.; Singh, Y. Silver nanoparticles: Biomedical applications, toxicity, and safety issues. Int. J. Res. Pharm. Pharm. Sci. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2015, 13, 12. [Google Scholar] [CrossRef] [Green Version]


| Sample | Sugar (%) | Proteins (%) | Phenolic c. (%) | Molar Ratio a | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fuc | Xyl | GluA | Gal | Man | Sulfate | ||||
| F0.5v | 88.2 ± 1.9 | 0.03 ± 0.04 | 0.01 ± 0.01 | 1.0 | 0.5 | 4.0 | nd | nd | 1.3 |
| F0.6v | 87.1± 0.9 | 0.04 ± 0.01 | <0.01 | 1.0 | 0.6 | 4.0 | 0.3 | 0.2 | 1.3 |
| F0.7v | 85.2 ± 2.3 | nd | <0.01 | 1.0 | 0.9 | 0.5 | 1.3 | 0.1 | 1.7 |
| F0.9v | 79.3 ± 3.9 | nd | <0.01 | 1.0 | 0.6 | 0.2 | 1.6 | nd | 2.0 |
| F1.1v | 78.7 ± 0.9 | nd | <0.01 | 1.0 | 0.7 | 0.1 | 1.3 | nd | 2.0 |
| F1.3v | 77.6 ± 1.7 | nd | nd | 1.0 | 1.1 | nd | 0.7 | nd | 2.2 |
| F2.0v | 77.0 ± 5.9 | 0.03 ± 0.01 | nd | 1.0 | 0.4 | nd | 1.0 | nd | 2.3 |
| Month | AgFuc Diameter (nm) | Polydispersity Index |
|---|---|---|
| 1 | 283.8 ± 15.20 | 0.32 ± 0.03 |
| 2 | 278.5 ± 21.02 | 0.33 ± 0.02 |
| 3 | 267.9 ± 19.72 | 0.30 ± 0.04 |
| 4 | 261.4 ± 15.41 * | 0.31 ± 0.02 |
| 5 | 245.1 ± 14.72 * | 0.35 ± 0.03 |
| % Inhibition | ||||
|---|---|---|---|---|
| Samples | 25 µg/mL | 50 µg/mL | 100 µg/mL | |
| 24 h | BNZ | 6.1± 1.3 *a | 20 ± 2.1 **a | 23 ± 1.3 **a |
| F0.5v | 0.0 | 0.0 | 43.0 ± 1.1 ***b | |
| AgFuc | 0.0 | 14.1 ± 2.2 **a | 58.9 ± 1.2 ***c | |
| Ag | 4.4 ± 1.3 *a | 10.0 ± 1.7 **a | 14.4 ± 0.85 **a | |
| F0.5v:Ag (9:1) | 0.0 | 0.0 | 40.0 ± 0.8 ***b | |
| Control # | 0.0 | 0.0 | 0.0 | |
| 48 h | BNZ | 20 ± 2.1 **a | 41.0 ± 1.4 ***a | 47.0 ± 1.4 ***a |
| F0.5v | 0.0 | 5.66 ± 1.4 *b | 59.4 ± 1.4 ***b | |
| AgFuc | 0.6 ± 1.0 | 51.0 ± 3.1 ***c | 67.3 ± 2.1 ***c | |
| Ag | 7.6 ± 1.7 *b | 13.2 ± 1.8 **d | 25.8 ± 2.1 **d | |
| F0.5v:Ag (9:1) | 0.0 | 4.3 ± 1.5 *b | 55.4 ± 2.4 ***b | |
| Control # | 0.0 | 0.0 | 0.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, A.O.; Oliveira, J.W.d.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity. Nanomaterials 2022, 12, 2059. https://doi.org/10.3390/nano12122059
Souza AO, Oliveira JWdF, Moreno CJG, de Medeiros MJC, Fernandes-Negreiros MM, Souza FRM, Pontes DL, Silva MS, Rocha HAO. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity. Nanomaterials. 2022; 12(12):2059. https://doi.org/10.3390/nano12122059
Chicago/Turabian StyleSouza, Adriana Oliveira, Johny Wysllas de Freitas Oliveira, Claudia Jéssica Gonsalves Moreno, Mayra Jane Campos de Medeiros, Marília Medeiros Fernandes-Negreiros, Flavia Roberta Monteiro Souza, Daniel Lima Pontes, Marcelo Sousa Silva, and Hugo Alexandre Oliveira Rocha. 2022. "Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity" Nanomaterials 12, no. 12: 2059. https://doi.org/10.3390/nano12122059