Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects
Abstract
:1. Introduction
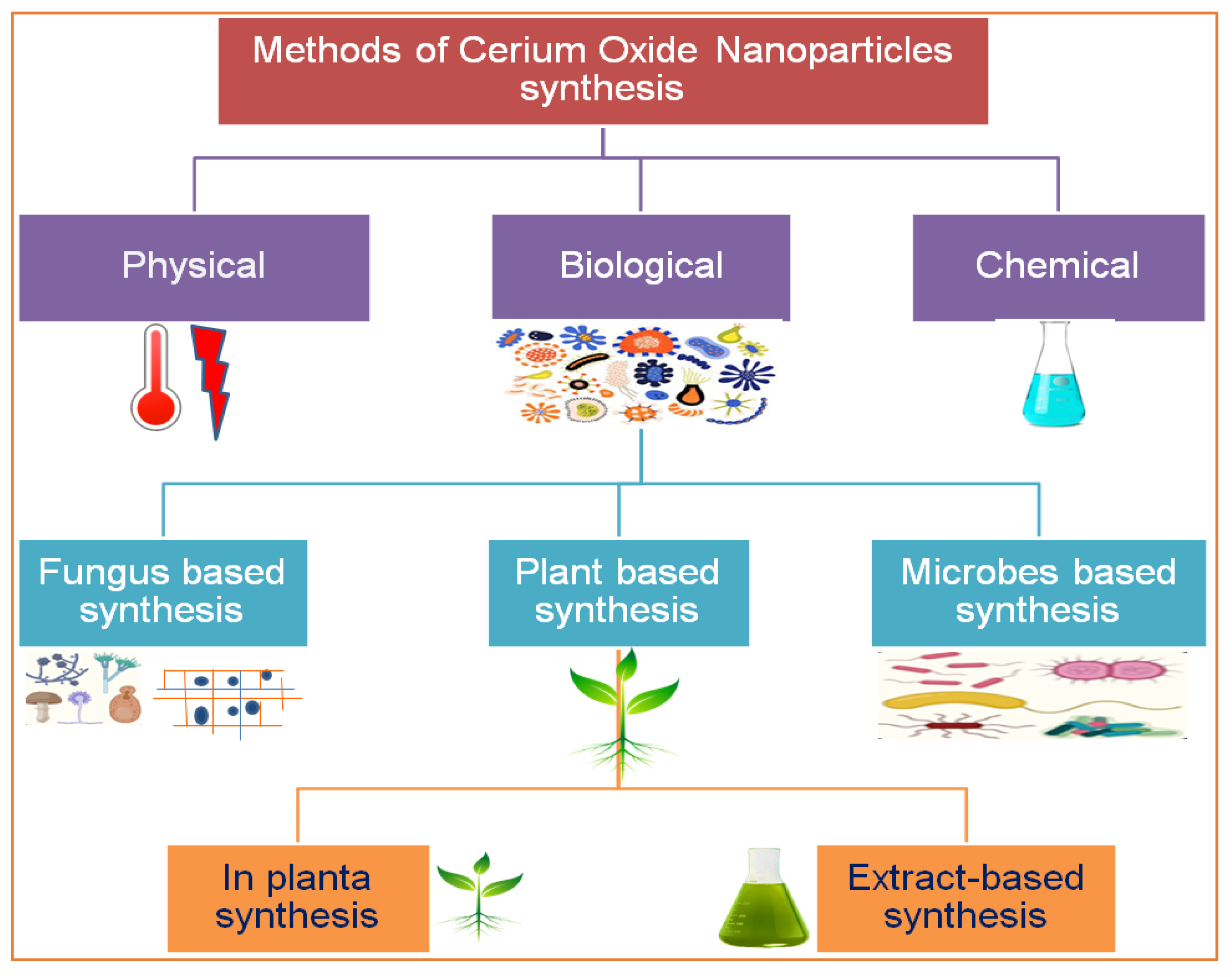
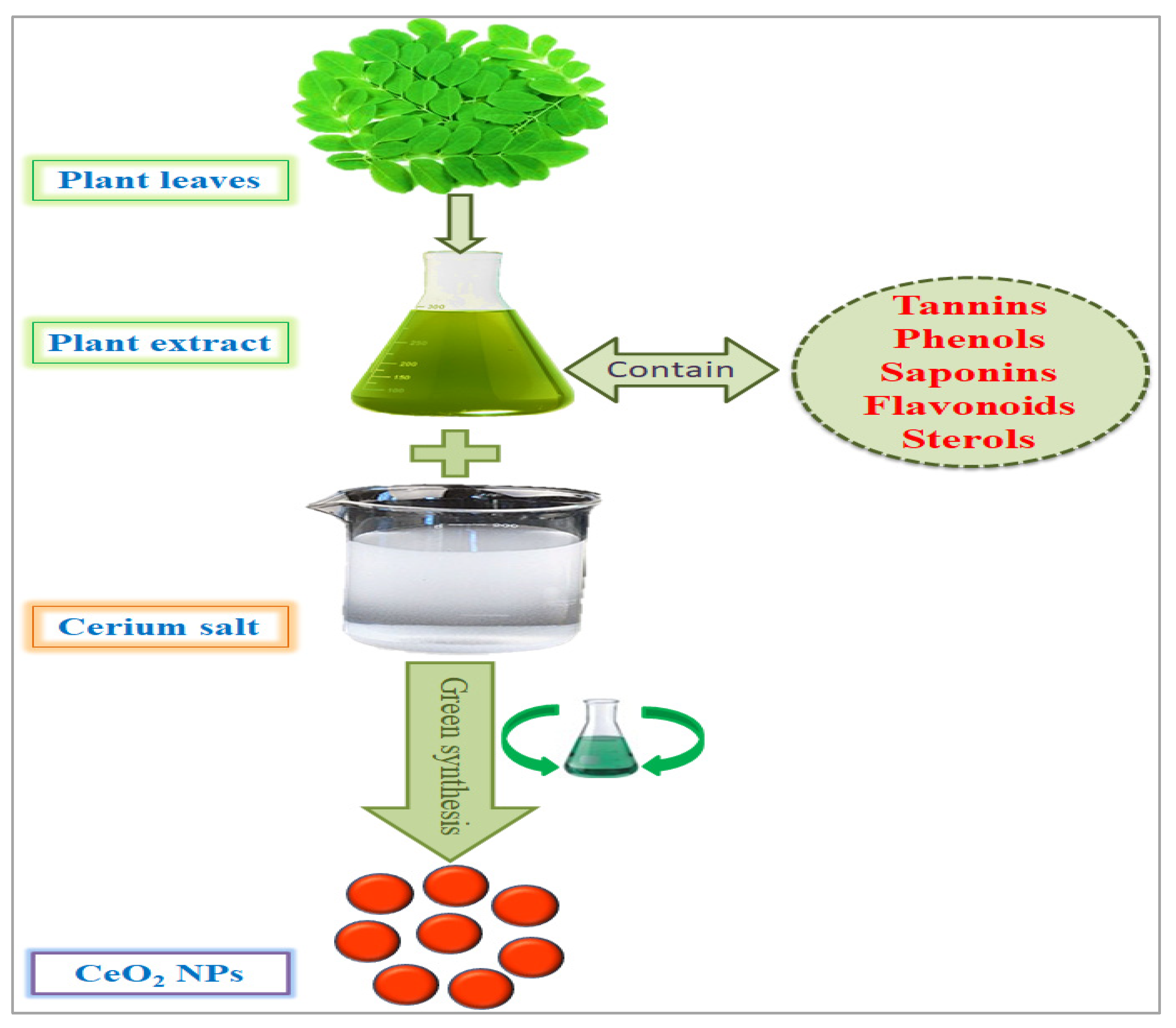
2. Phytosynthesis of CeO2 NPs and Other Alternative Approaches
3. Physicochemical Parameters Affecting the Synthesis of Green Cerium Oxide Nanoparticles
4. Green Cerium Oxide Nanoparticles as Strong Antioxidant Agents
5. Green Cerium Oxide Nanoparticles as Effective Anticancer Agents
6. Green Cerium Oxide Nanoparticles as a Potential Drug Delivery Vehicle
7. Antidiabetic Potential of Green Cerium Oxide Nanoparticles
8. Green Cerium Oxide Nanoparticles as Effective Potential Antimicrobial Agents
9. Green Cerium Oxide Nanoparticles as Potential Antifungal Agents
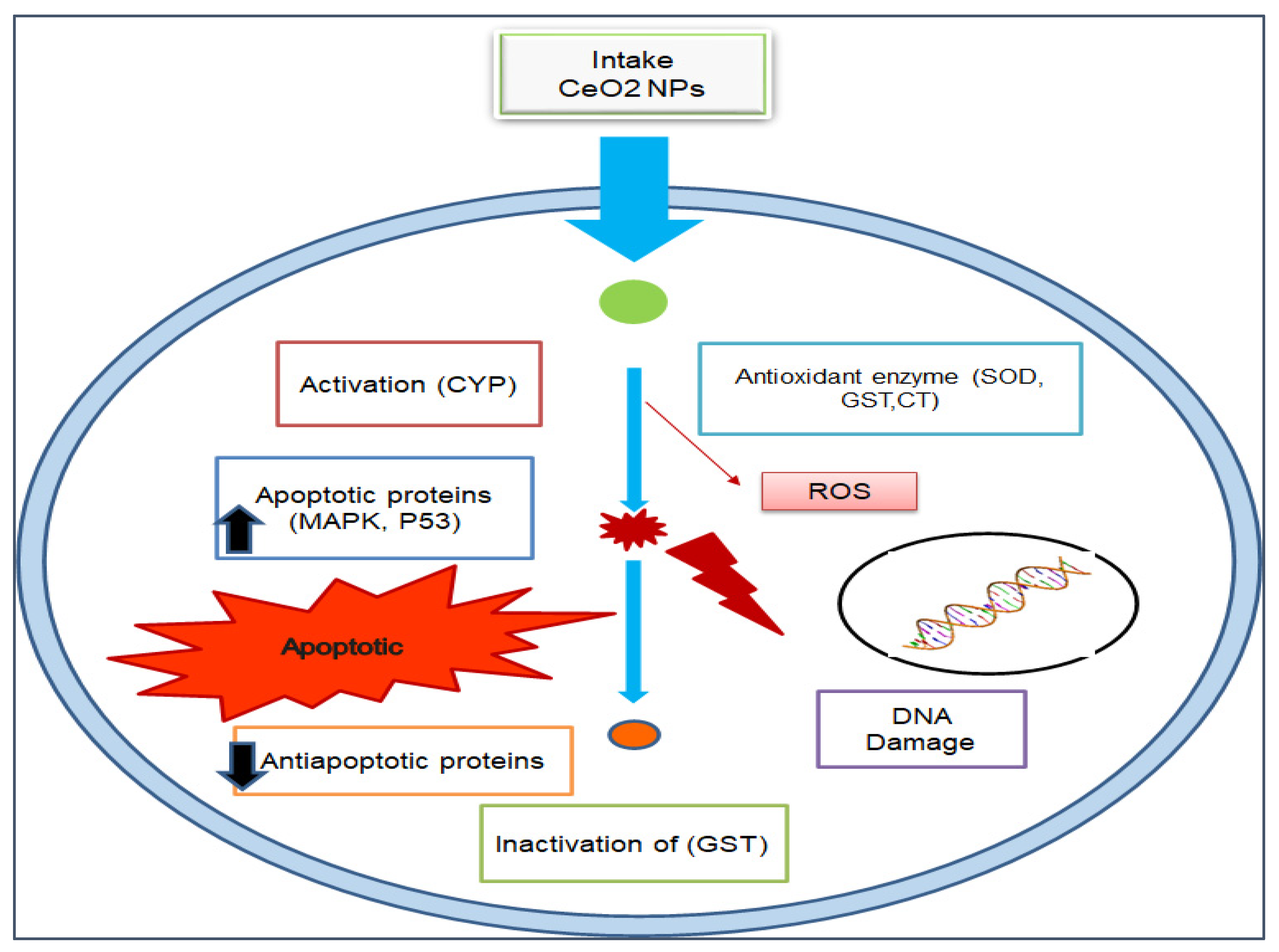
10. Cytotoxicity of Cerium Oxide Nanoparticles
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hembruff, S.L.; Cheng, N. Chemokine signaling in cancer: Implications on the tumor microenvironment and therapeutic targeting. Cancer Ther. 2009, 7, 254. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2907742/pdf/nihms124893.pdf (accessed on 10 May 2022). [PubMed]
- Gallucci, N.; Vitiello, G.; Di Girolamo, R.; Imbimbo, P.; Monti, D.M.; Tarallo, O.; Vergara, A.; Russo Krauss, I.; Paduano, L. Towards the development of antioxidant cerium oxide nanoparticles for biomedical applications: Controlling the properties by tuning synthesis conditions. Nanomaterials 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Es-haghi, A.; Javadi, F.; Yazdi, M.E.T.; Amiri, M.S. The expression of antioxidant genes and cytotoxicity of biosynthesized cerium oxide nanoparticles against hepatic carcinoma cell line. Avicenna J. Med. Biochem. 2019, 7, 16–20. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, E90. [Google Scholar] [CrossRef]
- Beaudoux, X.; Virot, M.; Chave, T.; Durand, G.; Leturcq, G.; Nikitenko, S.I. Vitamin C boosts ceria-based catalyst recycling. Green Chem. 2016, 18, 3656–3668. [Google Scholar] [CrossRef]
- Sathyaseelan, B.; Sambasivam, S.; Alagesan, T.; Sivakumar, K. Ex-situ studies on calcinations of structural, optical and morphological properties of post-growth nanoparticles CeO2 by HRTEM and SAED. Int. J. Nano Dimens. 2014, 5, 341–349. [Google Scholar] [CrossRef]
- Liying, H.; Yumin, S.; Lanhong, J.; Shikao, S. Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: A review. J. Rare Earths 2015, 33, 791–799. [Google Scholar] [CrossRef]
- Culcasi, M.; Benameur, L.; Mercier, A.; Lucchesi, C.; Rahmouni, H.; Asteian, A.; Casano, G.; Botta, A.; Kovacic, H.; Pietri, S. EPR spin trapping evaluation of ROS production in human fibroblasts exposed to cerium oxide nanoparticles: Evidence for NADPH oxidase and mitochondrial stimulation. Chem.-Biol. Interact. 2012, 199, 161–176. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, I.; Singh, N.; Singh, H. Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2019, 182, 109410. [Google Scholar] [CrossRef]
- Farias, I.A.P.; Santos, C.C.L.D.; Sampaio, F.C. Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: A systematic review. BioMed Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, J.-J.; Wang, H.; Li, Y.-R.; Zhu, J.-M.; Zhu, J.-J. Ultrasonic-induced synthesis of CeO2 nanotubes. J. Cryst. Growth 2005, 281, 525–529. [Google Scholar] [CrossRef]
- Morabito, K.; Shapley, N.; Steeley, K.; Tripathi, A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Moscato, S.; Ronca, F.; Nitti, S.; Mattoli, V.; Giorgi, M.; Ciofani, G. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Q.; Nazar, M.; Maqbool, A.; Pervez, M.T.; Jabeen, N.; Hussain, T.; Franklin, G. CuO and CeO2 nanostructures green synthesized using olive leaf extract inhibits the growth of highly virulent multidrug resistant bacteria. Front. Pharmacol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Srikar, S.; Giri, D.; Pal, D.; Mishra, P.; Upadhyay, S. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Dhall, A.; Self, W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Sisubalan, N.; Ramkumar, V.S.; Pugazhendhi, A.; Karthikeyan, C.; Indira, K.; Gopinath, K.; Hameed, A.S.H.; Basha, M.H.G. ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ. Sci. Pollut. Res. 2018, 25, 10482–10492. [Google Scholar] [CrossRef]
- Kargar, H.; Ghasemi, F.; Darroudi, M. Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceram. Int. 2015, 41, 1589–1594. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of cerium oxide nanoparticles—A review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.-U.-R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, T.; Karpagasundaram, M.; Rajarathinam, N. Ultrasound assisted green synthesis of cerium oxide nanoparticles using Prosopis juliflora leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Pol. 2017, 35, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Rajan, A.R.; Rajan, A.; Philip, D.; John, A. Antifungal activities of biogenic Au and CeO2 nanoparticles. AIP Conf. Proc. 2019, 2162, 020010. [Google Scholar]
- Senthilkumar, R.; Bhuvaneshwari, V.; Malayaman, V.; Chitra, G.; Ranjithkumar, R.; Dinesh, K.; Chandarshekar, B. Biogenic method of cerium oxide nanoparticles synthesis using wireweed (Sida acuta Burm. f.) and its antibacterial activity against Escherichia coli. Mater. Res. Express 2019, 6, 105026. [Google Scholar] [CrossRef]
- Patil, S.N.; Paradeshi, J.S.; Chaudhari, P.B.; Mishra, S.J.; Chaudhari, B.L. Bio-therapeutic potential and cytotoxicity assessment of pectin-mediated synthesized nanostructured cerium oxide. Appl. Biochem. Biotechnol. 2016, 180, 638–654. [Google Scholar] [CrossRef]
- Aseyd Nezhad, S.; Es-haghi, A.; Tabrizi, M.H. Green synthesis of cerium oxide nanoparticle using Origanum majorana L. leaf extract, its characterization and biological activities. Appl. Organomet. Chem. 2020, 34, e5314. [Google Scholar] [CrossRef]
- Qian, J.; Chen, F.; Zhao, X.; Chen, Z. China rose petal as biotemplate to produce two-dimensional ceria nanosheets. J. Nanopart. Res. 2011, 13, 7149–7158. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Mohana, V.; Manoharan, C. Green synthesis of cerium oxide nanoparticles using Calotropis procera flower extract and their photocatalytic degradation and antibacterial activity. Inorg. Chem. Commun. 2020, 119, 108086. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C.; Jayakumar, S.J. Synthesis of CeO2-NPs by chemical and biological methods and their photocatalytic, antibacterial and in vitro antioxidant activity. Res. Chem. Intermed. 2020, 46, 2705–2729. [Google Scholar] [CrossRef]
- Priya, G.S.; Kanneganti, A.; Kumar, K.A.; Rao, K.V.; Bykkam, S. Biosynthesis of cerium oxide nanoparticles using Aloe barbadensis miller gel. Int. J. Sci. Res. Publ. 2014, 4, 199–224. [Google Scholar]
- Maqbool, Q.; Nazar, M.; Naz, S.; Hussain, T.; Jabeen, N.; Kausar, R.; Anwaar, S.; Abbas, F.; Jan, T. Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. Int. J. Nanomed. 2016, 11, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.K.; Srivastava, P.; Ameen, S.; Akhtar, M.S.; Sengupta, S.; Singh, G. Phytoconstituents assisted green synthesis of cerium oxide nanoparticles for thermal decomposition and dye remediation. Mater. Res. Bull. 2017, 91, 98–107. [Google Scholar] [CrossRef]
- Arumugam, A.; Karthikeyan, C.; Hameed, A.S.H.; Gopinath, K.; Gowri, S.; Karthika, V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C 2015, 49, 408–415. [Google Scholar] [CrossRef]
- Reddy Yadav, L.; Manjunath, K.; Archana, B.; Madhu, C.; Raja Naika, H.; Nagabhushana, H.; Kavitha, C.; Nagaraju, G. Fruit juice extract mediated synthesis of CeO2 nanoparticles for antibacterial and photocatalytic activities. Eur. Phys. J. Plus 2016, 131, 154. [Google Scholar] [CrossRef]
- Nazaripour, E.; Mousazadeh, F.; Moghadam, M.D.; Najafi, K.; Borhani, F.; Sarani, M.; Ghasemi, M.; Rahdar, A.; Iravani, S.; Khatami, M. Biosynthesis of lead oxide and cerium oxide nanoparticles and their cytotoxic activities against colon cancer cell line. Inorg. Chem. Commun. 2021, 131, 108800. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M. Biosynthesis, characterization and cytotoxic activity of CeO2 nanoparticles. Ceram. Int. 2018, 44, 12642–12647. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, A. Fungus mediated synthesis of biomedically important cerium oxide nanoparticles. Mater. Res. Bull. 2013, 48, 4134–4138. [Google Scholar] [CrossRef]
- Miri, A.; Beiki, H.; Sarani, M. Cerium oxide nanoparticles: Biosynthesis, cytotoxic and UV protection. Preprints 2020, 2020070487. [Google Scholar] [CrossRef]
- Kannan, S.; Sundrarajan, M. A green approach for the synthesis of a cerium oxide nanoparticle: Characterization and antibacterial activity. Int. J. Nanosci. 2014, 13, 1450018. [Google Scholar] [CrossRef]
- Ashna, M.; Es-Haghi, A.; Karimi Noghondar, M.; Al Amara, D.; Yazdi, M.E.T. Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Mater. Technol. 2020, 1–8. [Google Scholar] [CrossRef]
- Gopinath, K.; Karthika, V.; Sundaravadivelan, C.; Gowri, S.; Arumugam, A. Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J. Nanostruct. Chem. 2015, 5, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Munusamy, S.; Bhakyaraj, K.; Vijayalakshmi, L.; Stephen, A.; Narayanan, V. Synthesis and characterization of cerium oxide nanoparticles using Curvularia lunata and their antibacterial properties. Int. J. Innov. Res. Sci. Eng. 2014, 2, 318. [Google Scholar]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surf. B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, A.M.; Borisovna, P.O.; Aleksandrovna, G.I.; Bagdasarovna, K.D.; Vladimirovich, B.D.; Vladimirovich, K.D.; Alexandrovich, F.A.; Yurievna, K.M.; Nikolaevna, B.E.; Aleksandrovich, K.D. “Green” Synthesis of Cerium Oxide Particles in Water Extracts Petroselinum crispum. Curr. Nanomater. 2019, 4, 176–190. [Google Scholar] [CrossRef]
- Miri, A.; Beiki, H.; Najafidoust, A.; Khatami, M.; Sarani, M. Cerium oxide nanoparticles: Green synthesis using Banana peel, cytotoxic effect, UV protection and their photocatalytic activity. Bioprocess Biosyst. Eng. 2021, 44, 1891–1899. [Google Scholar] [CrossRef]
- Altaf, M.; Manoharadas, S.; Zeyad, M.T. Green synthesis of cerium oxide nanoparticles using Acorus calamus extract and their antibiofilm activity against bacterial pathogens. Microsc. Res. Tech. 2021, 84, 1638–1648. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Mohamed, F.; Al-Quraishy, S.; Abdel-Baki, A.-A.S.; Abdel-Tawab, H. Green synthesis of Cerium oxide/Moringa oleifera seed extract nano-composite and its molluscicidsal activities against biomophalaria alexanderina. J. King Saud Univ. Sci. 2021, 33, 101368. [Google Scholar] [CrossRef]
- Thovhogi, N.; Diallo, A.; Gurib-Fakim, A.; Maaza, M. Nanoparticles green synthesis by Hibiscus sabdariffa flower extract: Main physical properties. J. Alloy. Compd. 2015, 647, 392–396. [Google Scholar] [CrossRef]
- Zafar, N.; Uzair, B.; Niazi, M.B.K.; Menaa, F.; Samin, G.; Khan, B.A.; Iqbal, H.; Menaa, B. Green Synthesis of Ciprofloxacin-Loaded Cerium Oxide/Chitosan Nanocarrier and its Activity against MRSA-Induced Mastitis. J. Pharm. Sci. 2021, 110, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Sebastiammal, S.; Sonia, S.; Henry, J.; Fathima, A.L. Green synthesis of cerium oxide nanoparticles using aloevera leaf extract and its optical properties. Songklanakarin J. Sci. Technol. 2021, 43, 582–587. Available online: https://rdo.psu.ac.th/sjstweb/journal/43-2/38.pdf (accessed on 10 May 2022).
- Sabouri, Z.; Sabouri, M.; Amiri, M.S.; Khatami, M.; Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles using Rheum turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef]
- Kalakotla, S.; Jayarambabu, N.; Mohan, G.K.; Mydin, R.B.S.; Gupta, V.R. A novel pharmacological approach of herbal mediated cerium oxide and silver nanoparticles with improved biomedical activity in comparison with Lawsonia inermis. Colloids Surf. B Biointerfaces 2019, 174, 199–206. [Google Scholar] [CrossRef]
- Chen, B.-H.; Stephen Inbaraj, B. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef]
- Miri, A.; Darroudi, M.; Sarani, M. Biosynthesis of cerium oxide nanoparticles and its cytotoxicity survey against colon cancer cell line. Appl. Organomet. Chem. 2020, 34, e5308. [Google Scholar] [CrossRef]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-mediated green synthesis of iron nanoparticles. J. Nanoparticles 2014, 2014, 140614. [Google Scholar] [CrossRef] [Green Version]
- Remya, V.; Abitha, V.; Rajput, P.S.; Rane, A.V.; Dutta, A. Silver nanoparticles green synthesis: A mini review. Chem. Int. 2017, 3, 165–171. [Google Scholar]
- Alamgir, A. Therapeutic Use of Medicinal Plants and Their Extracts; Springer Nature: Cham, Switzerland, 2017; Volume 1. [Google Scholar]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Samiee, S.; Nancarrow, P. Fabrication of cerium oxide nanoparticles: Characterization and optical properties. J. Colloid Interface Sci. 2011, 356, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Gupta, A.; Das, S.; Neal, C.J.; Seal, S. Controlling the surface chemistry of cerium oxide nanoparticles for biological applications. J. Mater. Chem. B 2016, 4, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Cappello, V.; Gemmi, M.; Athanassiou, A.; Mazzolai, B.; Mattoli, V. Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: Proliferation, differentiation, and dopamine secretion. Pharm. Res. 2013, 30, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Turin-Moleavin, I.-A.; Fifere, A.; Lungoci, A.-L.; Rosca, I.; Coroaba, A.; Peptanariu, D.; Nastasa, V.; Pasca, S.-A.; Bostanaru, A.-C.; Mares, M. In vitro and in vivo antioxidant activity of the new magnetic-cerium oxide nanoconjugates. Nanomaterials 2019, 9, 1565. [Google Scholar] [CrossRef] [Green Version]
- Estevez, A.Y.; Ganesana, M.; Trentini, J.F.; Olson, J.E.; Li, G.; Boateng, Y.O.; Lipps, J.M.; Yablonski, S.E.; Donnelly, W.T.; Leiter, J.C. Antioxidant enzyme-mimetic activity and neuroprotective effects of cerium oxide nanoparticles stabilized with various ratios of citric acid and EDTA. Biomolecules 2019, 9, 562. [Google Scholar] [CrossRef] [Green Version]
- Moskvin, M.; Marková, I.; Malínská, H.; Miklánková, D.; Hüttl, M.; Oliyarnyk, O.; Pop-Georgievski, O.; Zhigunov, A.; Petrovský, E.; Horák, D. Cerium oxide-decorated γ-Fe2O3 nanoparticles: Design, synthesis and in vivo effects on parameters of oxidative stress. Front. Chem. 2020, 8, 682. [Google Scholar] [CrossRef]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Javad Hoseini, S.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium oxide nanoparticles (Nanoceria): Hopes in soft tissue engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef]
- Mehta, A.; Scammon, B.; Shrake, K.; Bredikhin, M.; Gil, D.; Shekunova, T.; Baranchikov, A.; Ivanov, V.; Reukov, V. Nanoceria: Metabolic interactions and delivery through PLGA-encapsulation. Mater. Sci. Eng. C 2020, 114, 111003. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Javed, B. Synergistic effects of physicochemical parameters on bio-fabrication of mint silver nanoparticles: Structural evaluation and action against HCT116 colon cancer cells. Int. J. Nanomed. 2020, 15, 3621. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyata, S.; Nishimura, S.; Iijima, K.; Makita, M.; Akiyama, F.; Iwase, T. Malignant transformation of breast fibroadenoma to malignant phyllodes tumor: Long-term outcome of 36 malignant phyllodes tumors. Breast Cancer 2011, 18, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.-H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M.; Livney, Y.D.; Assaraf, Y.G. Targeted nanomedicine for cancer therapeutics: Towards precision medicine overcoming drug resistance. Drug Resist. Updates 2017, 31, 15–30. [Google Scholar] [CrossRef]
- Gao, R.; Mitra, R.N.; Zheng, M.; Wang, K.; Dahringer, J.C.; Han, Z. Developing Nanoceria-Based pH-Dependent Cancer-Directed Drug Delivery System for Retinoblastoma. Adv. Funct. Mater. 2018, 28, 1806248. [Google Scholar] [CrossRef]
- Sridharan, M.; Kamaraj, P.; Arockiaselvi, J.; Pushpamalini, T.; Vivekanand, P.; Kumar, S.H. Synthesis, characterization and evaluation of biosynthesized Cerium oxide nanoparticle for its anticancer activity on breast cancer cell (MCF 7). Mater. Today Proc. 2021, 36, 914–919. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; Khoshdel-Sarkarizi, H.; Nedaeinia, R.; Sadeghnia, H.R.; Hasanzadeh, L.; Darroudi, M.; Kazemi Oskuee, R. Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J. Cell. Physiol. 2019, 234, 4987–4996. [Google Scholar] [CrossRef]
- Nithya, P.; Sundrarajan, M. Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. J. Photochem. Photobiol. B Biol. 2020, 202, 111706. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Akbarpour Birjandi, S.; Sarani, M. Survey of cytotoxic and UV protection effects of biosynthesized cerium oxide nanoparticles. J. Biochem. Mol. Toxicol. 2020, 34, e22475. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, E.; Oskuee, R.K.; Hasanzadeh, L.; Mohajeri, M.; Hashemzadeh, A.; Rezayi, M.; Darroudi, M. Cytotoxic activity of greener synthesis of cerium oxide nanoparticles using carrageenan towards a WEHI 164 cancer cell line. Ceram. Int. 2018, 44, 19570–19575. [Google Scholar] [CrossRef]
- Hamidian, K.; Saberian, M.R.; Miri, A.; Sharifi, F.; Sarani, M. Doped and un-doped cerium oxide nanoparticles: Biosynthesis, characterization, and cytotoxic study. Ceram. Int. 2021, 47, 13895–13902. [Google Scholar] [CrossRef]
- Abbasi, N.; Homayouni Tabrizi, M.; Ardalan, T.; Roumi, S. Cerium oxide nanoparticles-loaded on chitosan for the investigation of anticancer properties. Mater. Technol. 2021, 1–11. [Google Scholar] [CrossRef]
- Wolkin, A.F.; Martin, C.A.; Law, R.K.; Schier, J.G.; Bronstein, A.C. Using poison center data for national public health surveillance for chemical and poison exposure and associated illness. Ann. Emerg. Med. 2012, 59, 56–61. [Google Scholar] [CrossRef]
- Qiu, W.-Y.; Wang, Y.-Y.; Wang, M.; Yan, J.-K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B. Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 2020, 134, 109853. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Hosseini, A.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Mohammadirad, A.; Pourkhalili, N.; Hassani, S.; Kamali, M.; Abdollahi, M. Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum. Exp. Toxicol. 2013, 32, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Di Liu, G.S.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; Du, Y. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; Mofarah, S.S.; Rawal, A.; Tomasetig, F.; Wang, X.; Yang, J.-L.; Koshy, P.; Sorrell, C.C. Green synthesis of zwitterion-functionalized nano-octahedral ceria for enhanced intracellular delivery and cancer therapy. ACS Sustain. Chem. Eng. 2019, 7, 9189–9201. [Google Scholar] [CrossRef]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome—Review of classical and new compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smokovski, I. Burden of Diabetes Prevalence. In Managing Diabetes in Low Income Countries; Smokovski, I., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Rahdar, A.; Aliahmad, M.; Hajinezhad, M.R.; Samani, M. Xanthan gum-stabilized nano-ceria: Green chemistry based synthesis, characterization, study of biochemical alterations induced by intraperitoneal doses of nanoparticles in rat. J. Mol. Struct. 2018, 1173, 166–172. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Shanker, K.; Naradala, J.; Mohan, G.K.; Kumar, G.; Pravallika, P. A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Adv. 2017, 7, 37158–37167. [Google Scholar] [CrossRef] [Green Version]
- Khurana, A.; Tekula, S.; Godugu, C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine 2018, 13, 1905–1922. [Google Scholar] [CrossRef]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W. Injectable, self-healable zwitterionic cryogels with sustained microRNA-cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Hirst, S.M.; Karakoti, A.; Singh, S.; Self, W.; Tyler, R.; Seal, S.; Reilly, C.M. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ. Toxicol. 2013, 28, 107–118. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Prashantha, S.; Sharma, S.; Dhananjaya, N.; Shivakumara, C.; Nagabhushana, B. Eco-friendly green synthesis, structural and photoluminescent studies of CeO2: Eu3+ nanophosphors using E. tirucalli plant latex. J. Alloy. Compd. 2014, 612, 425–434. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Bever, J.D.; Morton, J.B. Biogeography of arbuscular mycorrhizal fungi (Glomeromycota): A phylogenetic perspective on species distribution patterns. Mycorrhiza 2018, 28, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Ranque, S.; Prêcheur, I. Oral fungal-bacterial biofilm models in vitro: A review. Med. Mycol. 2018, 56, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerian, A.J. What is the Origin of Human Bacterial Flora? J. Appl. Environ. Microbiol. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.; Pillay, V. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasu, M.V.; Thirumamagal, R.; Srinivasan, M.; Al-Dhabi, N.A.; Ayeshamariam, A.; Kumar, D.S.; Punithavelan, N.; Jayachandran, M. Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol. B Biol. 2017, 173, 50–60. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Karasneh, G.A.; Al-Akhras, M.A.; Albiss, B.A.; Aljarah, K.M.; Al-Azzam, S.I.; Alzoubi, K.H. Cerium oxide and iron oxide nanoparticles abolish the antibacterial activity of ciprofloxacin against gram positive and gram negative biofilm bacteria. Cytotechnology 2015, 67, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Dong, Z.; Zhou, D.; Sun, K.; Zhao, Y.; Wang, B.; Chen, Y. Structure and immunostimulating activity of a galactofuranose-rich polysaccharide from the bamboo parasite medicinal fungus Shiraia bambusicola. J. Ethnopharmacol. 2020, 257, 112833. [Google Scholar] [CrossRef]
- Wani, S.H. Inducing fungus-resistance into plants through biotechnology. Not. Sci. Biol. 2010, 2, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Razmjou, A.; Yek, S.M.-G.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vartak, R.; Menon, S.; Patki, M.; Billack, B.; Patel, K. Ebselen nanoemulgel for the treatment of topical fungal infection. Eur. J. Pharm. Sci. 2020, 148, 105323. [Google Scholar] [CrossRef] [PubMed]
- Sinouvassane, D.; Wong, L.S.; Lim, Y.M.; Lee, P. A review on bio-distribution and toxicity of silver, titanium dioxide and zinc oxide nanoparticles in aquatic environment. Pollut. Res. 2016, 35, 701–712. [Google Scholar]
- Rahdar, A.; Beyzaei, H.; Askari, F.; Kyzas, G.Z. Gum-based cerium oxide nanoparticles for antimicrobial assay. Appl. Phys. A 2020, 126, 324. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Ali, M.; Zohra, T.; Akhtar, R.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Promising antiviral, antimicrobial and therapeutic properties of green nanoceria. Nanomedicine 2020, 15, 467–488. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhai, S.; Liu, Y.; Zhou, H.; Wu, J.; Jiao, Q.; Zhang, B.; Zhu, H.; Yan, B. Experimental modulation and computational model of nano-hydrophobicity. Biomaterials 2015, 52, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-related bioeffects induced by nanoparticles: The role of surface chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Zhang, H.; Zi, X.; Pan, X.; Chen, F.; Luo, W.; Li, J.; Zhu, H.; Hu, Y. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013, 4, e783. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| No. | Plant Name | Plant Part Used | Size of NPs | Activity | References |
|---|---|---|---|---|---|
| 1 | Calotropis procera | Flower | 20 nm | Biogenic CeO2 NPs exhibited important antibacterial activity against E. coli and Pseudomonas. | [30] |
| 2 | Solanum nigrum L. | Leaves | 20 nm | Biosynthesized CeO2 NPs exhibited the highest antibacterial activity against Gram-positive Bacillus subtilis and Gram-negative against E. coli. | [31] |
| 3 | Aloe barbadensis | Gel | 10 nm | Green CeO2 NPs showed high antioxidant potential. | [32] |
| 4 | Olea europaea L. | Leaves extract | 24 nm | Successful inhibition of fungal and bacterial strains. | [33] |
| 5 | Azadirachta indica | Leaves | 50 nm | CeO2 NPs exhibited a good photo-degradation rate. | [34] |
| 6 | Gloriosa superba L. | Leaves | 5 nm | CeO2 NPs exhibited good photoluminescence and antibacterial activities against Gram-positive and Gram-negative species. | [35] |
| 7 | Citrullus lanatus | Juice | 11.6 nm | Biosynthesized CeO2 NPs exhibited good photocatalytic activity and antibacterial potential by causing leakage of the bacterial membrane. | [36] |
| 8 | Prosopis fracta | Fruit | 15 nm | Green synthesized CeO2 NPs showed cellular toxicity against colon cancer cells. | [37] |
| 9 | Prosopis fracta | Aerial parts (leaves, branches) | 30 nm | Biosynthesized CeO2 NPs were found to be less effective against HT-29 cancer cells. | [38] |
| 10 | Camellia sinensis L. | Leaves | 5 nm | Biogenic CeO2 NPs were found to be protective against the oxidation of hepatic inflammation and oxidation of hepatic cells. | [38] |
| 11 | Humicola sp. | Fungus mycelia | 5 nm | Biosynthesized CeO2 NPs were found to be highly stable and did not agglomerate in an aqueous solution. | [39] |
| 12 | Salvadora persica L. | Whole plant extract | 20 nm | Green synthesized CeO2 NPs were found to be effective against a breast cancer cell line (MCF-7). | [119] |
| 13 | Musa sapientum L. | Fruit | 13 nm | Biosynthesized CeO2 NPs were found to be good sun-protective agents and anticancer agents against a lung (A549) cancer cell line. | [40] |
| 14 | Acalypha indica L. | Leaves | 15–30 nm | Biogenic CeO2 NPs showed antibacterial behavior against Gram-positive and Gram-negative species. | [41] |
| 15 | Brassica napus L. | Pollen grains | 4 nm | Green CeO2 NPs destroyed ovarian cancer cells (A2780). | [42] |
| 16 | Aspergillus niger | Fungus mycelia | 5–20 nm | Green CeO2 NPs exhibited high insecticidal potential against Aedes aegypti and antibacterial activity against Streptococcus pneumonia, Bacillus subtilis. | [43] |
| 17 | Origanum majorana L. | Leaves | 70 nm | Green synthesized CeO2 NPs could express SOD, CAT, POX, and antioxidant activities and were found to be highly cytotoxic against the MDA-MB-231 cancer cell line. | [28] |
| 18 | Prosopis juliflora | Leaves | 3.7 nm | Green synthesized CeO2 NPs were highly effective in killing both Gram-positive bacteria (Staphylococcus aureus, Streptococcus pneumonia) and Gram-negative bacteria (Pseudomonas aeruginosa, Proteus vulgaris). | [44] |
| 19 | Aloe vera (L.) | Leaves | 2–3 nm | Biogenic CeO2 NPs were found to be highly antioxidant agents. | [45] |
| 20 | Petroselinum crispum | Fruit | 25 nm | Green CeO2 NPs exhibited high antioxidative activity against various stresses in agricultural plants. | [46] |
| 21 | Musa sapientum L. | Peel extract | 4–13 nm | Green CeO2 NPs exhibited high photocatalytic activity. | [47] |
| 22 | Acorus calamus L. | Rhizome extract | 22.03 nm | Biogenic CeO2 NPs showed good antibacterial activity against Gram-positive and Gram-negative species. | [48] |
| 23 | Moringa oleifera | Seed | 30 nm | Green CeO2 NPs were found to express suitable insecticidal activity. | [49] |
| 24 | Hibiscus Sabdariffa L. | Flower | 3.9 nm | Green synthesized CeO2 NPs were found to be highly effective chelating agents. | [50] |
| 25 | Amomum subulatum | Seeds | 0.5 µm | Green CeO2 NPs were found to be highly effective against MRSA, methicillin-resistant S. aureus infection, which primarily affects animal mammary glands. | [51] |
| 26 | Aloe vera (L.) | Leaves | 7–12 nm | Green CeO2 NPs showed good optical properties at different concentrations of nanoparticles. | [52] |
| 27 | Sida acuta | Leaves | 8.2 nm | Green synthesized CeO2 NPs disrupted the cell membrane of E. coli and killed bacteria. | [26] |
| 28 | Rheum turkestanicum | Whole plant | 30 nm | Green synthesized CeO2 NPs exhibited photocatalytic and cytotoxic activities against PC12 cell lines. | [53] |
| 29 | Saccostrea cucullata | Whole mollusk | 15 nm | Biogenic CeO2 NPs exhibited suitable photocatalytic and cytotoxic activities. | [54] |
| 30 | Ceratoniasilique L. | Leaves | 100 nm | Green synthesized CeO2 NPs were found to be effective against the hepatic cancer cell line. | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Mashwani, Z.-u.-R.; Ikram, M.; Raja, N.I.; Mohamed, A.H.; Ren, G.; Omar, A.A. Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects. Nanomaterials 2022, 12, 2117. https://doi.org/10.3390/nano12122117
Khan M, Mashwani Z-u-R, Ikram M, Raja NI, Mohamed AH, Ren G, Omar AA. Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects. Nanomaterials. 2022; 12(12):2117. https://doi.org/10.3390/nano12122117
Chicago/Turabian StyleKhan, Maarij, Zia-ur-Rehman Mashwani, Muhammad Ikram, Naveed I. Raja, Azza H. Mohamed, Guogang Ren, and Ahmad A. Omar. 2022. "Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects" Nanomaterials 12, no. 12: 2117. https://doi.org/10.3390/nano12122117
APA StyleKhan, M., Mashwani, Z.-u.-R., Ikram, M., Raja, N. I., Mohamed, A. H., Ren, G., & Omar, A. A. (2022). Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects. Nanomaterials, 12(12), 2117. https://doi.org/10.3390/nano12122117









